1 Introduction
Ionic liquids (based imidazolium or other organic cations) have received considerable interest as eco-friendly solvents, catalysts and reagents in organic synthesis because of their unique properties, such as low volatility, non-flammability, high thermal stability, negligible vapor pressure and ability to dissolve a wide range of materials [1–9]. Among them, Brønsted acidic ionic liquids, with the useful characteristics of solid acids and mineral liquid acids, have designed to replace the traditional mineral liquid acids like sulfuric acid and hydrochloric acid in chemical procedures [10–14]. Considering the unique properties of imidazolium salts with Brønsted acidic property, and their successful applications in organic transformations [6–9], more recently, we have synthesized some sulfonic acid functionalized imidazolium salts, with Brønsted acidic property, including ionic liquid 3-methyl-1-sulfonic acid imidazolium chloride {[Msim]Cl} [10–13], ionic liquid 1,3-disulfonic acid imidazolium chloride {[Dsim]Cl} and 3-methyl-1-sulfonic acid imidazolium tetrachloroaluminate {[Msim]AlCl4} (as a solid) (Figs. 1 and 2) [13], and successfully applied them as highly efficient catalysts and reagents in organic synthesis [10–13]. Along this line, in this presented work, we have reported the preparation of two sulfonic acid functionalized imidazolium salts (SAFIS), 3-methyl-1-sulfonic acid imidazolium hexafluorophosphate(V) {[Msim]PF6} and 3-methyl-1-sulfonic acid imidazolium tetrafluoroborate {[Msim]BF4} as new ionic liquids (Fig. 2). We wish to use them as effective catalysts for different organic transformations. Herein, we have found that the preparation of 14-aryl-14H-dibenzo[a,j]xanthenes, tetrahydrobenzo[a]xanthene-11-ones and 1,8-dioxo-octahydroxanthenes can be efficiently performed in the presence of these ionic liquids under eco-friendly reaction conditions.
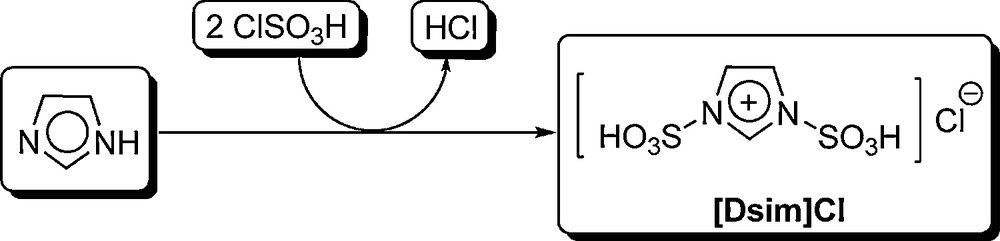
The preparation of sulfonic acid functionalized imidazolium salt [Dsim]Cl.
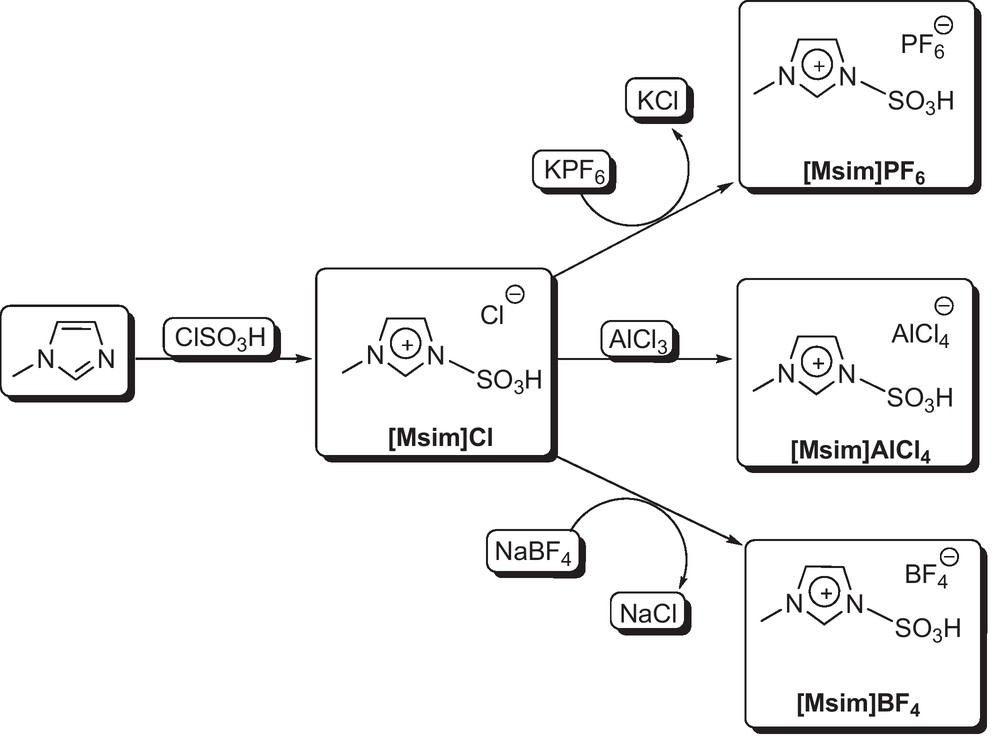
The preparation of the sulfonic acid functionalized imidazolium salts {[Msim]X}.
Design of highly efficient chemical reactions, which provide maximum structural complexity and diversity with a minimum number of synthetic steps to assemble compounds, is a major challenge of modern drug discovery [14]. Recently, multicomponent reactions have emerged as a highly valuable synthetic tool in the context of modern drug discovery. Atom economy and convergent character, simplicity of a one-pot procedure, possible structural variations, accessible complexity of molecules, and very large number of accessible compounds are among the described advantages of multicomponent reactions [15]. Thus, they are perfectly amenable to automation for combinatorial synthesis [16,17].
Xanthene derivatives are of importance as they have various biological activities such as antiplasmodial [18], and anti-inflammatory properties [19], and have been utilized as antagonists for drug-resistant leukemia lines [20]. Furthermore, these heterocyclic molecules have been widely used as dyes [21], and in pH-sensitive fluorescent materials [22] and laser technologies [23]. Many benzoxanthene derivatives are also potent nonpeptidic inhibitors of recombinant human calpain I [24], and novel CCR1 receptor antagonists [25]. Therefore, synthesis of xanthenes is of great importance. Synthesis of 14-aryl-14H-dibenzo[a,j]xanthenes has been achieved by the one-pot multicomponent condensation of β-naphthol (2 eq.) with aldehydes (1 eq.) (Scheme 1), in the presence of some catalysts such as selectfluor™ [26], KAl(SO4)2, 12H2O (alum) [27], Nano-TiO2 [28], MeSO3H [29], iodine [30], silica sulfuric acid [31], cyanuric chloride [32] and [Et3N-SO3H]Cl [33]. The best method for preparation of tetrahydrobenzo[a]xanthene-11-ones is the one-pot the multicomponent condensation reaction between β-naphthol, arylaldehydes and dimedone (5,5-dimethyl-1,3-cyclohexanedione) (Scheme 2) using some catalysts, e.g. sulfamic acid [34], NaHSO4/ionic liquid ([bmim]BF4) [35], dodecatungstophosphoric acid [36], iodine [37], InCl3/P2O5 [38] and p-toluenesulfonic acid/ionic liquid ([bmim]BF4) [39]. The most common protocol for the preparation of 1,8-dioxo-octahydroxanthenes involves the reaction of two molecules of dimedone with one molecule of aldehydes (Scheme 3) [40–42], in the presence of several catalysts, including [Et3N-SO3H]Cl [33], p-dodecylbenzenesulfonic acid [43], triethylbenzylammonium chloride [44], diammonium hydrogen phosphate [45], p-dodecylbenzenesulfonic acid under ultrasonic irradiation [46], MCM-41-SO3H using ultrasonic irradiation [47], SbCl3/SiO2 [48], silica-supported preyssler nano particles [49], sulfonic acid on silica gel (SiO2-R-SO3H) [50], and trimethylsilylchloride [51]. Nevertheless, many of the reported methods are associated with one or more of the following drawbacks: (i) low yields, (ii) long reaction times, (iii) the use of large amount of catalyst, (iv) the use of toxic or expensive catalysts, and (v) inefficiency of method.
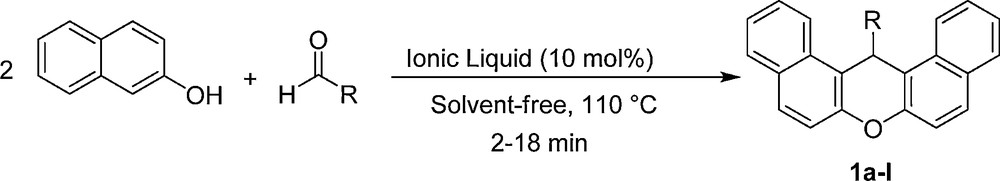
The reaction of β-naphthol with aldehydes catalyzed by the sulfonic acid functionalized imidazolium salts.
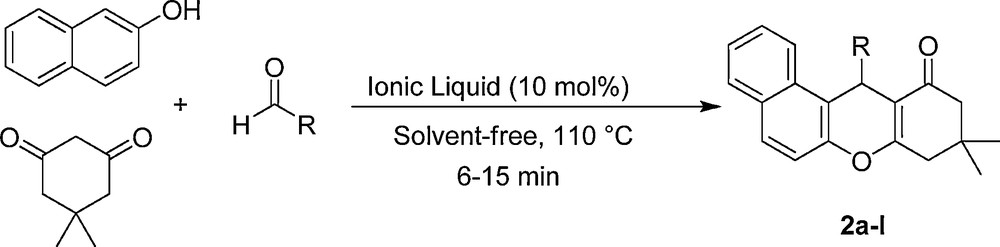
The reaction of β-naphthol, aldehydes and dimedone catalyzed by the SAFIS.
The reaction of dimedone with aldehydes under using the SAFIS.
Having the above points in mind, we report here our results on the efficient solvent-free synthesis of 14-aryl-14H-dibenzo[a,j]xanthenes, tetrahydrobenzo[a]xanthene-11-ones and 1,8-dioxo-octahydroxanthenes in the presence of a catalytic amount of ionic liquids 1,3-disulfonic acid imidazolium chloride {[Dsim]Cl}, 3-methyl-1-sulfonic acid imidazolium hexafluorophosphate(V) {[Msim]PF6} or 3-methyl-1-sulfonic acid imidazolium tetrafluoroborate {[Msim]BF4} under solvent-free conditions (Schemes 1, 2 and 3). Interestingly, these methods for the preparation of the xanthene derivatives have none of the above-mentioned drawbacks at all.
2 Results and discussion
At first, ionic liquid 1,3-disulfonic acid imidazolium chloride {[Dsim]Cl} was prepared by the reaction of imidazole (1 eq.) with chlorosulfonic acid (2 eq.) in CH2Cl2 (Fig. 1) [13]. In the next step, 3-methyl-1-sulfonic acid imidazolium chloride {[Msim]Cl} was prepared by the reaction of 1-methylimidazole with chlorosulfonic acid in CH2Cl2 with almost 100% atom economy [10–13]. Then, the other SAFIS, 3-methyl-1-sulfonic acid imidazolium hexafluorophosphate(V) {[Msim]PF6} and 3-methyl-1-sulfonic acid imidazolium tetrafluoroborate {[Msim]BF4}, were prepared by anion exchange procedure (Fig. 2). As it is shown in Fig. 2, [Msim]Cl was reacted with Lewis acids KPF6 and NaBF4 to afford ionic liquids [Msim]PF6 and [Msim]BF4, respectively. The structures of the SAFIS were identified by 1H, 13C and 31P NMR as well as mass spectra. The corresponding spectral data are reported in the Experimental section.
In another study, to confirm that [Msim]Cl was completely converted to [Msim]BF4, a solution of AgNO3 in distilled water was added to a solution of [Msim]BF4 in distilled water. The absence of AgCl precipitate indicates complete conversion of the [Msim]Cl to [Msim]BF4.
Thermal gravimetric analysis (TGA) of the sulfonic acid functionalized imidazolium salts was also studied (Fig. 3). As thermal gravimetry (TG) and differential thermal gravimetric (DTG) diagrams indicate, weight losses of [Dsim]Cl happened in four steps after 150 °C, and [Msim]PF6 and [Msim]BF4 were decomposed after 200 °C and 230 °C, respectively. The TG pattern of [Msim]PF6 and [Msim]BF4 is similar to single stage decomposition in which no intermediate was exactly identified. But, we observed multistage decomposition pattern in [Dsim]Cl. Some weight losses were observed about 10%, 30%, 15% and 44% which can be related to loss of CH2 = CH2 or HCl, SO3, CH3CN and ClSO3H, correspondingly. Therefore, [Msim]PF6, [Msim]BF4 and [Dsim]Cl could be applied as catalysts under 200 °C, 230 °C and 150 °C.
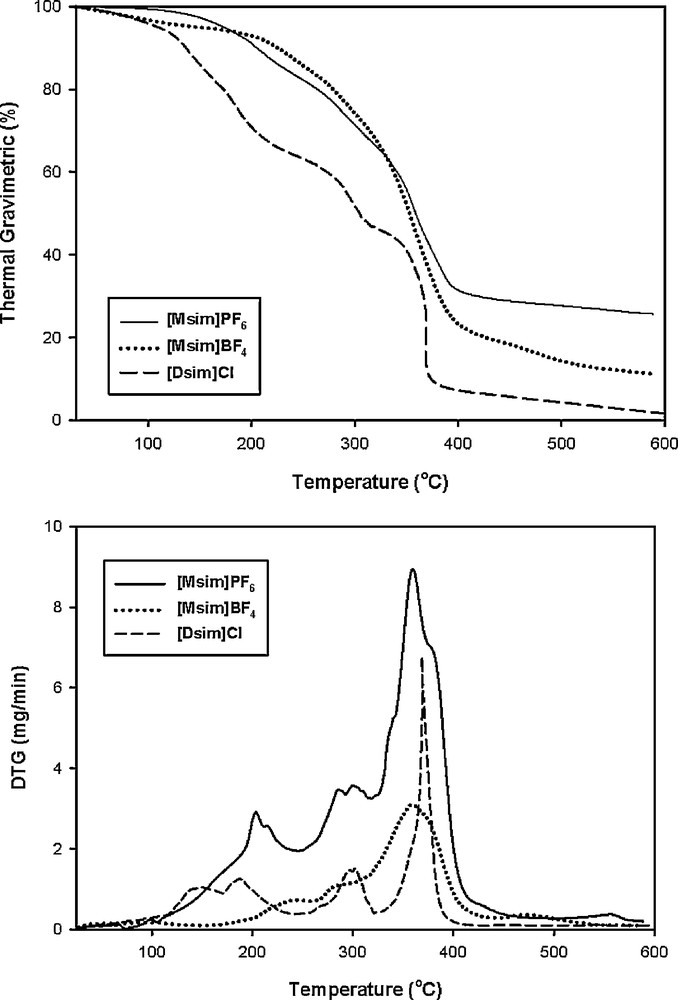
Thermal gravimetry (TG) and differential thermal gravimetry (DTG) of the [Dsim]Cl, [Msim]PF6 and [Msim]BF4.
Satisfactory application of these catalysts in organic synthesis encouraged us to use them for preparation of xanthene derivatives.
In our initial study on the applicability of the SAFIS in organic synthesis, we investigated the preparation of 14-aryl-14H-dibenzo[a,j]xanthenes from β-naphthol and aldehydes in the presence of them (Scheme 1). For this purpose, the condensation of β-naphthol (2 mmol) with 4-cholorobenzaldehyde (1 mmol) was examined using different amounts of [Dsim]Cl, [Msim]PF6 or [Msim]BF4 at various temperatures under solvent-free conditions. The results are summarized in Table 1. Interestingly, the three SAFIS were highly efficient, and 10 mol% of them was sufficient to afford the product in excellent yields and in very short reaction times at 110 °C (Table 1, entry 3). No improvement in the reaction results was observed by increasing the amount of the catalysts and the temperature. The solvent-free condensation was also tested at 110 °C without catalyst in which the reaction did not significantly progress even after long reaction time (12 h).
Effect of amounts of the catalysts and temperature on the condensation of β-naphthol (2 mmol with 4-cholorobenzaldehyde) (1 mmol).
| Entry | Catalyst amount (mol%) | Temp. (°C) | [Dsim]Cl | [Msim]PF6 | [Msim]BF4 | |||
| Time (min) | Yielda (%) | Time (min) | Yielda (%) | Time (min) | Yielda (%) | |||
| 1 | – | 110 | 720 | 32 | 720 | 29 | 720 | 30 |
| 2 | 5 | 110 | 8 | 88 | 14 | 89 | 17 | 91 |
| 3 | 7.5 | 110 | 5 | 90 | 9 | 93 | 10 | 90 |
| 4 | 10 | 110 | 3 | 98 | 6 | 98 | 7 | 97 |
| 5 | 10 | 120 | 3 | 97 | 6 | 99 | 6 | 95 |
| 6 | 10 | 100 | 5 | 92 | 8 | 90 | 11 | 91 |
| 7 | 10 | 90 | 9 | 93 | 11 | 91 | 12 | 92 |
| 8 | 10 | 80 | 14 | 90 | 15 | 89 | 18 | 87 |
| 9 | 15 | 110 | 3 | 98 | 6 | 98 | 7 | 98 |
a Isolated yield.
Under the optimal reaction conditions, β-naphthol was condensed with different aromatic aldehydes (including aldehydes bearing electron-releasing substituents, electron-withdrawing substituents or halogens on their aromatic ring) using [Dsim]Cl, [Msim]PF6 or [Msim]BF4 (Table 2). As it can be seen in Table 2, all catalysts were highly efficient and general, and gave the desired 14-aryl-14H-dibenzo[a,j]xanthenes in high yields and short reaction times.
The solvent-free synthesis of 14-aryl-14H-dibenzo[a,j]xanthenes using the sulfonic acid functionalized imidazolium salts.
| Productb | [Dsim]Cl | [Msim]PF6 | [Msim]BF4 | Mp °C (Lit.) | |||
| Time (min) | Yielda (%) | Time (min) | Yielda (%) | Time (min) | Yielda (%) | ||
| 5 | 90 | 7 | 85 | 8 | 88 | 186–188 (184–185) [28] | |
| 3 | 98 | 6 | 98 | 7 | 97 | 284–286 (289–290) [27] | |
| 2 | 97 | 4 | 97 | 5 | 98 | 207–209 (206–211) [28] | |
| 4 | 90 | 8 | 91 | 7 | 88 | 209–211 (214–216) [28] | |
| 3 | 98 | 5 | 95 | 6 | 93 | 186–188 (190–191) [28] | |
| 3 | 99 | 5 | 98 | 7 | 99 | 312–314 (311–312) [26] | |
| 3 | 96 | 4 | 99 | 7 | 94 | 215–217 (213–214) [26] | |
| 5 | 86 | 10 | 91 | 8 | 87 | 212–214 (214–215) [28] | |
| 6 | 99 | 7 | 98 | 8 | 96 | 225–227 (227–229) [28] | |
| 7 | 97 | 8 | 95 | 8 | 98 | 200–202 (203–205) [27] | |
| 10 | 90 | 15 | 88 | 13 | 91 | 289–292 (290–295) [53] | |
| 9 | 91 | 14 | 85 | 18 | 88 | 162–164 (163–165) [53] |
a Isolated yield.
b These reactions were carried out by the reaction of β-naphthol (2 mmol) with 4-cholorobenzaldehyde (1 mmol) using SAFIS (10 mol%) at 110 °C.
In a plausible mechanism (Scheme 4), at first, aromatic aldehyde is activated by the acidic group of [Dsim]Cl (or the other SAFIS) to produce I. Then, β-naphthol attacks to the carbonyl group of the activated aldehyde, and affords intermediate II. Next, by removing H2O from II, orthoquinone methide (o-QM, III) is prepared. [Dsim]Cl again activates intermediate III to give IV as a Michael acceptor. Afterward, Michael addition of another β-naphthol to intermediate IV affords V. Intermediate V converts to VI after tautomerisation. Finally, by removing H2O from VI, 14-aryl-14H-dibenzo[a,j]xanthene forms.
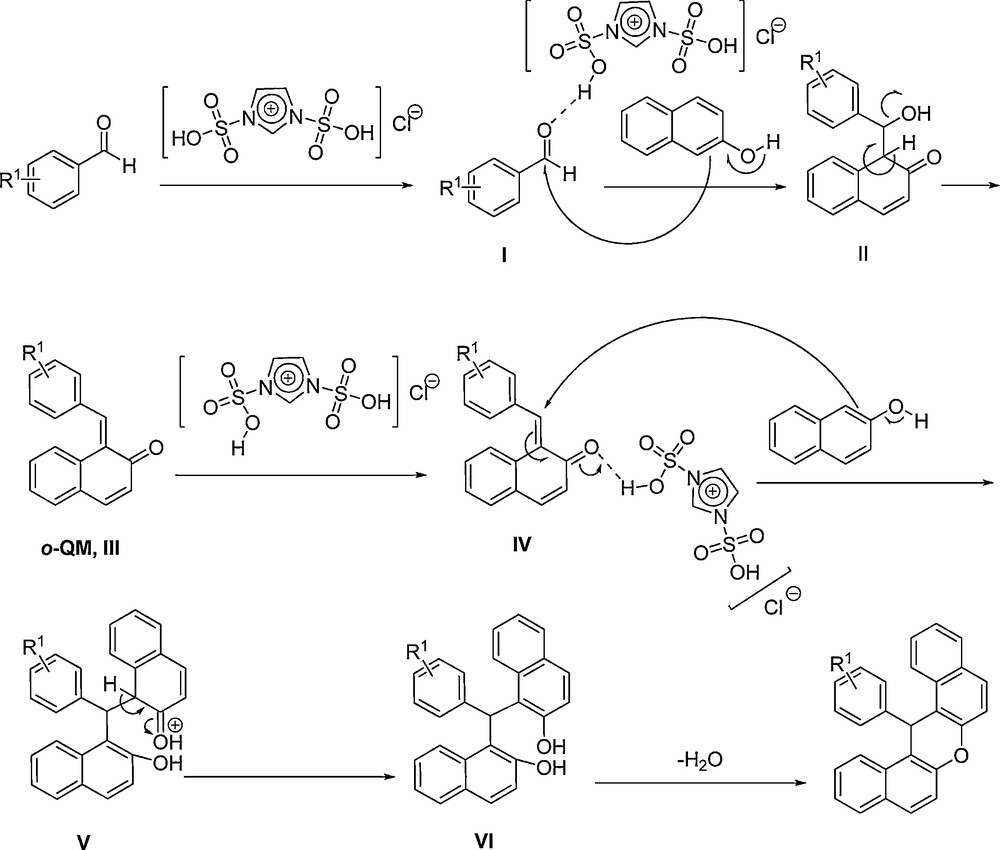
The plausible mechanism for the condensation reaction of β-naphthol with aldehydes using the SAFIS.
In another study, the reaction of aldehyde, β-naphthol and dimedone, to produce tetrahydrobenzo[a]xanthene-11-ones, in the presence of the SAFIS was investigated (Scheme 2). In this regard, the condensation of β-naphthol (1 mmol) with dimedone (1 mmol) and 4-cholorobenzaldehyde (1 mmol) was checked using different amounts of [Dsim]Cl, [Msim]PF6 or [Msim]BF4, at range of 90–120 °C in the absence of solvent (Table 3). As it is clear from Table 3, 10 mol% of the SAFIS was sufficient to promote the reaction efficiently at 110 °C, and afford the product in excellent yields and in very short reaction times (Table 3, entry 3). Moreover, [Msim]PF6 and [Msim]BF4 were more effective than [Dsim]Cl. The solvent-free condensation was also tested at 110 °C without catalyst in which the reaction did not significantly progress even after long reaction time (12 h).
Effect of amounts of the catalysts and temperature on the condensation of β-naphthol (1 mmol), dimedone (1 mmol) and 4-cholorobenzaldehyde (1 mmol).
| Entry | Catalyst amount (mol%) | Temp. (°C) | [Dsim]Cl | [Msim]PF6 | [Msim]BF4 | |||
| Time (min) | Yielda (%) | Time (min) | Yielda (%) | Time (min) | Yielda (%) | |||
| 1 | – | 110 | 720 | 26 | 720 | 22 | 720 | 21 |
| 2 | 5 | 110 | 15 | 80 | 13 | 90 | 12 | 88 |
| 3 | 7.5 | 110 | 13 | 86 | 10 | 89 | 10 | 91 |
| 4 | 10 | 110 | 10 | 88 | 8 | 93 | 7 | 95 |
| 5 | 10 | 120 | 9 | 70 | 8 | 90 | 6 | 91 |
| 6 | 10 | 100 | 11 | 81 | 12 | 88 | 15 | 90 |
| 7 | 10 | 90 | 17 | 84 | 14 | 88 | 17 | 91 |
| 8 | 10 | 80 | 20 | 80 | 17 | 86 | 22 | 89 |
| 9 | 15 | 110 | 10 | 88 | 8 | 92 | 7 | 95 |
a Isolated yield.
To assess the efficiency and the scope of the SAFIS in the synthesis of tetrahydrobenzo[a]xanthene-11-ones, various aromatic aldehydes (including aldehydes with electron-releasing substituents, electron-withdrawing substituents and halogens on their aromatic ring) were reacted with β-naphthol and dimedone in the optimal reaction conditions to furnish the corresponding products in high yields and in short reaction times. The results are tabulated in Table 4.
The solvent-free synthesis of tetrahydrobenzo[a]xanthene-11-ones using the sulfonic acid functionalized imidazolium salts.
| Productb | [Dsim]Cl | [Msim]PF6 | [Msim]BF4 | Mp °C (Lit.) | |||
| Time (min) | Yielda (%) | Time (min) | Yielda (%) | Time (min) | Yielda (%) | ||
| 15 | 85 | 12 | 90 | 10 | 93 | 148–151 (151–153) [38] | |
| 10 | 88 | 8 | 93 | 7 | 95 | 178–179 (180–182) [38] | |
| 14 | 88 | 8 | 93 | 7 | 95 | 175–178 (–) [39] | |
| 7 | 95 | 9 | 93 | 9 | 95 | 222–224 (223–225) [52] | |
| 6 | 93 | 8 | 92 | 7 | 93 | 179–181 (181–182) [36] | |
| 15 | 88 | 8 | 93 | 8 | 95 | 185–187 (186–187) [37] | |
| 13 | 90 | 8 | 92 | 10 | 92 | 161–164 (–) [39] | |
| 14 | 85 | 11 | 83 | 13 | 85 | 169–171 (170–172) [35] | |
| 13 | 88 | 10 | 95 | 8 | 97 | 175–178 (178–180) [38] | |
| 15 | 86 | 11 | 91 | 10 | 93 | 167–170 (168–170) [38] | |
| 10 | 88 | 9 | 90 | 8 | 92 | 237–239 (240–241) [38] | |
| 14 | 83 | 10 | 90 | 8 | 93 | 158–160 (159–162) [34] |
a Isolated yield.
b These reactions were carried out by the condensation of β-naphthol (1 mmol), dimedone (1 mmol) and 4-cholorobenzaldehyde (1 mmol) using SAFIS (10 mol%) at 110 °C.
Mechanistically (Scheme 5), at first, aromatic aldehyde is activated by acidic group of [Msim]X (X = PF6 or BF4) or [Dsim]Cl to produce I. Then, β-naphthol attacks to the carbonyl group of the activated aldehyde, and affords intermediate II. Next, by removing H2O from II, orthoquinone methide (o-QM, III) is prepared. [Msim]X again activates intermediate III to give IV as a Michael acceptor. Afterward, Michael addition of dimedone to intermediate IV affords V. Intermediate V converts to VI after tautomerisation. Finally, tetrahydrobenzo[a]xanthene-11- one produces by removing H2O from VI.
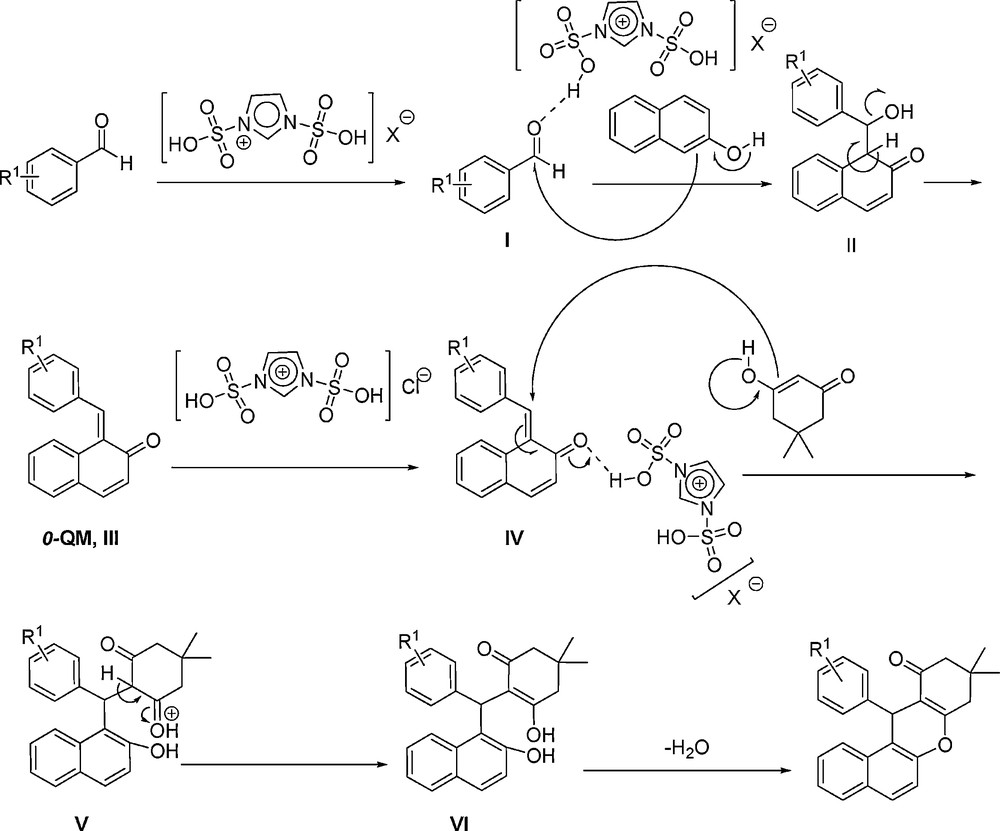
The plausible mechanism for the condensation reaction of β-naphthol with aldehydes and dimedone catalyzed by the SAFIS.
The last group of xanthenes mentioned in the present work is 1,8-dioxo-octahydroxanthenes. To optimize the reaction conditions, the solvent-free condensation of dimedone (2 mmol) with 4-cholorobenzaldehyde (1 mmol) (Scheme 3), using different molar ratios of [Dsim]Cl, [Msim]PF6 or [Msim]BF4, at range of 25–75 °C was studied (Table 5). As it can be seen in Table 5, [Dsim]Cl were more effective than [Msim]PF6 or [Msim]BF4, and the best results were obtained using 10 mol% of the ionic liquids at 70 °C (Table 5, entry 6). This condensation was also examined at 70 °C without catalyst under solvent-free condition in which the reaction did not significantly progress even after long reaction time (12 h).
Effect of amounts of the catalysts and temperature on the condensation of dimedone (2 mmol) and 4-cholorobenzaldehyde (1 mmol).
| Entry | Catalyst Amount (mol%) | Temp. (°C) | [Dsim]Cl | [Msim]PF6 | [Msim]BF4 | |||
| Time (min) | Yielda (%) | Time (min) | Yielda (%) | Time (min) | Yielda (%) | |||
| 1 | – | 70 | 720 | 14 | 720 | 13 | 720 | 10 |
| 2 | 5 | 70 | 20 | 70 | 25 | 65 | 22 | 60 |
| 3 | 7.5 | 70 | 8 | 73 | 15 | 70 | 17 | 71 |
| 4 | 10 | 25 | 60 | 40 | 60 | 35 | 60 | 38 |
| 5 | 10 | 45 | 15 | 50 | 30 | 44 | 30 | 48 |
| 6 | 10 | 65 | 4 | 70 | 10 | 66 | 12 | 68 |
| 7 | 10 | 70 | 3 | 98 | 5 | 95 | 6 | 94 |
| 8 | 10 | 75 | 3 | 96 | 4 | 92 | 5 | 93 |
| 9 | 15 | 70 | 3 | 98 | 4 | 96 | 6 | 94 |
a Isolated yield.
After optimization the reaction conditions, the efficacy of the catalysts was studied using various aromatic aldehydes to produce 1,8-dioxo-octahydroxanthenes (Table 6). As it is shown in Table 6, [Dsim]Cl, [Msim]PF6 as well as [Msim]BF4 were highly efficient and general, and the reaction efficiently proceeded using all kinds of aldehydes.
The solvent-free synthesis of 1,8-dioxo-octahydroxanthenes using the sulfonic acid functionalized imidazolium salts.
| Productb | [Dsim]Cl | [Msim]PF6 | [Msim]BF4 | Mp °C (Lit.) | |||
| Time (min) | Yielda (%) | Time (min) | Yielda (%) | Time (min) | Yielda (%) | ||
| 6 | 98 | 8 | 95 | 10 | 96 | 199–201 (201–203) [51] | |
| 3 | 97 | 13 | 91 | 15 | 95 | 224–226 (228–230) [49] | |
| 5 | 94 | 15 | 90 | 13 | 93 | 164–166 (168–170) [50] | |
| 7 | 85 | 11 | 87 | 13 | 81 | 220–222 (217–218) [48] | |
| 13 | 95 | 15 | 87 | 12 | 92 | 243–245 (248–250) [49] | |
| 11 | 91 | 14 | 91 | 17 | 88 | 178–180 (175–176) [48] | |
| 10 | 94 | 5 | 98 | 7 | 97 | 216–218 (219–221) [51] | |
| 7 | 90 | 10 | 83 | 13 | 85 | 249–251 (250–252) [33] | |
| 3 | 98 | 5 | 95 | 6 | 94 | 229–231 (230–232) [51] | |
| 5 | 96 | 10 | 93 | 9 | 89 | 186–187 (183–185) [48] | |
| 4 | 96 | 6 | 91 | 8 | 95 | 242–244 (240–242) [51] | |
| 6 | 80 | 3 | 84 | 5 | 85 | 172–174 (177–178) [50] |
a Isolated yield.
b These reactions were carried out by the reaction of dimedone (2 mmol) with 4-cholorobenzaldehyde (1 mmol) using SAFIS (10 mol%) at 70 °C.
According to the application of the SAFIS in the above-mentioned mechanisms, initially, aromatic aldehyde is activated by acidic group of [Dsim]Cl (or the others) to give I. Then, dimedone attacks to the carbonyl group of the activated aldehyde, and affords intermediate II. Next, by removing H2O from II, III is prepared. [Msim]X again activates intermediate III to give IV as a Michael acceptor. Afterward, Michael addition of dimedone to intermediate IV affords V. Intermediate V converts to VI after tautomerisation. Finally, 1,8-dioxo-octahydroxanthenes forms by removing H2O from VI (Scheme 6).
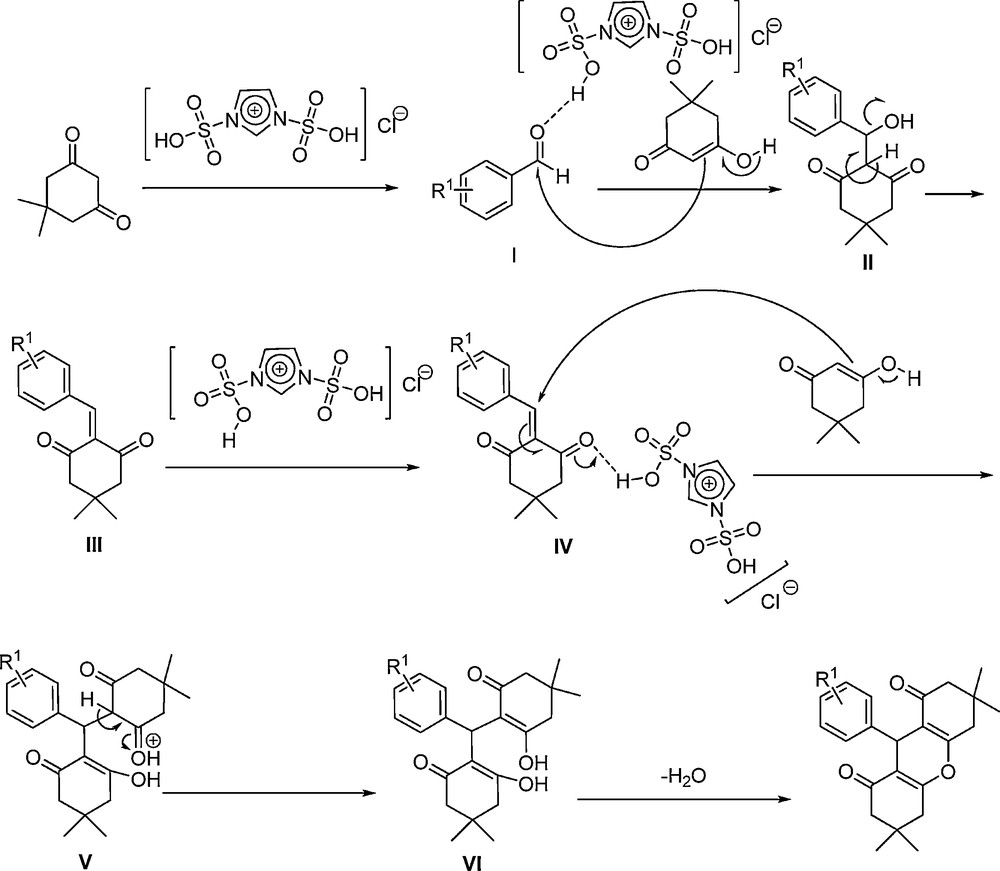
The plausible mechanism for the condensation reaction of dimedone and aldehydes in the presence of the SAFIS.
As previsously showed, [Dsim]Cl, [Msim]PF6 and [Msim]BF4 were highly efficient and general for the synthesis of the xanthene derivatives. To raise the catalysts worth, their recoverability and reusability were studied. For this purpose, the reaction of β-naphthol with 4-chlorobenzaldehyde using [Msim]PF6 was carried out several times, and the reaction mixtures were combined. Afterward, H2O was added to the combined reaction mixtures, stirred for 5 min, and filtered [Msim]PF6 is soluble in H2O; however, the reaction mixture is not soluble in H2O. In the aqueous media, a quantity of [Msim]PF6 hydrolyzed to 1-methylimidazole (as monitored on TLC) and H2SO4. To complete hydrolysis of [Msim]PF6, and consequently formation of 1-methylimidazole, a solution of NaOH (10%) was added to the filtrate, and stirred for 5 min. The solution was extracted with t-butylmethyl ether, washed with H2O and dried. Evaporation of the solvent gave 1-methylimidazole. The recovered 1-methylimidazole was reacted with chlorosulfonic acid to give [Msim]Cl. [Msim]Cl was reacted with NaPF6 to produce [Msim]PF6. The catalytic activity of the reproduced [Msim]PF6 was as same as the first one. The regeneration of this catalyst is summarized in Fig. 4. [Dsim]Cl and [Msim]BF4 were also reproduced accordingly.
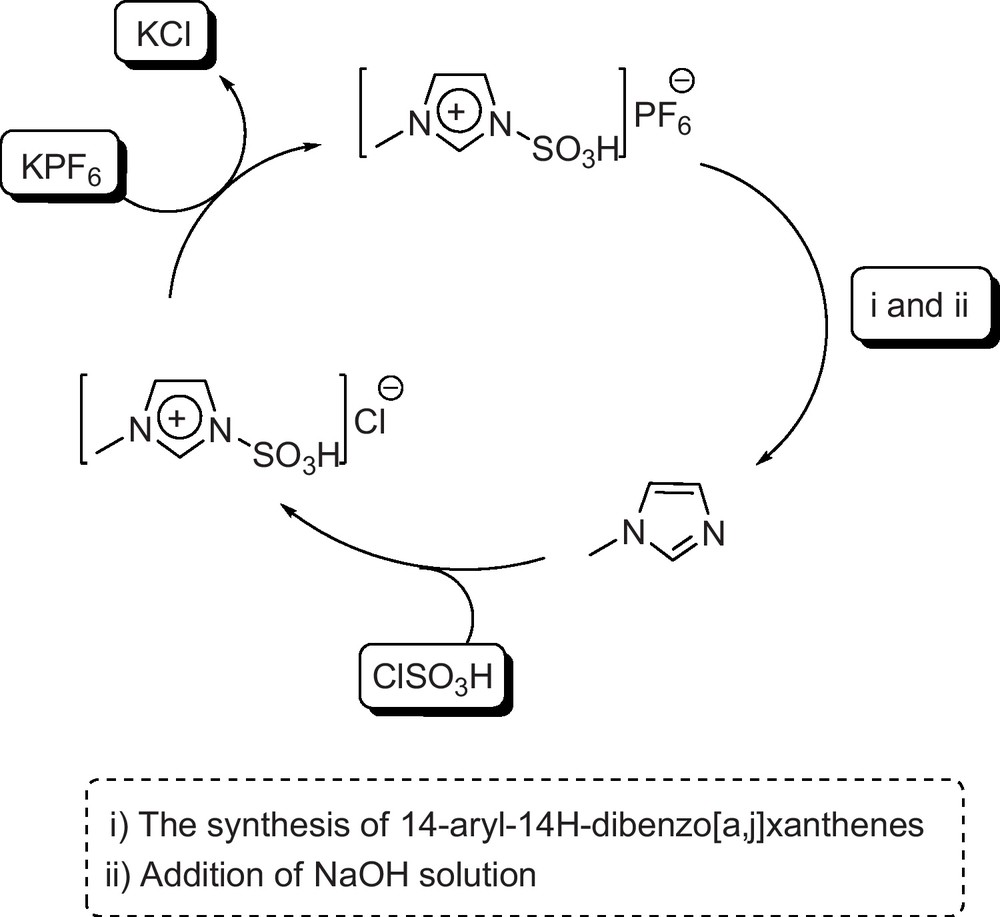
The regeneration of [Msim]PF6.
It should be mentioned that [Msim]Cl and [Msim]AlCl4 were also successfully employed in the synthesis of the xanthenes derivatives, and the results were similar to [Dsim]Cl, [Msim]PF6 and [Msim]BF4. Therefore, we did not mention the results of the application of [Msim]Cl and [Msim]AlCl4 in the presented work.
The mentioned xanthenes derivatives which produce in achiral media were racemic. To confirm this subject, optical activity of the compounds were measured by polarimeter. This study clearly confirmed the racemization of the xanthene derivatives in our protocol.
3 Conclusions
In summary, we have introduced some novel sulfonic acid functionalized imidazolium salts including [Dsim]Cl, [Msim]PF6 and [Msim]BF4 as highly efficient, regenerable catalysts for organic transformations. For instance, in this work, the synthesis of 14-aryl-14H-dibenzo[a,j]xanthenes, tetrahydrobenzo[a]xanthene-11-ones and 1,8-dioxo-octahydroxanthenes, by the one-pot multicomponent condensation reaction, were efficiently catalyzed by these imidazolium salts. The promising points for the presented methodology are efficiency, generality, high yields, very short reaction times, cleaner reaction profile, simplicity, ease of preparation of the catalyst.
4 Experimental
4.1 General
All chemicals were purchased from Merck or Fluka Chemical Companies. The known products were identified by comparison of their melting points and spectral data with those reported in the literature. Progress of the reactions was monitored by TLC using silica gel SIL G/UV 254 plates. IR spectra were run on a Shimadzu FTIR-8300 spectrophotometer. The 1H NMR (500 or 300 MHz) and 13C NMR (125 or 75 MHz) were run on a Bruker Avance DPX. FT-NMR spectrometer (δ in ppm). Melting points were recorded on a Büchi B-545 apparatus in open capillary tubes. Optical rotations were measured in spectral grade solvents using a Perkin–Elmer 341 polarimeter.
4.2 Preparation of ionic liquid [Dsim]Cl
To a round-bottomed flask (100 mL) containing imidazole (0.340 g, 5 mmol) in CH2Cl2 (50 mL), was added chlorosulfonic acid (1.177 g, 10.2 mmol) dropwise over a period of 20 min at room temperature (Fig. 1). After the addition was completed, the reaction mixture was stirred for 12 h under pressure of nitrogen (to remove the produced HCl), stand for 5 min, and the CH2Cl2 was decanted. The residue was washed with dry CH2Cl2 (3 × 50 mL) and dried under vacuum to give [Dsim]Cl as a viscous pale yellow oil in 95% yield, 1.257 g [13].
4.3 Preparation of ionic liquid [Msim]Cl
A round-bottomed flask (100 mL) was charged with 1-methylimidazole (0.410 g, 5 mmol) in dry CH2Cl2 (50 mL), and then chlorosulfonic acid (0.583 g, 5 mmol) was added dropwise over a period of 5 min at room temperature (Fig. 2). After the addition was completed, the reaction mixture was stirred for 20 min, stand for 5 min, and the CH2Cl2 was decanted. The residue was washed with dry CH2Cl2 (3 × 50 mL) and dried under vacuum to give [Msim]Cl as a viscous colorless oil in 97% yield, 0.964 g [13].
4.4 Preparation of ionic liquids [Msim]PF6 and [Msim]BF4
A mixture of [Msim]Cl (0.993 g, 5 mmol) and KPF6 (0.92 g, 5 mmol) or NaBF4 (0.548 g, 5 mmol) in a round-bottomed flask (100 mL) was stirred for 12 h at 60 °C (Fig. 2). Afterward, to separate produced ionic liquid from KCl or NaCl, absolute ethanol (25 mL) was added to the reaction mixture, stirred for 2 min, and filtered (KCl and NaCl are insoluble in absolute ethanol). The solvent of the filtrate was evaporated under vacuum to give [Msim]PF6 or [Msim]BF4 in 92% (1.421 g) and 94% (1.173 g) yields, respectively.
4.5 Spectral data of [Msim]PF6 and [Msim]BF4
4.5.1 3-Methyl-1-sulfonic acid imidazolium hexafluorophosphate(V) {[Msim]PF6}
1H NMR (300 MHz, DMSO-d6): δ (ppm) 3.82 (s, 3H, CH3), 7.55 (s, 2H), 8.90 (s, 1H), 14.15 (s, 1H); 13C NMR (75 MHz, DMSO-d6): δ (ppm) 35.6, 119.9, 123.4, 136.0; CHN Analysis: Anal. Calcd for C4H7F6N2O3PS: C, 15.59; H, 2.29; N, 9.09. Found: C, 15.67; H, 2.37; N, 8.94; MS: m/z = 309 (M+ + 1), 308 (M+), 163 (M+-PF6), 293 (M+-CH3), 227 (M+-SO3H); 31P NMR (121 MHz, DMSO-d6): −143.7 (septet, 1J (P,F) = 711 Hz, PF6−).
4.5.2 3-Methyl-1-sulfonic acid imidazolium tetrafluoroborate {[Msim]BF4}
1H NMR (300 MHz, DMSO-d6): δ (ppm) 3.83 (s, 3H, CH3), 7.51 (s, 1H), 7.56 (s, 1H), 8.89 (s, 1H), 14.05 (s, 1H); 13C NMR (75 MHz, DMSO-d6): δ (ppm) 35.7, 119.9, 123.5, 135.9; CHN Analysis: Anal. Calcd for C4H7BF4N2O3S: C, 19.22; H, 2.82; N, 11.21. Found: C, 18.97; H, 2.94; N, 11.03; MS: m/z = 251 (M+ + 1), 250 (M+), 163 (M+-BF4), 235 (M+-CH3), 169 (M+-SO3H).
4.6 General procedure for the synthesis of 14-aryl-14H-dibenzo[a,j]xanthene derivatives
A mixture of β-naphthol (0.42 g, 2 mmol), aldehyde (1 mmol) and sulfonic acid functionalized imidazolium salt (0.1 mmol) in a 10 mL round-bottomed flask connected to a reflux condenser, was stirred in an oil-bath (110 °C). After completion of the reaction, as monitored by TLC, the reaction mixture was cooled to room temperature, H2O (15 mL) was added to it, stirred for 3 min to remove ionic liquid, and filtered. Then, hot EtOH (2 mL) was added to the resulting solid (crude product), stirred for 3 min, and filtered (the product is insoluble in EtOH; however, the starting materials dissolve in EtOH). The solid residue was washed with EtOH (2 mL) to give the pure product.
4.7 Spectral data of 14-aryl-14H-dibenzo[a,j]xanthenes
4.7.1 14-Phenyl-14H-dibenzo[a,j]xanthene (1a)
Rf (EtOAc/n-hexane: 1/9) = 0.40; 1H NMR (400 MHz, DMSO-d6): δ (ppm) 6.74 (s, 1H), 6.97 (t, J = 7.6 Hz, 1H), 7.14 (t, J = 7.6 Hz, 2H), 7.45 (t, J = 7.2 Hz, 2H), 7.57 (d, J = 8.8 Hz, 2H), 7.62–7.66 (m, 4H), 7.91–7.93 (m, 4H), 8.70 (d, J = 8.8, 2H); 13C NMR (100 MHz, DMSO-d6): δ (ppm) 37.0, 117.9, 118.2, 123.9, 124.9, 126.7, 127.4, 128.4, 128.8, 129.1, 129.5, 131.1, 131.4, 146.0, 148.5.
4.7.2 14-(4-Chlorophenyl)-14H-dibenzo[a,j]xanthene (1b)
Rf (EtOAc/n-hexane: 1/9) = 0.50; 1H NMR (300 MHz, DMSO-d6): δ (ppm) 6.76 (s, 1H), 7.18 (d, J = 6.8 Hz, 2H), 7.46–7.64 (m, 10H), 8.92 (d, J = 7.79 Hz, 2H), 8.66 (d, J = 7.6 Hz, 2H); 13C NMR (75 MHz, DMSO-d6): δ (ppm) 36.3, 117.4, 118.1, 123.4, 123.7, 125.0, 127.4, 128.8, 129.1, 129.6, 130.1, 131.1, 131.2, 144.8, 148.4.
4.7.3 14-(3-Chlorophenyl)-14H-dibenzo[a,j]xanthene (1c)
Rf (EtOAc/n-hexane: 1/9) = 0.20; 1H NMR (300 MHz, DMSO-d6): δ (ppm) 6.74 (s, 1H), 7.01 (d, J = 8.1, 1H), 7.13 (t, J = 7.8, 1H), 7.42 (t, J = 7.2, 2H), 7.52–7.66 (m, 6H), 7.91 (d, J = 8.7, 4H), 8.67 (d, J = 8.7, 2H); 13C NMR (75 MHz, DMSO-d6): δ (ppm) 36.5, 117.2, 118.1, 123.7, 125.1, 126.8, 127.0, 127.5, 127.9, 129.1, 129.7, 130.7, 131.1, 131.2, 133.5, 148.2, 148.5.
4.7.4 14-(2-Chlorophenyl)-14H-dibenzo[a,j]xanthene (1d)
Rf (EtOAc/n-hexane: 1/9) = 0.75; 1H NMR (300 MHz, DMSO-d6): δ (ppm) 6.64 (s, 1H), 6.91–7.03 (m, 2H), 7.27 (d, J = 7.7 Hz, 2H), 7.38–7.50 (m, 5H), 7.57–7.70 (m, 2H), 7.76–7.90 (m, 4H), 8.54 (d, J = 8.4 Hz, 1H); 13C NMR (75 MHz, DMSO-d6): δ (ppm) 34.8, 116.9, 118.2, 123.3, 124.9, 127.4, 128.5, 128.8, 129.2, 129.8, 130.2, 130.3, 130.9, 131.4, 132.0, 143.2, 148.7.
4.7.5 14-(3-Bromophenyl)-14H-dibenzo[a,j]xanthene (1e)
Rf (EtOAc/n-hexane: 1/9) = 0.25; 1H NMR (300 MHz, DMSO-d6): δ (ppm) 6.63 (s, 1H), 6.90–7.02 (m, 2H), 7.26 (d, J = 7.7 Hz, 2H), 7.37–7.49 (m, 5H), 7.58 (t, J = 7.9 Hz, 2H), 7.85–7.89 (m, 4H), 8.53 (d, J = 8.5 Hz, 1H); 13C NMR (75 MHz, DMSO-d6): δ (ppm) 34.8, 116.9, 118.2, 123.3, 124.9, 127.4, 128.5, 128.8, 129.1, 129.8, 130.2, 130.3, 130.9, 131.4, 132.0, 143.2, 148.6.
4.7.6 14-(4-Nitrophenyl)-14H-dibenzo[a,j]xanthene (1f)
Rf (EtOAc/n-hexane: 1/9) = 0.44; 1H NMR (300 MHz, DMSO-d6): δ (ppm) 6.95 (s, 1H), 7.43 (t, J = 7.2 Hz, 3H), 7.58–7.65 (m, 4H), 7.81 (d, J = 7.5 Hz, 1H), 7.90–7.95 (m, 4H), 8.14 (d, J = 7.8, 1H), 8.45 (s, 1H), 8.72 (d, J = 8.4 Hz, 2H); 13C NMR (75 MHz, DMSO-d6): δ (ppm) 36.4, 116.9, 118.2, 122.0, 123.6, 125.2, 127.7, 129.1, 130.0, 130.4, 131.11, 134.7, 147.9, 148.3, 148.6.
4.7.7 14-(3-Nitrophenyl)-14H-dibenzo[a,j]xanthene (1g)
Rf (EtOAc/n-hexane: 1/9) = 0.20; 1H NMR (300 MHz, DMSO-d6): δ (ppm) 6.91 (s, 1H), 7.11–7.25 (m, 1H), 7.43–7.48 (m, 2H), 7.55–7.71 (m, 4H), 7.88–8.03 (m, 7H), 8.66 (d, J = 8.4 Hz, 2H); 13C NMR (75 MHz, DMSO-d6): δ (ppm) 36.7, 116.6, 118.2, 120.1, 123.6, 124.2, 125.2, 127.6, 129.1, 129.5, 130.0, 131.1, 131.2, 134.2, 146.3, 148.4, 153.1.
4.7.8 14-(2-Nitrophenyl)-14H-dibenzo[a,j]xanthene (1h)
Rf (EtOAc/n-hexane: 1/9) = 0.36; 1H NMR (300 MHz, DMSO-d6): δ (ppm) 6.94 (s, 1H), 7.41–7.46 (m, 3H), 7.56–7.65 (m, 4H), 7.79 (d, J = 2.1, 1H), 7.89–7.94 (m, 4H), 8.13 (d, J = 7.8, 1H), 8.45 (s, 1H), 8.70 (d, J = 6, 2H); 13C NMR (75 MHz, DMSO-d6): δ (ppm) 36.4, 116.9, 118.1, 122.0, 122.5, 123.6, 125.2, 127.7, 129.1, 130.0, 130.4, 131.1, 134.7, 138.2, 147.9, 148.3, 148.6.
4.7.9 14-(4-Methylphenyl)-14H-dibenzo[a,j]xanthene (1i)
Rf (EtOAc/n-hexane: 1/9) = 0.42; 1H NMR (300 MHz, DMSO-d6): δ (ppm) 2.01 (s, 3H), 6.63 (s, 1H), 6.90 (d, J = 7.2, 2H), 7.40–7.61 (m, 8H), 7.88 (t, J = 2.7, 4H), 8.61 (d, J = 8.7, 2H); 13C NMR (75 MHz, DMSO-d6): δ (ppm) 20.8, 37.4, 117.9, 118.1, 123.8, 124.9, 127.3, 128.2, 129.0, 129.3, 129.3, 131.1, 131.3, 135.8, 143.0, 148.3.
4.7.10 14-(4-Methoxyphenyl)-14H-dibenzo[a,j]xanthene (1j)
Rf (EtOAc/n-hexane: 1/9) = 0.44; 1H NMR (300 MHz, DMSO-d6): δ (ppm) 3.52 (s, 3H), 6.63 (s, 1H), 6.67 (d, J = 7.5, 2H), 7.19–7.62 (m, 8H), 7.87–7.92 (m, 4H), 8.64 (d, J = 8.7, 2H); 13C NMR (75 MHz, DMSO-d6): δ (ppm) 36.0, 55.3, 114.1, 118.1, 123.9, 124.9, 127.9, 124.9, 127.3, 129.2, 131.1, 135.7, 138.1, 147.0, 149.4, 157.9.
4.7.11 4-(14H-dibenzo[a,j]xanthen-14-yl)-N,N-dimethylaniline (1k)
Rf (EtOAc/n-hexane: 1/9) = 0.32; 1H NMR (DMSO, 300 MHz): δ 2.60 (s, 6H), 6.42 (d, J = 7.2 Hz, 2H), 6.56 (s, 1H), 7.37–7.92 (m, 12H), 8.64 (d, J = 8, 2H), 13C NMR (CDCl3, 75 MHz): δ 36.0, 40.8, 112.6, 118.1, 118.4, 123.9, 124.8, 127.2, 128.8, 128.9, 129.0, 131.1, 131.4, 133.8, 148.3, 149.0. MS: m/z = 402 (M+ + 1), 401 (M+), 357 (M+-N(CH3)2), 281 (M+-N,N-dimethylaniline).
4.7.12 14-(4-Benzyloxyphenyl)-14H-dibenzo[a,j]xanthene (1l)
Rf (EtOAc/n-hexane: 1/9) = 0.30; 1H NMR (300 MHz, DMSO-d6): δ (ppm) 4.42 (s, 2H), 4.82 (s, 1H), 6.65 (s, 1H), 6.75 (d, J = 7.8 Hz, 1H), 7.27 (s, 5H), 7.41–7.78 (m, 8H), 7.88 (t, J = 6.4, 4H) 8.66 (d, J = 8.3, 2H); 13C NMR (75 MHz, DMSO-d6): δ (ppm) 36.2, 62.5, 114.9, 118.0, 123.8, 124.8, 127.2, 127.9, 128.1, 128.7, 129.0, 129.2, 129.3, 131.1, 131.3, 137.3, 138.3, 148.3, 157.1. MS: m/z = 465 (M+ + 1), 464 (M+), 373 (M+-C7H7), 281 (M+-C13H11O).
4.8 General procedure for the synthesis of tetrahydrobenzo[a]xanthene-11-ones derivatives
A mixture of β-naphthol (0.144 g, 1 mmol), dimedone (0.140 g, 1 mmol), aldehyde (1 mmol) and SAFIS (0.1 mmol) in a 10 mL round-bottomed flask connected to a reflux condenser, was stirred in an oil-bath (110 °C). After completion of the reaction, as monitored with TLC, the reaction mixture was cooled to room temperature, H2O (15 mL) was added to it, stirred for 3 min to remove ionic liquid, and filtered. The crude product was recrystallized from ethanol to afford the pure product.
4.9 Spectral data of tetrahydrobenzo[a]xanthene-11-ones
4.9.1 9,9-dimethyl-12-phenyl-9,10-dihydro-8H-benzo[a]xanthen-11(12H)-one (2a)
Rf (EtOAc/n-hexane: 1/9) = 0.40; 1H NMR (300 MHz, DMSO-d6): δ (ppm) 0.84 (s, 3H), 1.02 (s, 3H), 2.09 (d, J = 16.2 Hz, 1H), 2.30 (d, J = 16.2 Hz, 1H), 2.60 (Distorted AB System, 2H), 5.54 (s, 1H), 7.00 (t, J = 7.2 Hz, 1H), 7.14 (t, J = 7.8 Hz, 2H), 7.27 (d, J = 7.8 Hz, 2H), 7.36–7.48 (m, 3H), 7.89 (d, J = 8.5 Hz 2H), 8.01 (d, J = 8.4 Hz, 1H), 13C NMR (75 MHz, DMSO-d6): δ (ppm) 26.6, 29.2, 32.3, 34.5,40.7, 50.5, 113.6, 117.6, 117.7, 123.7, 125.4, 126.6, 127.6, 128.5, 128.6, 129.0, 129.5, 131.0, 131.5, 145.3, 147.6, 164.3, 196.2.
4.9.2 12-(4-chlorophenyl)-9,9-dimethyl-9,10-dihydro-8H-benzo[a]xanthen-11(12H)-one (2b)
Rf (EtOAc/n-hexane: 1/9) = 0.50; 1H NMR (500 MHz, DMSO-d6): δ (ppm) 0.86 (s, 3H), 1.04 (s, 3H), 2.12 (d, J = 16.1 Hz, 1H), 2.32 (d, J = 16.1 Hz, 1H), 2.56 (d, J = 17.3 Hz, 1H), 2.66 (d, J = 17.4 Hz, 1H), 5.58 (s, 1H), 7.23 (d, J = 8.4 Hz, 2H), 7.30 (d, J = 8.4 Hz, 2H), 7.41–7.49 (m, 3H), 7.89–7.92 (m, 2H), 7.99 (d, J = 8.3 Hz, 1H), 13C NMR (125 MHz, DMSO-d6): δ (ppm) 27.0, 29.6, 32.7, 32.4, 41.0, 50.9, 113.6, 117.5, 118.0, 124.0, 125.9, 128.1, 128.9, 129.4, 130.2, 130.8, 131.3, 131.6, 131.9, 144.6, 148.0, 164.8, 196.7.
4.9.3 12-(3-chlorophenyl)-9,9-dimethyl-9,10-dihydro-8H-benzo[a]xanthen-11(12H)-one (2c)
Rf (EtOAc/n-hexane: 1/9) = 0.22; 1H NMR (500 MHz, DMSO-d6): δ (ppm) 0.88 (s, 3H), 1.03 (s, 3H), 2.11 (d, J = 12.8 Hz, 1H), 2.32 (d, J = 16.1 Hz, 1H), 2.61 (Distorted AB System, 2H), 5.60 (s, 1H), 7.11 (s, 1H), 7.19 (d, J = 4.4 Hz, 2H), 7.36 (s, 1H), 7.40–7.50 (m, 3H), 7.89–8.01 (m, 2H), 8.02 (d, J = 8.3 Hz, 1H), 13C NMR (125 MHz, DMSO-d6): δ (ppm) 27.0, 29.6, 32.7, 34.7, 41.0, 50.9, 113.5, 117.3, 118.0, 124.0, 125.9, 127.2, 127.6, 128.2, 128.8, 129.4, 130.3, 130.9, 131.3, 131.9, 133.5, 148.03, 148.07, 165.0, 196.7.
4.9.4 12-(2,3-dichlorophenyl)-9,9-dimethyl-9,10-dihydro-8H-benzo[a]xanthen-11(12H)-one (2d)
Rf (EtOAc/n-hexane: 1/9) = 0.35; 1H NMR (500 MHz, DMSO-d6): δ (ppm) 0.88 (s, 3H), 1.06 (s, 3H), 2.10 (d, J = 16.0 Hz, 1H), 2.32 (d, J = 16.0 Hz, 1H), 2.57 (d, J = 17.3 Hz, 1H), 2.69 (d, J = 17.3 Hz, 1H), 5.86 (s, 1H), 7.16–7.22 (m, 1), 7.27 (s, 1H), 7.36 (d, J = 7.7 Hz, 1H), 7.41–7.43 (m, 2H), 7.50 (t, J = 7.8 Hz, 1H), 7.91 (t, J = 6.6 Hz, 2H), 8.04 (d, J = 8.4 Hz, 1H), 13C NMR (125 MHz, DMSO-d6): δ (ppm) 27.0, 29.6, 32.6, 32.6, 41.1, 51.0, 113.3, 118.1, 123.7, 125.9, 126.3, 127.7, 128.1, 128.8, 129.5, 129.6, 130.5, 131.0, 131.7, 131.8, 132.9, 137.3, 148.2, 165.2, 196.6.
4.9.5 12-(2,4-Dichlorophenyl)-9,9-dimethyl-8,9,10,12- tetrahydrobenzo[a]xanthen-11-one (2e)
Rf (EtOAc/n-hexane: 1/9) = 0.50; 1H NMR (500 MHz, DMSO-d6): δ (ppm) 0.89 (s, 3H), 1.05 (s, 3H), 2.09 (d, J = 16.0 Hz, 1H), 2.31 (d, J = 16.0 Hz, 1H), 2.65 (d, J = 16.8 Hz, 1H), 2.67 (d, J = 17.3 Hz, 1H), 5.77 (s, 1H), 7.22–7.28 (m, 2H), 7.37–7.42 (m, 3H), 7.47–7.50 (m, 1H), 7.88–7.91 (m, 2H), 8.03 (d, J = 8.4 Hz, 1H), 13C NMR (125 MHz, DMSO-d6): δ (ppm) 27.0, 29.5, 32.5, 32.6, 41.1, 51.0, 118.0, 123.7, 125.9, 127.5, 128.1, 128.3, 129.6, 129.7, 130.5, 131.6, 131.8, 132.5, 133.7, 140.7, 148.2, 165.1, 196.5.
4.9.6 12-(4-bromophenyl)-9,9-dimethyl-9,10-dihydro-8H-benzo[a]xanthen-11(12H)-one (2f)
Rf (EtOAc/n-hexane: 1/9) = 0.40; 1H NMR (500 MHz, DMSO-d6): δ (ppm) 0.86 (s, 3H), 1.04 (s, 3H), 2.12 (d, J = 16.0 Hz, 1H), 2.32 (d, J = 16.0 Hz, 1H), 2.61 (Distorted AB System, 2H), 5.56 (s, 1H), 7.24 (d, J = 8.3 Hz, 2H), 7.36 (d, J = 8.3 Hz, 2H), 7.40–7.49 (m, 3H), 7.90 (t, J = 5.0 Hz 2H), 7.98 (d, J = 8.4 Hz, 1H), 13C NMR (125 MHz, DMSO-d6): δ (ppm) 27.1, 29.6, 32.7, 34.5, 41.0, 50.9, 113.5, 117.4, 118.0, 120.1, 124.0, 125.9, 128.1, 129.4, 130.2, 131.2, 131.3, 131.91, 131.96, 145.0, 148.0, 164.8, 196.7.
4.9.7 12-(3-bromophenyl)-9,9-dimethyl-9,10-dihydro-8H-benzo[a]xanthen-11(12H)-one (2g)
Rf (EtOAc/n-hexane: 1/9) = 0.35; 1H NMR (500 MHz, DMSO-d6): δ (ppm) 0.88 (s, 3H), 1.05 (s, 3H), 2.13 (d, J = 16.1 Hz, 1H), 2.31 (d, J = 16.1 Hz, 1H), 2.62 (Distorted AB System, 2H), 5.52 (s, 1H), 6.85 (d, J = 6.2 Hz, 1H), 7.03–7.07 (m, 2H), 7.11 (s, 1H), 7.40–7.50 (m, 3H), 7.89 (d, J = 8.7 Hz 2H), 8.04 (d, J = 8.4 Hz, 1H), 13C NMR (125 MHz, DMSO-d6): δ (ppm) 27.0, 29.6, 32.7, 34.9, 41.1, 51.3, 114.1, 118.0, 118.2, 124.1, 125.8, 126.2, 127.7, 127.9, 128.8, 129.3, 129.5, 129.8, 131.5, 131.9, 137.9, 145.7, 148.0, 164.9, 196.7.
4.9.8 12-(2-bromophenyl)-9,9-dimethyl-9,10-dihydro-8H-benzo[a]xanthen-11(12H)-one (2h)
Rf (EtOAc/n-hexane: 1/9) = 0.36; 1H NMR (500 MHz, DMSO-d6): δ (ppm) 0.89 (s, 3H), 1.06 (s, 3H), 2.09 (d, J = 16 Hz, 1H), 2.32 (d, J = 16 Hz, 3H), 2.57 (d, J = 17.4 Hz, 1H), 2.70 (d, J = 17.3 Hz, 1H), 5.76 (s, 1H), 6.97–7.00 (m, 1H), 7.18 (t, J = 7.2 Hz, 1H), 7.22 (s, 1H), 7.41–7.43 (m, 2H), 7.47–7.51 (m, 2H), 7.89–7.92 (m, 2H), 8.20 (d, J = 16 Hz, 1H), 13C NMR (125 MHz, DMSO-d6): δ (ppm) 27.0, 29.7, 32.6, 35.5, 41.2, 51.1, 118.1, 123.5, 124.3, 125.9, 127.9, 128.7, 129.1, 129.5, 130.3, 131.83, 131.87, 133.8, 148.1, 164.8, 196.5.
4.9.9 9,9-dimethyl-12-(4-nitrophenyl)-9,10-dihydro-8H-benzo[a]xanthen-11(12H)-one (2i)
Rf (EtOAc/n-hexane: 1/9) = 0.20; 1H NMR (300 MHz, DMSO-d6): δ (ppm) 0.82 (s, 3H), 1.02 (s, 3H), 2.11 (d, J = 15.9 Hz, 1H), 2.32 (d, J = 16.2 Hz, 1H), 2.63 (Distorted AB System, 2H), 5.75 (s, 1H), 7.38–7.48 (m, 4H), 7.72 (d, J = 7.5 Hz, 1H), 7.88–7.94 (m, 3H), 8.02 (d, J = 8.4 Hz, 1H), 8.15 (s, 1H), 13C NMR (75 MHz, DMSO-d6): δ (ppm) 26.6, 29.1, 32.4, 34.4, 40.8, 50.4, 112.8, 116.4, 117.6, 121.9, 123.0, 123.6, 125.6, 127.9, 129.1, 130.2, 130.8, 131.5, 135.3, 147.3, 147.7, 148.0, 164.8, 196.4.
4.9.10 9,9-dimethyl-12-(3-nitrophenyl)-9,10-dihydro-8H-benzo[a]xanthen-11(12H)-one (2j)
Rf (EtOAc/n-hexane: 1/9) = 0.15; 1H NMR (500 MHz, DMSO-d6): δ (ppm) 0.84 (s, 3H), 1.04 (s, 3H), 2.13 (d, J = 16.0 Hz, 1H), 2.33 (d, J = 16.1 Hz, 1H), 2.64 (Distorted AB System, 2H), 5.77 (s, 1H), 7.40–7.49 (m, 4H), 7.73 (d, J = 7.8 Hz, 1H), 7.89–7.95 (m, 3H), 8.03 (d, J = 8.4 Hz, 1H), 8.17 (t, J = 1.8 Hz, 1H), 13C NMR (125 MHz, DMSO-d6): δ (ppm) 27.0, 29.5, 32.7, 34.8, 41.7, 50.8, 113.2, 116.7, 117.0 118.0, 122.3, 123.4, 124.0, 126.0, 128.3, 129.5, 130.6, 131.2, 132.0, 135.6, 147.7, 148.1, 148.4, 165.2, 196.8.
4.9.11 12-(3-hydroxyphenyl)-9,9-dimethyl-9,10-dihydro-8H-benzo[a]xanthen-11(12H)-one (2k)
Rf (EtOAc/n-hexane: 1/9) = 0.19; 1H NMR (300 MHz, DMSO-d6): δ (ppm) 0.90 (s, 3H), 1.05 (s, 3H), 2.15 (d, J = 16.1 Hz, 1H), 2.32 (d, J = 16.1 Hz, 1H), 2.54 (Distorted AB System, 2H), 5.49 (s, 1H), 6.45 (dd, J = 6.3, J = 1.5, 4H), 6.66 (s, 1H), 6.73 (d, J = 7.1 Hz, 1H), 6.96 (t, J = 7.8 Hz, 1H), 7.42–7.89 (m, 3H), 7.90 (d, J = 9.0 Hz, 2H), 8.01 (d, J = 8.4 Hz, 1H), 9.20 (s, 1H), 13C NMR (75 MHz, DMSO-d6): δ (ppm) 27.1, 29.6, 32.7, 34.8, 41.1, 51.0, 114.1, 115.9, 118.0, 118.2, 119.8, 124.1, 125.8, 127.9, 129.3, 129.8, 131.6, 131.9, 147.0, 148.0, 157.9, 164.5. 196.7.
4.9.12 9,9-dimethyl-12-o-tolyl-9,10-dihydro-8H-benzo[a]xanthen-11(12H)-one (2l)
Rf (EtOAc/n-hexane: 1/9) = 0.45; 1H NMR (500 MHz, DMSO-d6): δ (ppm) 0.87 (s, 3H), 1.06 (s, 3H), 2.11 (d, J = 15.5 Hz, 1H), 2.12 (s, 3H), 2.31 (d, J = 16.1 Hz, 1H), 2.56 (d, J = 17.2 Hz, 1H), 2.66 (d, J = 17.3 Hz, 1H), 5.52 (s, 1H), 6.96 (d, J = 7.9 Hz, 2H), 7.16 (d, J = 7.9 Hz, 2H), 7.39–7.49 (m, 3H), 7.89 (d, J = 8.6 Hz, 2H), 8.02 (d, J = 8.4 Hz, 1H) 13C NMR (125 MHz, DMSO-d6): δ (ppm) 21.3, 27.1, 29.7, 32.7, 34.5, 41.1, 51.0, 114.1, 118.0, 118.3, 124.1, 125.7, 127.9, 128.8, 129.3, 129.5, 129.8, 131.5, 131.9, 136.0, 142.8, 147.9, 164.5, 196.7.
4.10 General procedure for the synthesis of 1,8-dioxo-octahydroxanthene derivatives
To a mixture of dimedone (2 mmol) and aldehyde (1 mmol) in a 10 mL round-bottomed flask connected to a reflux condenser, SAFIS (0.1 mmol) was added. The resulting mixture was stirred in an oil-bath (70 °C), and the reaction was monitored by TLC. After completion of the reaction, H2O (5 mL) added to the mixture, stirred for 3 min to remove ionic liquid and filtered. The solid residue was recrystallized from EtOH to give the product.
4.11 Spectral data of 1,8-dioxo-octahydroxanthenes
4.11.1 3,3,6,6-Tetramethyl-9-(phenyl)-1,8-dioxo-octahydroxanthene (3a)
Rf (EtOAc/n-hexane: 1/9) = 0.25; 1H NMR (500 MHz, DMSO-d6): δ (ppm) 0.90 (s, 6H), 1.04 (s, 6H), 2.09 (d, J = 16.1 Hz, 2H), 2.27 (d, J = 16.2 Hz, 2H), 2.53 (d, J = 17.1 Hz, 2H), 2.58 (d, J = 17.7 Hz, 2H), 4.53 (s, 1H), 7.10 (t, J = 7.0 Hz, 1H), 7.18 (d, J = 7.0 Hz, 2H), 7.21 (t, J = 7.20 Hz, 2H); 13C NMR (125 MHz, DMSO-d6): δ (ppm) 27.7, 29.6, 32.3, 32.6, 41.3, 51.2, 116.1, 126.8, 128.4, 128.8, 144.5, 162.7, 196.8.
4.11.2 3,3,6,6-Tetramethyl-9-(4-nitro-phenyl)-1,8-dioxo-octahydroxanthene (3b)
Rf (EtOAc/n-hexane: 1/9) = 0.36; 1H NMR (500 MHz, CDCl3): δ (ppm) 0.99 (s, 6H), 1.12 (s, 6H), 2.16 (d, J = 16.3 Hz, 2H), 2.26 (d, J = 16.3 Hz, 2H), 2.51 (t, J = 18.7 Hz, 4H), 4.83 (s, 1H), 7.48 (d, J = 8.2 Hz, 2H), 8.08 (2H, J = 8.2 Hz, 2H); 13C NMR (125 MHz, CDCl3): δ (ppm) 27.7, 29.6, 32.6, 32.8, 41.2, 51.0, 114.9, 123.8, 129.8, 146.8, 152.0, 163.5, 196.7.
4.11.3 3,3,6,6-Tetramethyl-9-(3-nitro-phenyl)-1,8-dioxo-octahydroxanthene (3c)
Rf (EtOAc/n-hexane: 1/9) = 0.30; 1H NMR (500 MHz, CDCl3): δ (ppm) 1.01 (s, 6H), 1.13 (s, 6H), 2.18 (d, J = 16.3 Hz, 2H), 2.27 (d, J = 16.3 Hz, 2H), 2.53 (t, J = 18.5 Hz, 4H), 4.85 (s, 1H), 7.41 (t, J = 7.9 Hz, 1H), 7.81 (d, J = 7.5 Hz, 1H), 7.99 (d, J = 8.2 Hz, 1H), 8.06 (s, 1H); 13C NMR (125 MHz, CDCl3): δ (ppm) 27.7, 29.6, 32.5, 32.7, 41.2, 51.0, 114,9, 122.0, 123.1, 129.2, 136.0, 146.8, 148.7, 163.5, 196.8.
4.11.4 3,3,6,6-Tetramethyl-9-(4-cyano-phenyl)-1,8-dioxo-octahydroxanthene (3d)
Rf (EtOAc/n-hexane: 1/9) = 0.33; 1H NMR (400 MHz, CDCl3): δ (ppm) 0.99 (s, 6H), 1.13 (s, 6H), 2.17 (d, J = 16.4 Hz, 2H), 2.26 (d, J = 16.4, 2H), 2.52 (t, J = 15.4 Hz, 4H), 4.78 (s, 1H), 7.43 (d, J = 8.4 Hz, 2H), 7.53 (2H, J = 8.0 Hz, 2H); 13C NMR (100 MHz, CDCl3): δ (ppm) 27.3, 29.2, 32.2, 32.4, 40.8, 50.6, 110.2, 114.6, 119.1, 129.3, 131.9, 149.5, 162.9, 196.3.
4.11.5 3,3,6,6-Tetramethyl-9-(4-methoxy-phenyl)-1,8-dioxo-octahydroxanthene (3e)
Rf (EtOAc/n-hexane: 1/9) = 0.40; 1H NMR (400 MHz, CDCl3): δ (ppm) 1.01 (s, 6H), 1.12 (s, 6H), 2.18 (d, J = 16.4 Hz, 2H), 2.25 (d, J = 16.4 Hz, 2H), 2.48 (s, 4H), 3.75 (s, 3H), 4.72 (s, 1H), 6.77 (d, J = 8.8 Hz, 2H), 7.22 (d, J = 8.8 Hz, 2H); 13C NMR (100 MHz, CDCl3): δ (ppm) 27.3, 29.3, 30.9, 32.2, 40.9, 50.8, 55.1, 113.5, 115.8, 129.3, 136.5, 157.9, 162.1, 196.5.
4.11.6 3,3,6,6-Tetramethyl-9-(3,4-dimethoxy-phenyl)-1,8-dioxo-octahydroxanthene (3f)
Rf (EtOAc/n-hexane: 1/9) = 0.31; 1H NMR (500 MHz, DMSO-d6): δ (ppm) 0.92 (s, 6H), 1.04 (s, 6H), 2.10 (d, J = 16.1 Hz, 2H), 2.27 (d, J = 16.1 Hz, 2H), 2.51 (d, J = 17.6 Hz, 2H), 2.57 (d, J = 17.4 Hz, 2H), 3.68 (s, 6H), 4.48 (s, 1H), 6.67 (dd, J = 8.3, 1.9 Hz, 1H), 6.72 (d, J = 1.9 Hz, 1H), 6.80 (d, J = 8.31 Hz, 1H); 13C NMR (125 MHz, DMSO-d6): δ (ppm) 27.2, 29.6, 31.4, 32.7, 50.9, 56.3, 56.3, 112.2, 112.9, 115.4, 120.9, 137.7, 148.1, 148.9, 163.6, 196.9.
4.11.7 3,3,6,6-Tetramethyl-9-(4-methyl-phenyl)-1,8-dioxo-octahydroxanthene (3g)
Rf (EtOAc/n-hexane: 1/9) = 0.33; 1H NMR (500 MHz, CDCl3): δ (ppm) 1.02 (s, 6H), 1.25 (s, 6H), 2.17–2.27 (m, 7H), 2.49 (s, 4H), 4.74 (s, 1H), 7.04 (d, J = 7.5 Hz, 2H), 7.20 (d, J = 6.9 Hz, 2H); 13C NMR (125 MHz, CDCl3): δ (ppm) 21.5, 27.8, 29.7, 31.9, 32.6, 41.3, 51.2, 116.2, 128.7, 129.2, 136.1, 141.6, 162.5, 196.8.
4.11.8 3,3,6,6-Tetramethyl-9(5-bromo-2-hydroxy-Phenyl)-1,8-dioxo-octahydroxanthene (3h)
Rf (EtOAc/n-hexane: 1/9) = 0.22; 1H NMR (500 MHz, DMSO-d6): δ (ppm) 0.90 (s, 6H), 0.97 (s, 3H), 1.05 (s, 3H), 2.04 (d, J = 15.8 Hz, 2H), 2.25 (d, J = 15.9 Hz, 2H), 2.34 (d, J = 17.3 Hz, 2H), 2.55 (d, J = 17.4 Hz, 2H), 5.04 (s, 1H), 6.95 (d, J = 8.64 Hz, 1H), 7.04 (s, 1H), 7.28 (d, J = 8.60 Hz, 1H), 10.59 (s, 1H); 13C NMR (125 MHz, DMSO-d6): δ (ppm) 26.9, 28.4, 30.0, 32.5, 51.2, 100.4, 111.3, 116.3, 129.1, 130.6, 131.4, 149.8, 165.3, 196.5.
4.11.9 3,3,6,6-Tetramethyl-9-(4-chloro-phenyl)-1,8-dioxo-octahydroxanthene (3i)
Rf (EtOAc/n-hexane: 1/9) = 0.35; 1H NMR (500 MHz, DMSO-d6): δ (ppm) 0.90 (s, 6H), 1.04 (s, 6H), 2.09 (d, J = 16.1 Hz, 2H), 2.27 (d, J = 16.1 Hz, 2H), 2.52 (d, 2H), 2.57 (d, J = 17.6 Hz, 2H), 4.51 (s, 1H), 7.19 (d, J = 8.3 Hz, 2H), 7.29 (d, J = 8.2 Hz, 2H); 13C NMR (125 MHz, DMSO-d6): δ (ppm) 27.7, 29.7, 31.8, 32.6, 41.3, 51.1, 115.6, 128.6, 130.2, 132.4, 143.1, 162.8, 196.7.
4.11.10 3,3,6,6-Tetramethyl-9-(3-chloro-phenyl)-1,8-dioxo-octahydroxanthene (3j)
Rf (EtOAc/n-hexane: 1/9) = 0.20; 1H NMR (500 MHz, CDCl3): δ (ppm) 1.02 (s, 6H), 1.12 (s, 6H), 2.18–2.27 (Distorted AB system, 4H), 2.52 (t, J = 14.0 Hz, 4H), 4.74 (s, 1H), 7.09 (d, J = 7.6 Hz, 1H), 7.16 (t, J = 7.9 Hz, 2H), 7.25 (s, 1H); 13C NMR (125 MHz, CDCl3): δ (ppm)27.8, 29.6, 32.2, 32.6, 41.2, 51.1, 115.5, 127.0, 127.4, 128.8, 129.6, 134.3, 146.6, 163.1, 196.7.
4.11.11 3,3,6,6-Tetramethyl-9-(4-bromo-phenyl)-1,8-dioxo-octahydroxanthene (3k)
Rf (EtOAc/n-hexane: 1/9) = 0.35; 1H NMR (500 MHz, DMSO-d6): δ (ppm) 0.91 (s, 6H), 1.04 (s, 6H), 2.07 (d, J = 16.1 Hz, 2H), 2.25 (d, J = 16.1 Hz, 2H), 2.50–2.59 (Distorted AB system, 4H), 4.49 (s, 1H), 7.13 (d, J = 8.4 Hz, 2H), 7.42 (d, J = 8.4 Hz, 2H).
4.11.12 3,3,6,6-Tetramethyl-9-(2-phenyl-ethylene)-1,8-dioxo-octahydroxanthene (3l)
Rf (EtOAc/n-hexane: 1/9) = 0.37; 1H NMR (500 MHz, DMSO-d6): δ (ppm) 1.04 (s, 6H), 1.06 (s, 6H), 2.25 (d, J = 16.04 Hz, 2H), 2.31 (d, J = 16.02 Hz, 2H), 2.52 (s, 4H), 4.15 (d, J = 5.29 Hz, 1H), 6.19 (d, J = 16.05 Hz, 1H), 6.21 (dd, 1H), 7.19 (m, 1H), 7.27 (d, J = 3.7 Hz, 4H); 13C NMR (125 MHz, DMSO-d6): δ (ppm) 27.7, 28.3, 29.4, 32.8, 50.9, 114.1, 126.8, 128.2, 129.4, 130.4, 132.2, 137.5, 164.4, 197.1.
Acknowledgements
The authors gratefully acknowledge partial support of this work by the Research Affairs Office of Bu-Ali Sina University (Grant number 32-1716 entitled development of chemical methods, reagents and molecules), and Center of Excellence in Development of Chemical Method (CEDCM), Hamedan, I.R. Iran.


