1 Introduction
In recent years, utilization of ionic liquids (ILs) in organic synthesis and industry received great attention as alternative green solvents due to their unusual properties compared with traditional organic solvents, such as lower vapor pressure, thermal stability; broad rang of solubility for many organic or inorganic compounds, and favorable environments. However, most ILs have the disadvantage of high cost for large-scale production and are environmentally unsafe. Recently, a new versatile reaction medium has emerged, known as deep eutectic solvents (DESs). They are made by simple heating of a quaternary ammonium salt and a metal halide or hydrogen bond donor, with 100% atom economy. Relative to conventional ILs, DESs have significant advantages, such as being easier and cheaper to prepare, nonreactive with water and many are biodegradable, but still exhibit chemical stability, non-flammability and conductivity [1–7].
Protection of amines as formamides is an important reaction in synthetic organic chemistry [8]. Formamides have wide applications as intermediates in the preparation of pharmaceuticals and as important reagents for Vilsmeier formylation [9–11]. They are also in use as Lewis base catalysts in organic transformations of carbonyl compounds [12,13].
In the literature, various approaches with some of the useful formylation reagents have been reported on N-formylation of amines [14–26]. Many of the N-formylation methods have disadvantages such as expensive reagents, formation of side products, thermal instability and difficult accessibility to reagents.
2 Experimental
Reactions were monitored by TLC and GC. FT-IR spectra were recorded using KBr disks on a Bruker Vector 22 FT-IR Spectrometer, 1H NMR spectra were recorded on 500 MHz NMR spectrometer and 13C NMR spectra were recorded on 125 MHz NMR spectrometer using CDCl3 or DMSO as a solvent, and chemical shifts have been expressed in ppm downfield from TMS. Melting points were recorded on Buchi 535 melting point apparatus and are uncorrected. All starting materials, choline chloride and Tin chloride are commercially available and were purchased and used without further purification. Water and other solvent were distilled before used.
2.1 General procedure for preparation of deep eutectic solvent
Choline chloride (100 mmol) was mixed with tin (II) chloride (200 mmol) and heated to ca. 130 °C in air with stirring until a clear liquid was obtained1.
2.2 General procedure for N-formylation of amine
To a mixture of aniline (2 mmol) and formic acid (3 mmol) or trimethyl orthoformate (3 mmol) in water (0.5 mL), tin (II) chloride–choline chloride (2:1) (30 mol %) was added into a test tube with magnetic stirring. The test tube was heated in an oil bath at 70 °C for 20 min and then was cooled to room temperature slowly and, ethyl acetate (20 mL) was added and filtered off to extract the product from the deep eutectic solvent. In some cases, purification was not necessary and the products were analyzed by 1H NMR spectroscopy; however, where appropriate, short flash column chromatography and recrystallization in an appropriate solvent was used for further purification. All compounds are known and characterized on the basis of their spectroscopic data (IR, NMR) and melting point by comparison with those reported in the literature.
2.3 General procedure for the preparation of symmetric formamidines
To a mixture of aniline (2 mmol), trimethyl orthoformate (1 mmol), in a test tube with magnetic stirring, tin (II) chloride–choline chloride (2:1) (30 mol %) ionic liquid was added. The test tube was heated in an oil bath at 90 °C for 20 minutes and then was cooled to room temperature and ethyl acetate (20 mL) was added slowly and filtered off to extract the product from the deep eutectic solvent. Then the solvent and other volatile compounds were removed under reduced pressure to give solid crude products. Further purification was carried out by simple silica gel with the mixture of ethyl acetate and hexane (4:1) or recrystallization in hexane or the mixture of acetone and tert-butylmethylether providing a colorless or yellow powder.
3 Results and discussion
During the course of our study aimed for improving the ecocompatibility of certain organic processes, we evaluated the possibility of performing basic organic transformations by using water and a deep eutectic solvent as green solvent to develop environmentally benign reactions [27–31]. Herein, we report, a simple and practical synthesis of Formamides and N,N’-diarylamidines promoted by deep eutectic solvent under mild reaction conditions.
First, we embarked upon a series of experiments to establish the optimum conditions. In the initial study, five deep eutectic solvents were screened under different reaction conditions (Scheme 1). First findings indicated that simply mixing aniline (2 mmol), formic acid (3 mmol) and choline chloride•2SnCl2 (30 mol %) at 70 °C provided N-formyl aniline 3 in 98% yield within 20 minutes.
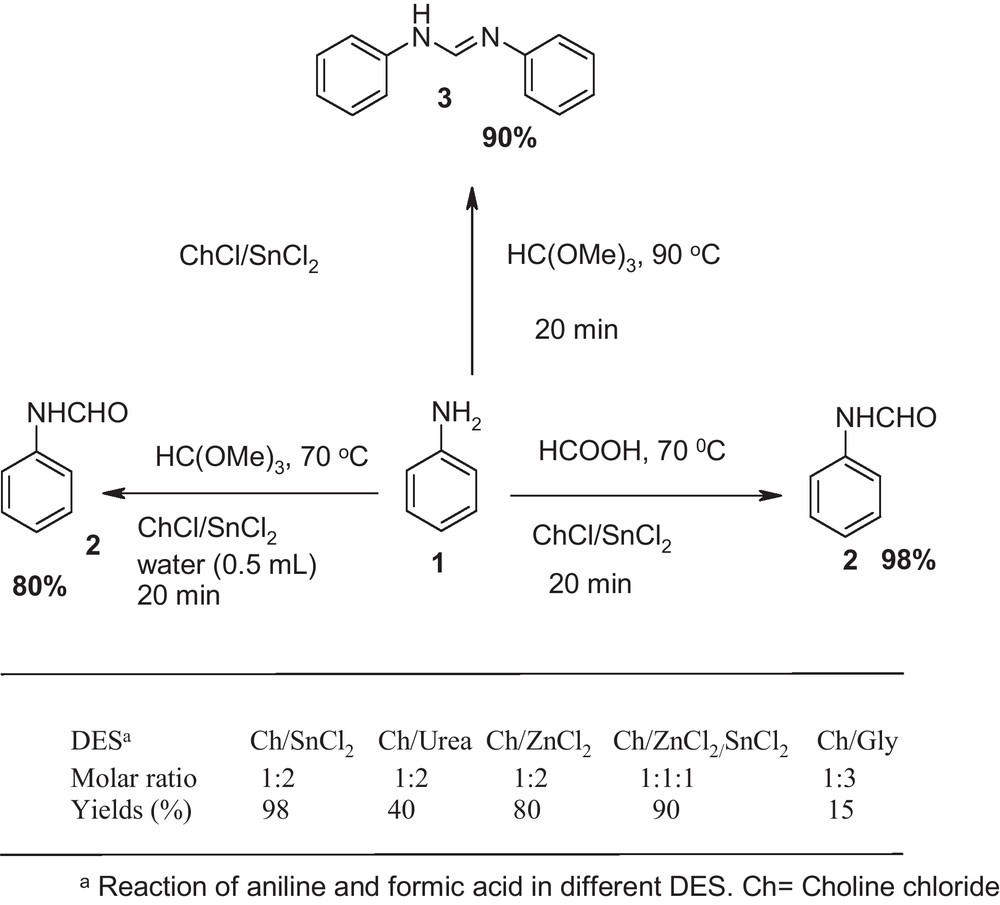
Optimization of reaction condition.
Among different deep eutectic solvents tested in this reaction system, choline chloride ChCl and urea are both the naturally occurring biocompatible compound as they are easily biodegradable and are not hazardous if they are released back into nature. Thus, we further optimized the reaction condition (temperature, time and amount of reagent) in urea-based deep eutectic solvent. So we prolonged the reaction time to 4 h and 12 h and obtained N-formyl aniline, with 78% and 85% yields respectively. Then the reaction was performed at different temperatures. We next investigated the effect of temperature on the yields of reaction. At room temperature only starting materials were recovered. When, the reaction was carried at higher temperatures, i.e., 60 °C, 80 °C, 100 °C and 140 °C. The yield was improved to 90% when the reaction was run at 100 °C. However, increasing the temperature to 140 °C failed to enhance the reaction rate substantially. In point of fact, higher temperatures lowered the product yield slightly, accompanied by some impurities. Furthermore, 4-nitroaniline did not give any products at choline chloride/urea based deep eutectic solvent.
To demonstrate the versatility of this green method, we next investigated the scope of this reaction under the optimized conditions with the orthoformate as protecting group in deep eutectic solvent. In DES, reaction of aniline with trimethyl orthoformate, depending on the reaction conditions, provided either compound 2 or 3 as the major product with satisfactory yield. Under anhydrous conditions in the presence of DES (30 mol %), reaction of aniline (2 mmol) and trimethyl orthoformate (1 mmol) at 90 °C gave N,N’-diarylamidines 3 at 90% yields within 20 minutes. When same reaction condition was carried out in the presence of water (0.5 mL), formamide 2 were obtained in 80% yields (Scheme 1).
This finding has encouraged us to investigate the N-formylation of a variety of aryl, and heteroaryl amines with formic acid and trimethyl orthoformate under similar conditions using DES as a catalyst and reaction condition and the results of this investigation are shown in Table 1. All the reactions with substituted aromatic amines proceeded very cleanly, and no undesirable side-reactions were observed, although the yields were highly dependent on the substituents and formylating agent. Anilines containing either electron-donating or electron-withdrawing groups such as chloro, fluoro, bromo and iodo favored the formation of product with formic acid and orthoester. In contrast, strong electron-withdrawing group and deactivated amines gave the slightly lower yield with longer reaction times (Table 1). Secondary aromatic amines, such as N-ethyl aniline gave excellent yields with both formyl sources. With aliphatic amines no N-formylation occurred with both formyl sources whereas diphenylamine gave moderate yield with formic acid. When o-phenylenediamine was used, instead of N-formylation, cyclization occurred to give benzimidazole as a sole product with formic acid and orthoester. In the all cases formic acid is more efficient formylating agent when compared to orthoester.
Synthesis of Formamides (2) in DESs.
| Yields (%)a | ||||
| Entry | Product (2) | HCO2H | HC(OMe)3 | |
| 1 | X = H | 98 | 80 | |
| 2 | X = Cl | 95 | 78 | |
| 3 | X = Br | 90 | 72 | |
| 4 | X = I | 92 | 74 | |
| 5 | X = OH | 95 | 70 | |
| 6 | X = CH3 | 95 | 68 | |
| 7 | X = n-Bu | 90 | 64 | |
| 8 | X = OMe | 97 | 60 | |
| 9 | X = Me2CH | 97 | 72 | |
| 10 | X = NO2 | 90 | 40 | |
| 11 | X = Cl | 90 | 58 | |
| 12 | X = Br | 82 | 62 | |
| 13 | X = OH | 88 | 60 | |
| 14 | X = CH3 | 90 | 74 | |
| 15 | X = OMe | 92 | 76 | |
| 16 | X = C2H5 | 88 | 68 | |
| 17 | X = Cl | 95 | 55 | |
| 18 | X = Br | 90 | 60 | |
| 19 | X = CH3 | 92 | 70 | |
| 20 | X = C2H5 | 85 | 72 | |
| 21 | 88 | 50 | ||
| 22 | 72 | 40 | ||
| 23 | 80 | 45 | ||
| 24 | 70 | 30 | ||
| 25 | 72 | 35 | ||
| 26 | 90 | 60 |
a Isolated yields.
Amidines and their derivatives have important industrial applications in catalyst chemistry [31], material science [32], biological chemistry [33–35], nitrogen based superbase [36] promoted organic reactions and switchable solvents [37–41]. A review of synthetic methods in the literature indicates that nitriles, amides and dithiocarbamates in the presence of either protic acid or Lewis acid are the most common building blocks for the synthesis of unsymmetrical amidines. Furthermore, the condensation of primary amine with carboxylic acids and their derivatives or ortho-esters can be used for the synthesis of symmetrical amidines in low yields and long reaction time [42,43].
To expand the scope of this reaction further, eco-friendly preparation of symmetrical amidines using anhydrous eutectic salts was carried out with a primary aryl amine and orthoformate (Table 2). Notably, most aromatic amines performed very well and led to symmetrical formamidines in good yields. As shown in Table 2, a series of aromatic amines bearing either electron-donating or electron-withdrawing groups on the aromatic ring was investigated. In general, electron-rich arylamines and mild electron-withdrawing groups such as halides afforded good to excellent yields of products in short reaction times. However, when strongly electron-withdrawing groups (such as CF3) were placed in the ortho-, meta- and parapositions, higher reaction temperatures and longer reaction times were required to access the products in reasonable yields. Furthermore, heteroaromatic amine such as 4-aminopyridine in deep eutectic solvent gives the desired products within prolonged reaction time in good yields (Table 2).
Simple ppreparation of symmetric formamidines in deep eutectic solvent.a
| Entry | Products | Yields (%)a,b | Entry | Products | Yields (%)a,b |
| 1 | 90 | 10 | 80 | ||
| 2 | 80 | 11 | 75 | ||
| 3 | 82 | 12 | 88 | ||
| 4 | 78 | 13 | 90 | ||
| 5 | 85 | 14 | 78 | ||
| 6 | 85 | 15 | 72 | ||
| 7 | 80 | 16 | 45c | ||
| 8 | 78 | 17 | 64c | ||
| 9 | 74 | 18 | 66c |
a Isolated yields.
b Reaction condition: amine (2 mmol), trimethyl orthoformate (1 mmol) and tin (II) chloride–choline chloride (2:1) (30 mol %) at 90 °C.
c Reaction run at 130 °C for 4 h.
4 Conclusion
In summary, we have developed that an environmentally friendly, and practical procedure for the synthesis of N,N’-diarylamidines and formamides in the presence of deep eutectic solvent and readily available starting material in high yields. We believe that originality, time/cost savings and experimental simplicity, concepts that are considered by modern academic and industrial synthetic chemists in reaching the maximum efficiency of a process, are clearly represented this very simple catalytic transformation that provides an appealing methodology for the synthesis of amidine derivatives. Exploitation of this novel reaction media for the other organic synthesis is currently under way in our laboratory.
Acknowledgments
The financial support of this work provided by Chemistry and Chemical Research Center of Iran and Iran National Science Foundation (INSF) is gratefully appreciated.


