1 Introduction
The construction of CC bonds through CH activation techniques is on the rise, as it typically obviates the need for costly and tedious pre-activation steps [1]. These transformations are highly atom economical as they usually produce little or no waste (in the case of redox neutral CH activation cross-couplings). These reactions are usually easy to implement, operator friendly, and scalable to some extent [2]. Even when large amounts of oxidant are required, techniques have been elaborated to use air, or O2 as terminal oxidant, making these promising leads for the future of organic chemistry [1,3]. Nevertheless, these reactions still suffer from selectivity issues, an obvious problem as most substrates have many different CH positions between which the catalyst must discriminate. In this account, we wish to revisit the selectivity issues, which we have encountered in several of our works on Rh catalyzed CH activation oxidative cross-couplings. We chose to highlight in particular three topics: the pyrrole synthesis and its reactivity switch between vinylic CH and allylic CH activation (section 2.1), the double oxidative annulated lactam synthesis and its peculiar trisubstituted exocyclic double bond formation (section 2.2), and finally the linear diene synthesis, and its many possible diastereomers (section 2.3).
2 Results and discussions
2.1 CH activation for pyrrole synthesis
While studying the CH activation of a series of protected β-amino acid precursors with RhCp* catalysts, we discovered that the nature of the carboxylic acid protecting groups was essential for directing which CH position would be functionalized (see molecules 1 and 3, Scheme 1) [4]. Indeed, with an ester protected substrate (molecule 1), only the allylic CH functionalization is observed, whereas with a nitrile protecting group (molecule 3), only the vinylic one is functionalized (Scheme 1). This is explained by a metallacycle rearrangement. Through deuterium control experiments, it was established that the vinylic CH activation occurs first (intermediate I) and rearranges to the allylic metallacycle, provided that an ester group can stabilize it by chelation (intermediate II). The subsequent alkyne insertion, rearrangement and reductive elimination lead to a series of substituted pyrroles with exclusive selectivity. The identity of these compounds was first tackled through a series of advanced NMR techniques, and then later in the X-ray structure of compounds 2b, 4a and 4b (Figs. 1–3, respectively). Structure 4a especially, is interesting because it is the only tetra-substituted pyrrole of the series, bearing four different substituents. Intriguingly, only the depicted isomer is detectable in the reaction mixture, which is arguably outstanding for a dehydrogenative one step cross-coupling of two very simple components. The reader should also note that in the case of unsymmetrical internal alkynes as coupling partners, the aryl moiety is always on the side of the N atom (position 2 in the case of 4a), whereas the alkyl chain takes up position 3.

Pyrrole synthesis.

X-ray structure (Ortep view 30% probability level) of pyrrole 2b. Hydrogen atoms have been omitted for clarity.
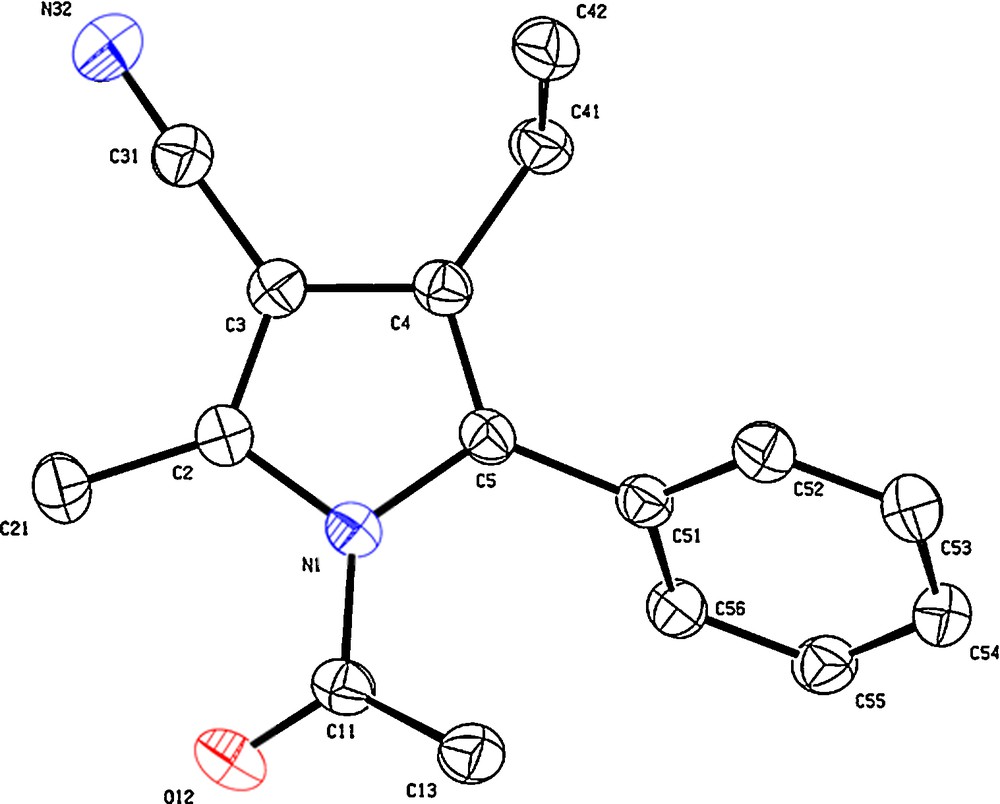
X-ray structure (Ortep view 30% probability level) of pyrrole 4a.
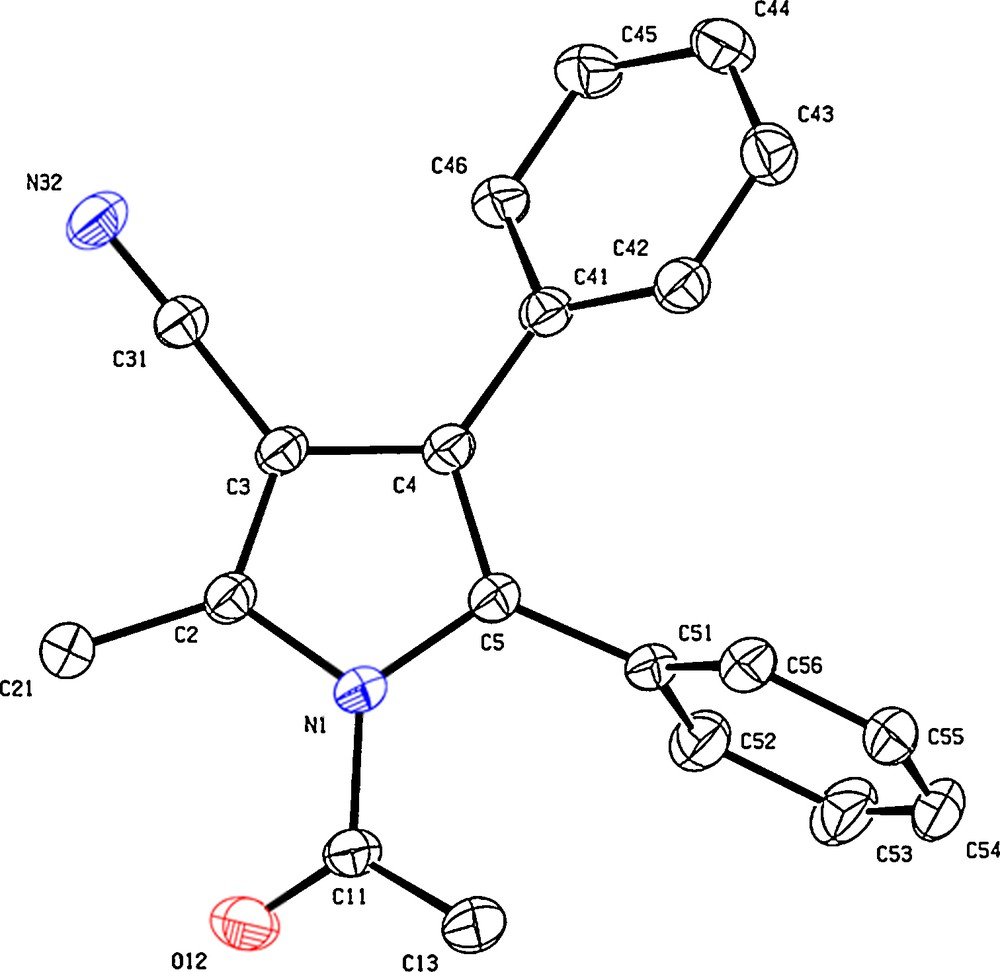
X-ray structure (Ortep view 30% probability level) of pyrrole 4b.
2.2 Double CH activation for annulated lactam synthesis
In the course of studying the oxidative olefination of primary benzamides with Michael acceptors such as n-butylacrylate, we came across a peculiar cyclization reaction (Scheme 2) [5–7]. Through control experiments, we established that: 1) the first step of the reaction is a classical oxidative olefination (indeed, when 7 is engaged under the catalytic conditions, the intramolecular cyclization product 8 is formed); 2) the second step does not go through a Michael type addition, as the cyclic conjugate addition adduct 9 did not lead to the product when submitted to the reaction conditions. Thus, the product may have formed through a second, vinylic CH activation event, followed by reductive elimination with the benzamide part. Nevertheless, an electrophilic mechanism cannot be excluded. The configuration of the exo double bond can be assigned through astute NMR experiments. We were also fortunate enough to obtain crystals suitable for X-ray crystal structural analysis (Fig. 4). This confirmed that the double bond is exclusively Z-configured in lactam product 6, a diasteroselectivity that is linked to the intramolecular H-bond between NH and ester moieties. Indeed, usage of a substituted benzamide 10 leads to a mixture of E and Z-configured products 11.
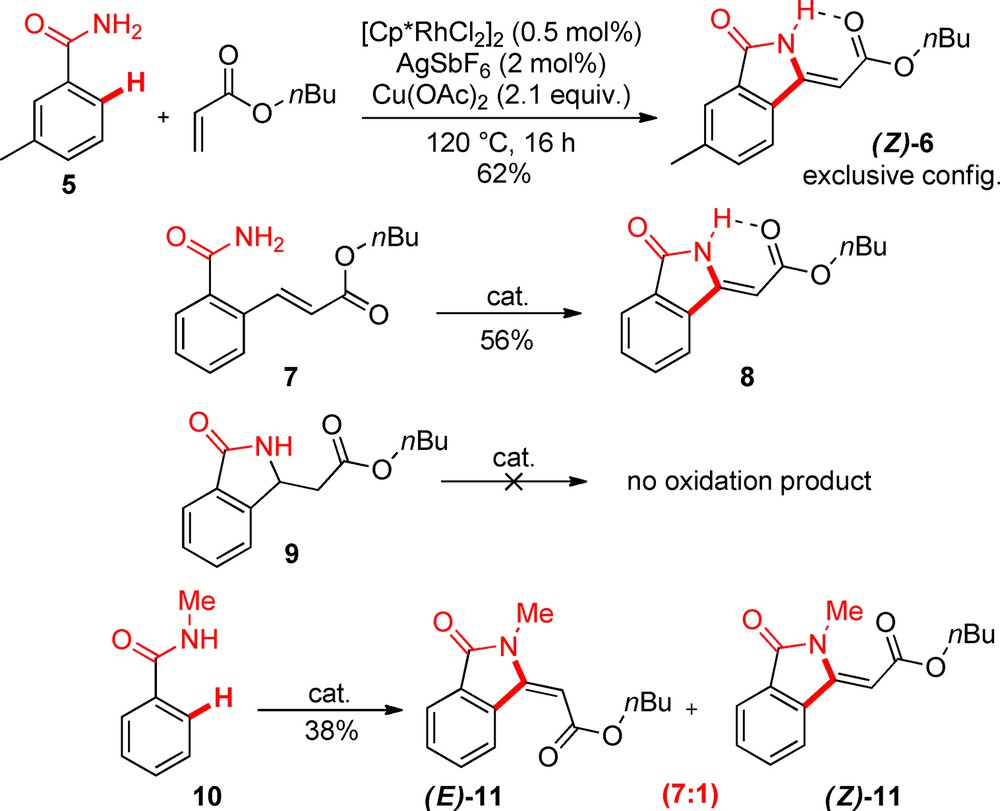
Annulated lactam synthesis.
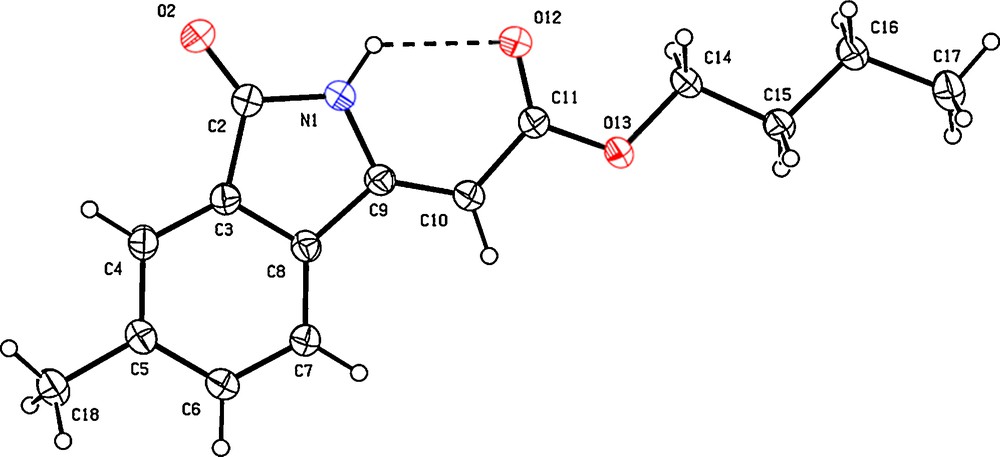
X-ray structure (Ortep view 30% probability level) of lactam 6. Selected atomic distances (Å): N1-H1 = 0.90(2), O12-H1 = 2.13(2), C9-C10 = 1.346(2), C10-C11 = 1.453(2). Selected dihedral angle: C9-N1-012-C11 = -1.4(2)°.
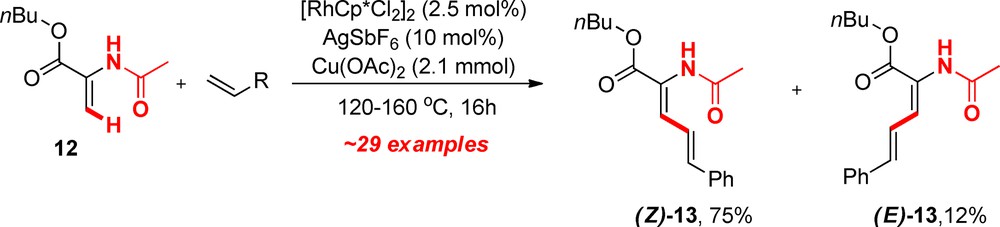
2.3 CH activation for linear diene synthesis
While we discovered that a number of acrylate derivatives were suitable substrates for CH activation and oxidative olefination, we faced a new type of selectivity problem with competing β-hydride elimination pathways [8,9]. Especially in substrates in which two functional groups are competing for the rhodacycle intermediate, such as α amino-acid precursors like methyl acetamidoacrylate derivatives (12, Scheme 3). The products are typically composed of a mixture of major Z and minor E configured linear dienes, usually separable through Silica gel column chromatography. Interestingly, no branched isomer was ever detected in this sort of reaction using these conditions, significantly simplifying the issue of selectivity. Because these molecules are highly functionalized, it is generally trivial to determine the absolute configurations of every single isomer through standard NOE experiments, even within mixtures. In the particular case of molecule E-13, an X-ray structure confirmed the stereochemistry, also assigned by the help of other methods (Fig. 5). A slight H-bonding effect between the ester group and one of the vinylic CH is visible (H5-O2 = 2.51 Å), which may partly be linked to the systematic formation of this minor isomer, through a H-bonding directed β-hydride elimination (Table 1). We have attempted to tune the electronic properties of the substituents, but this only had a minor effect on the regioselective outcome, and the resulting yields are usually lower (Table 1)
Unnatural amino-acid precursor synthesis.
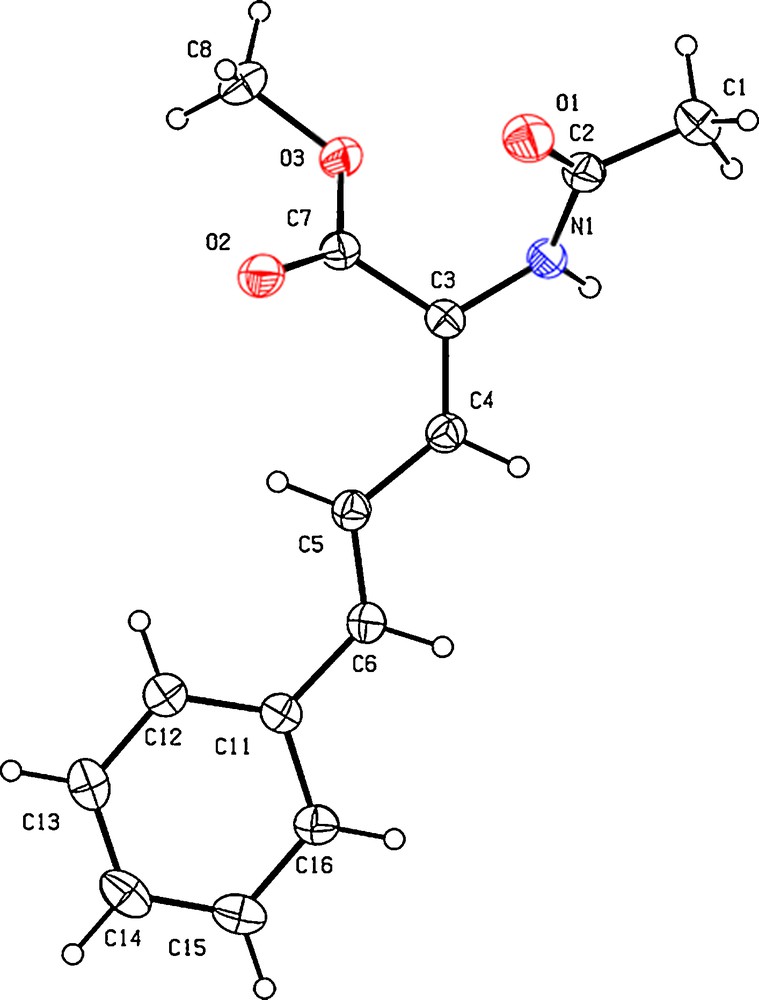
X-ray structure (Ortep view 30% probability level) of α amino-acid precursor E-13. Selected atomic distances (Å): C3-C4 = 1.338(3), C4-C5 = 1.436(3), C5-C6 = 1.341(4), C6-C11 = 1.459(3), H5-O2 = 2.51(3). Selected dihedral angles: O2-C7-C3-C4 = 36.5(4)°, C4-C3-N1-C2 = −131.5(3)°, C6-C5-C4-C3 = 174.8(3)°, C16-C11-C6-C5 = 175.4(3)°.
Crystallographic data.
| 2b | 4a | 4b | 6 | E-13 | |
| Formula | C21H19NO3 | C20H16N2O | C16H16N2O | C15H17NO3 | C14H15NO3 |
| M | 333.37 | 300.35 | 252.31 | 259.30 | 245.27 |
| Crystal size (mm) Color | 0.35 × 0.10 × 0.02 colorless | 0.25 × 0.20 × 0.10 colorless | 0.25 × 0.20 × 0.07 colorless | 0.35 × 0.07 × 0.05 colorless | 0.35 × 0.05 × 0.03 colorless |
| a (Å) | 6.5120 (7) | 8.3207 (2) | 7.4414 (4) | 4.0426 (3) | 33.2953 (17) |
| b (Å) | 7.5339 (8) | 20.3346 (8) | 13.5131 (7) | 11.7557 (6) | 5.8094 (2) |
| c (Å) | 17.536 (2) | 9.8166 (5) | 14.2305 (5) | 14.3134 (12) | 13.9664 (8) |
| α (°) | 90 | 90 | 90 | 101.941 (4) | 90 |
| β (°) | 94.574 (5) | 107.628 (2) | 103.936 (5) | 95.094 (7) | 109.925 (7) |
| γ (°) | 90 | 90 | 90 | 91.029 (3 | 90 |
| V (Å3) | 857.59 (16) | 1582.96 (11) | 1388.85 (11) | 662.42 (8) | 2539.7 (2) |
| λ (Å) | 1.54178 | 1.54178 | 1.54178 | 1.54178 | 1.54178 |
| ρcalc (g cm−3) | 1.291 | 1.260 | 1.207 | 1.300 | 1.283 |
| μ (mm−1) | 0.696 | 0.621 | 0.604 | 0.738 | 0.741 |
| Z | 2 | 4 | 4 | 2 | 8 |
| Crystal system/space group | Monoclinic/P21 (No. 4) | Monoclinic/P21/n (No. 14) | Monoclinic/P21/c (No. 14) | Triclinic/P1 bar (No. 2) | Monoclinic/C2/c (No. 15) |
| Reflections collected | 5121 | 12312 | 10008 | 7107 | 8548 |
| Reflections unique/Rmerge | 2329/0.069 | 2755/0.037 | 2412/0.053 | 2235/0.037 | 2138/0.046 |
| Reflection observed (I ≥ 2 σ(I)) | 1861 | 2470 | 2043 | 2018 | 1818 |
| Refined parameter | 228 | 210 | 176 | 177 | 168 |
| R1 (oberserved data) | 0.056 | 0.038 | 0.044 | 0.044 | 0.054 |
| wR2 (all data) | 0.153 | 0.107 | 0.117 | 0.124 | 0.155 |
| Flack parameter | 0.0(5) | – | – | – | – |
| Max./min. residual electron density | 0.20/–0.16 | 0.15/–0.17 | 0.17/–0.18 | 0.29/–0.19 | 0.19/–0.21 |
| CCDC | 864553 | 864554 | 864555 | 864556 | 864557 |
3 Summary and conclusion
Herein, we have collected a few elements concerning the study of the selectivity in three different Rh catalyzed CH activation cross-couplings. We think they bring some more perspectives on these works, and clarify some of the issues related with regioselectivity.
Data sets were collected with a Nonius KappaCCD diffractometer. Programs used: data collection COLLECT (Nonius B.V., 1998), data reduction Denzo-SMN (Z. Otwinowski, W. Minor, Methods in Enzymology, 276 (1997) 307–26), absorption correction Denzo (Z. Otwinowski, D. Borek, W. Majewski & W. Minor, Acta Cryst. A59 (2003) 228–34), structure solution SHELXS-97 (G.M. Sheldrick, Acta Cryst. A46 (1990), 467–73), structure refinement SHELXL-97 (G.M. Sheldrick, Acta Cryst. A64 (2008) 112–22), graphics ORTEP.
Acknowledgements
This work was supported by the European Research Council under the European Community's Seventh Framework Program (FP7 2007–2013)/ERC Grant agreement no 25936 and the Alexander von Humboldt Foundation (F.P.). The research of F.G. has been supported by the Alfried Krupp Prize for Young University Teachers of the Alfried Krupp von Bohlen und Halbach Foundation.


