1 Introduction
Epoxidation of olefins is an important class of industrial reactions since epoxides are used as chemical intermediates [1,2]. Because of the unique properties of polyoxometalates (POMs) such as tunable size, shape, charge density, acidity, redox potential, stability and solubility, these compounds have attracted much attention for many years [3–5]. These compounds have many applications in electrochemistry [6], catalysis [7–9], medicine [10], and material science [11]. An important subclasses of POM clusters include lacunary POMs in which one or more metal atoms are substituted by low valent-transition-metals [12]. These compounds so called transition-metal-substituted polyoxometalates (TMSPs), which have been used as oxidation catalysts [13–19]. Keggin-type polyoxometalates such as Zn, Ti, Fe, and Mn-substituted polyoxometalates can act as effective catalysts for H2O2-based epoxidation [20–23]. The [PZnMo2W9O39]5− (ZnPOM) has been reported to be an active catalyst for epoxidation although its stability has not been clear [20,24]. Although most current uses of TMSPs are in homogeneous catalysis [12–19], but heterogeneous catalysts incorporated the properties of both the polyoxometalate (oxidation catalysis, ion-exchange) and a high surface area support. In addition, they can be easily separated from the reaction mixture and are of high stability and reusability [25–29]. Ionic liquids (ILs) have many applications; such as high thermal and chemical stability, high polarity, non-flammability and tunable acidity make them suitable reaction media for a broad range of catalytic applications [30]. Supported ionic liquid catalysis is a concept that combines the advantages of ionic liquids with those of heterogeneous catalysts [31]. First, Davis recognized that functionalized ionic liquids can serve, not only as reaction media, but as catalyst as well [32,33]. Ionic liquid-modified supported catalysts provide the hydrophobic environment for organic reactions [34–36].
In this work, the catalytic activity of [(n-Bu4N)5PZnMo2W9O39] supported on ionic liquid-modified silica, Im-SiO2, in the alkene epoxidation with aqueous H2O2 is reported (Scheme 1).
Epoxidation of alkenes with hydrogen peroxide catalyzed by [PZnMo2W9O39]5- immobilized on ionic liquid-modified silica.
2 Experimental
2.1 Materials
All chemicals were of commercial reagent grade and were used without further purification. Hydrogen peroxide (30%) was titrated by a standard KMnO4 solution. Polyoxometalate and SìO2-Im were prepared according to the literature [24,35]. The prepared catalyst was characterized by elemental analysis, X-ray diffraction (XRD), scanning electron microscopy (SEM), FT-IR and UV–Vis spectroscopic methods. FT-IR spectra were obtained as potassium bromide pellets in the range 400–4000 cm−1 with a Jasco Impact 6300D spectrometer. Scanning electron micrographs of the catalyst and support were taken on a SEM Philips XL 20 instrument. UV–Vis spectra were obtained in the range 200-800 cm−1 with a Jasco V-670 spectrometer. Powder X-ray diffraction patterns were obtained on a D8 Advanced Bruker using Cu Kα radiation (2θ = 5–60∘). Gas chromatography experiments (GC) were performed with a Shimadzu GC-16A instrument using a 2 m column packed with silicon DC-200 or Carbowax 20M. In GC experiments, n-decane was used as internal standard. 1H NMR spectra were recorded on a Bruker-Avance AQS 300 MHz.
2.2 Preparation of ZnPOM immobilized on ionic liquid-modified silica [ZnPOM@Im-SiO2-Im]
To a solution of [(n-Bu4N)5PZnMo2W9O39] (1 g) in acetonitrile, SiO2-Im (4 g) was added and refluxed for 24 hours. Finally, the catalyst was filtered and dried at room temperature.
2.3 General procedure for epoxidation of alkenes with H2O2 catalyzed by [PZnMoW@SiO2-Im] under reflux conditions
In a 25 mL round-bottom flask equipped with a magnetic stirring bar, to a mixture of alkene (0.5 mmol), and the [ZnPOM@Im-SiO2] (200 mg, 0.01 mmol) in acetonitrile (10 mL) was added H2O2 (30%, 0.5 ml) and refluxed. The progress of the reactions was monitored by GC. At the end of the reaction, the catalyst was filtered and washed with Et2O (20 ml). The organic layer was separated, concentrated and after chromatography on a short column of silica gel, the pure product was obtained. IR and 1H NMR spectral data confirmed the identities of the products.
3 Results and discussion
3.1 Preparation and characterization of catalyst, ZnPOM@Im-SiO2
The preparation route for the catalyst is shown in Scheme 2. First 3-chloropropyl functionalized silica, SiO2, was reacted with N-methylimidazole at 80 °C to prepare the supported ionic liquid. The IR spectrum of SiO2-Im (Fig. 1a) showed the characteristic band of the parent ionic liquid (νC=N) at 1638 cm−1. The four typical skeletal vibration of the polyoxometalates appear at 1057 (νP-O), 953 (νM=O), 889 (νM–Ob–M) and 807 (νM–Oc–M) (Ob: corner-sharing; Oc: edge-sharing), respectively [24]. After an ion-exchange with polyoxometalate anion, owing to the overlap of P–O band with those of Si-O-Si stretching vibrations of silica gel, only the bands corresponding to Mo-Ob-Mo (887 cm−1) and Mo-Oc-Mo (807 cm−1) and M = O (946 cm−1) are observed in the supported catalyst (Fig 1b). The FT-IR spectra indicated that ZnPOM had been successfully supported on ionic liquid-modified SiO2. The support and the catalyst were characterized by elemental analysis. The nitrogen content of the SiO2-Im was about 0.86%. According to this value, the amount of the ionic liquid fragment, which was attached to SiO2, was 0.31 mmol/g of support. The amounts of Mo and W were measured by ICP. Both values showed that the catalyst loading is about 0.05 mmol/g. The XRD patterns of Im-SiO2, ZnPOM and [ZnPOM@Im-SiO2] are shown in Fig. 2. From these patterns, it is clear that PVMo has been introduced in the substitutional position of SìO2-Im. Diffuse reflectance (DR) UV–Vis spectra of the ZnPOM showed a broad absorption peak at 258 nm which can be attributed to a ligand metal charge transfer (LMCT). This absorption band presents in the UV–Vis spectra of [ZnPOM@Im-SiO2] (Fig. 3B) and since pure SìO2-Im shows no UV absorption peak in this area (Fig. 3A), it is clear that ZnPOM has been supported on the Im-SiO2. The SEM images of SiO2-Im and [ZnPOM@Im-SiO2] are shown in Fig. 4. The clear change in the morphology of catalysts indicated that the PZnMoW has been supported on the SiO2-Im.

The preparation route for catalyst.
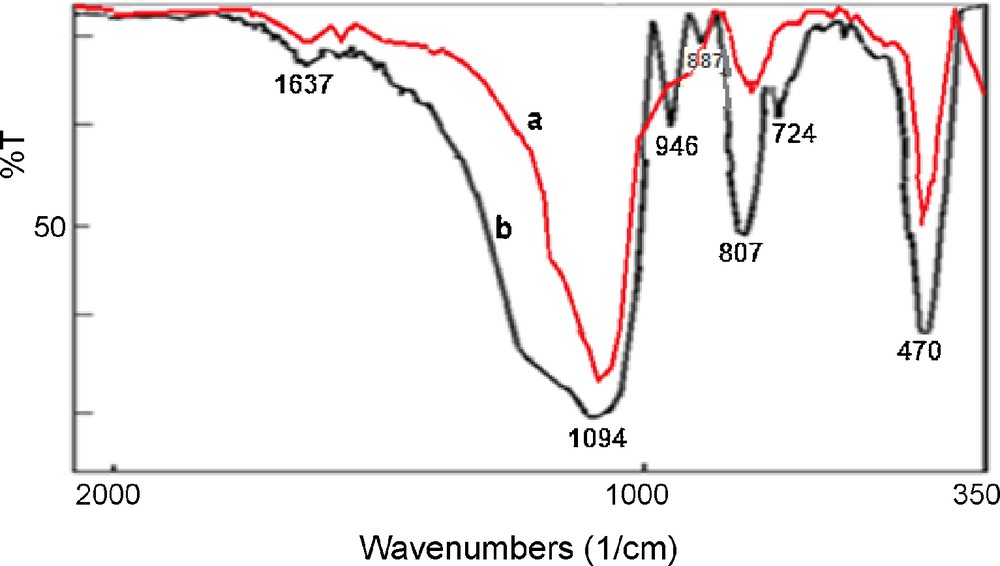
FT-IR spectra of: (a) SiO2-Im and (b) [ZnPOM@Im-SiO2].

XRD patterns of: (A) SìO2-Im; (B) ZnPOM and (C) [ZnPOM@Im-SiO2] composite.
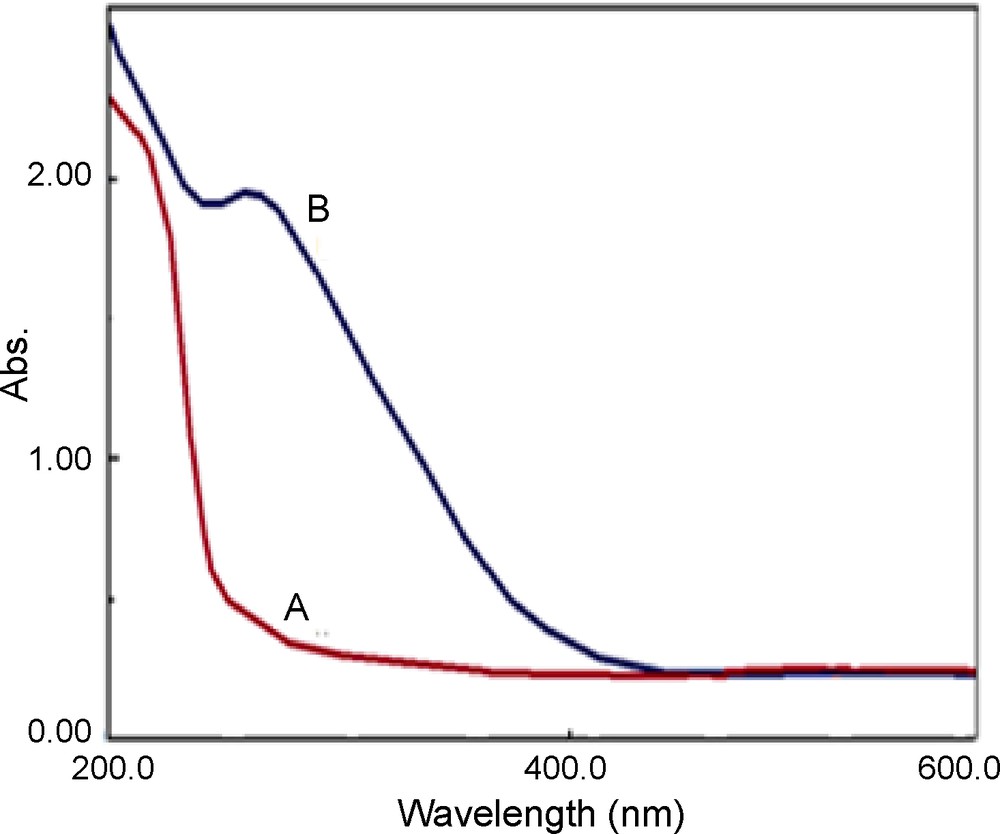
DR UV–Vis spectra of: (A) SiO2-Im and (B) [ZnPOM@Im-SiO2].
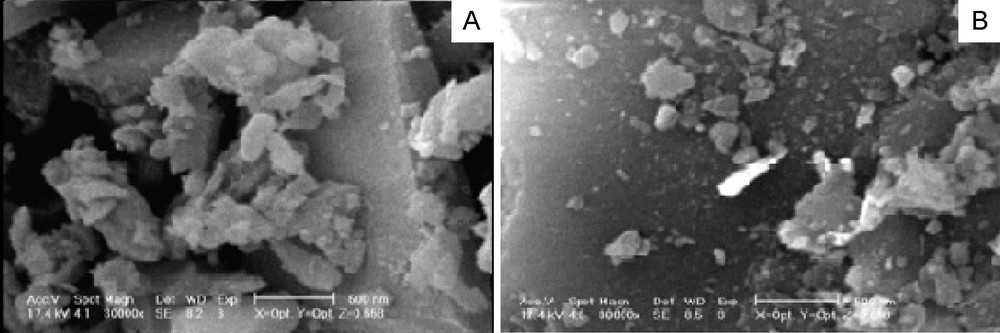
Scanning electron micrographs of: (A) SiO2-Im and (B) [ZnPOM@Im-SiO2].
3.2 Catalytic activity
The synthesized catalyst [ZnPOM@Im-SiO2] was used in the oxidation of various alkenes with aqueous H2O2. First, the effect of different solvents such as dichloromethane, chloroform, acetonitrile, acetone and methanol were checked on the epoxidation of cis-cyclooctene. Amongst them, acetonitrile was chosen as reaction media because the highest epoxide yield was observed (Table 1). The catalytic activity of this catalyst was investigated in the epoxidation of different olefins (Table 2). The reactions were continued until no further progress was observed. In these reactions, cis-cyclooctene, cyclohexene, 1-heptene and 1-octene were completely converted to their corresponding epoxides with 100% selectivity. In the case of indene, the major product was epoxide in 90% yield, and indene-1-one was produced as by-product. In the oxidation of α-pinene, the major product was α-pinene oxide (60%), while verbenone and verbenol were produced as minor products. Control experiments in the absence of the catalyst were also investigated and it was observed that the amount of product is negligible.
The effect of solvent on the epoxidation of cis-cyclooctene catalyzed by [ZnPOM@Im-SiO2] under reflux conditions.a
| Entry | Solvent | Epoxide yield (%)b | Time (h) |
| 1 | CH2Cl2 | 8 | 2 |
| 2 | CHCl3 | 7 | 2 |
| 3 | (CH3)2CO | 50 | 2 |
| 4 | CH3OH | 42 | 2 |
| 5 | CH3CN | 98 | 2 |
a Reaction conditions: cis-cyclooctene (0.5 mmol), catalyst (200 mg, 0.01 mmol), solvent(10 mL), H2O2 (0.5 ml).
b GC yield based on the starting cyclooctene.
Oxidation of alkenes with H2O2 catalyzed by [ZnPOM@Im-SiO2] under reflux conditions.a
| Entry | Substrate | Product | Time (h) | Conversion(%)b | Epoxide selectivity (%) | TOF(h−1) | ||
| 1 | 2 | 98 | 100 | 24.5 | ||||
| 2 | 5 | 90 | 100 | 9.0 | ||||
| 3 | 1.5 | 100 | 90 | 33.3 | ||||
| 4 | 2.5 | 100 | 60 | 20 | ||||
| 5 | 5 | 55 | 100 | 5.5 | ||||
| 6 | 5 | 50 | 100 | 5.0 |
a Reaction conditions: alkene (0.5 mmol), H2O2 (0.5 ml), catalyst (200 mg, 0.01 mmol), acetonitrile (10 ml).
b GC yield based on the starting alkene.
3.3 Catalyst reuse and stability
The stability of the [ZnPOM@Im-SiO2] catalyst was monitored using multiple sequential oxidation of cyclo-octene with hydrogen peroxide under reflux conditions. To assess stability and reusability, the catalyst was separated from the reaction mixture after each experiment, washed thoroughly with acetonitrile and n-hexane successively and dried before being used in the subsequent run. The results of the recycling experiments indicated that the yields reduced in three first runs and after then no significant loss of its catalytic activity was observed (Fig. 5). The filtrates were used for determination of the catalyst leaching by atomic absorption spectroscopy (AAS). The results showed that in two first runs some catalyst is leached from support (4.45% in the first run and 0.74% in the second run), but in the next runs no leaching was observed. The FT-IR spectroscopy of the recovered catalyst after using for 10 times showed no change in its IR spectra (Fig. 6), which confirmed the fact that the catalytic activity of the catalyst did not change significantly compared to fresh catalyst.

The results obtained from catalyst reuse and stability in the oxidation of cyclooctene with H2O2 by [ZnPOM@Im-SiO2] under reflux conditions.
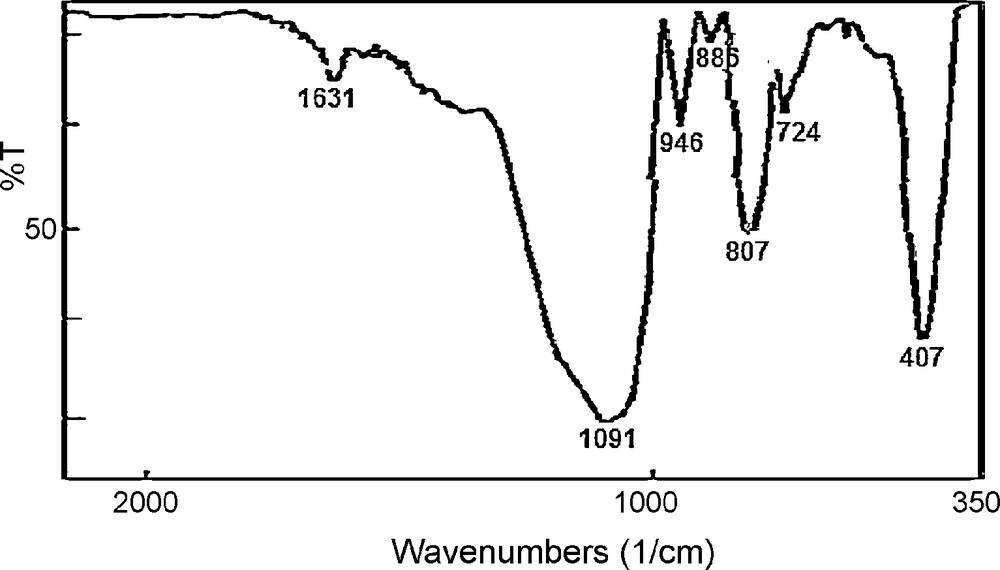
FT-IR spectra of: recovered [ZnPOM@Im-SiO2].
4 Conclusion
In summary, we have designed an efficient olefins epoxidation system with H2O2 in which [(n-C4H9)4N]5PZnMo2W9O39.3H2O has been immobilized on ionic liquid-modified silica. The activity and selectivity of this catalytic system were comparable to its homogeneous analogue. In addition, the catalyst can be easily separated from the reaction mixture and reused.
Acknowledgements
The support of this work by the Center of Excellence of Chemistry of University of Isfahan (CECUI) is acknowledged.


