1 Introduction
The synthesis of 2-amino-3,5-dicarbonitrile-6-thio-pyridine derivatives has attracted much attention of organic chemists for their various biological activities. Compounds containing a 2-amino-3,5-dicarbonitrile-6-thio-pyridine ring system are useful as anti-prion [1], anti-hepatitis B virus [2], anti-bacterial [3], and anticancer [4] agents and as potassium channel openers for treatment of urinary incontinence [5]. These compounds were reported to inhibit PrPSc accumulation in scrapie-infected mouse neuroblastoma cells (ScN2a) [1a], MAPK-activated PK-2 [4b], IKK-2 for treating HBV infection [2], and modulate androgen receptor function [6]. In addition, it was found that several of these compounds are selective ligands towards adenosine receptors [7]. They were recently recognized as potential targets in the development of new drugs for the treatment of diseases such as Parkinson, hypoxia/ischemia, asthma, epilepsy [8] and Creutzfeldt-Jacob [1a,b] and also kidney problems [8]. One of the most significant existing methods for the synthesis of 2-amino-3,5-dicarbonitrile-6-thio-pyridines involves the three-component condensation of aldehyde, malononitrile, and thiol [9]. The condensation has been carried out under basic conditions using various bases including Et3N, DABCO [10], DBU [11], TBAH, piperidine [12], nanocrystaline MgO (NAP-MgO) [13], nano silica [14], KF/alumina [15], basic alumina [16], TBAF [17], sodium silicate [18], K2CO3 [19] and microporous molecular sieves [20]. Some acids such as ZrOCl2.8H2O [21], ZnCl2 [22], o-iodoxybenzoic acid (IBX) [23] and H3BO3 [24] were also addressed for the promotion of this reaction. Moreover, ionic liquids such as [bmIm]OH [25] and [bmIm]Br [26] were found to catalyze the synthesis of polysubstituted pyridines. However, some of these protocols suffer from drawbacks such as formation of inevitable side products, prolonged reaction times, low yields, harsh reaction conditions, tedious workup and use of expensive and environmentally toxic catalysts as well as solvents. Thus, organic chemists have struggled to overwhelm these shortcomings and develop efficient methods for these nucleus using milder non-hazardous and inexpensive reagents.
In recent years, room temperature ionic liquids have received considerable attention as an alternative green reaction medium for numerous organic reactions due to their favorable properties, such as good solvating capability, wide liquid range, negligible vapor pressure, tunable polarity, high thermal stability and ease of recyclability [27]. Ionic liquids have also played a significant role in controlling the reactions as catalysts [28]. The major concern in using ionic liquids as reaction media in industrial process is the cost of the ionic liquid, which would be directly dependent on the price of the cations and anions that are used for their production [29]. The currently popular ionic liquids incorporate expensive cations such as alkyl methyl imidazolium or dialkyl imidazolium and expensive anions such as tetrafluroborate or hexaflurophosphate. Thus, introducing of cost-effective ionic liquids as reaction media is indispensable. Moreover, despite the great attention for methylimidazolium (mIm) based-ionic liquids, taken from EC50 data, the other existed ionic liquids are better candidates [30].
As part of our continued interest on the development of new eco-friendly methods for the synthesis of organic compounds [31], we have recently synthesized β-phosphonomalonates, 4-substituted 2-amino-4H-chromenes and bis(pyrazolyl)methanes in the presence of task-specific ionic liquids [32]. In this connection, herein, we wish to report 2-hydroxyethylammonium acetate (2-HEAA) [33] as a task-specific ionic liquid for the synthesis of 2-amino-3,5-dicarbonitrile-6-thio-pyridines at room temperature (Scheme 1).

Synthesis of 2-amino-3,5-dicarbonitrile-6-thio-pyridines.
2 Results and discussion
Initially, we have optimized different reaction parameters for the synthesis of 2-amino-4-phenyl-6-(phenylsulfanyl)-3,5-pyridinedicarbonitrile (1) using one-pot three-component reaction of benzaldehyde, malononitrile and thiophenol (Table 1).
Synthesis of 2-amino-4-phenyl-6-(phenylsulfanyl)-3,5-pyridinedicarbonitrile (1) in the presence of different [X(CH2)nNH3+][Y−].
| Entry | [X(CH2)nNH3+][Y−] | Amount of IL (mL) | Time (min) | Yielda (%) |
| 1 | n = 2, X = OH, Y = AcO | 0.125 | 5 | 88 |
| 2 | n = 2, X = OH, Y = AcO | 0.25 | 5 | 88 |
| 3 | n = 2, X = OH, Y = AcO | 0.5 | 5 | 96 |
| 4 | n = 2, X = H, Y = AcO | 0.5 | 2.5 h | 48b |
| 5 | – | – | 24 h | 0 |
| 6 | n = 2, X = OH, Y = CF3CO2 | 0.5 | 5 | 95 |
| 7 | n = 2, X = OH, Y = HCO2 | 0.5 | 5 | 96 |
| 8 | n = 2, X = OH, Y = CH3SO3 | 0.5 | 5 | 95 |
| 9 | n = 2, X = OH, Y = NO3 | 0.5 | 5 | 96 |
| 10 | n = 3, X = OH, Y = AcO | 0.5 | 5 | 96 |
| 11 | n = 5, X = OH, Y = AcO | 0.5 | 5 | 98 |
| 12c | n = 2, X = OH, Y = AcO | 0.5 | 5 | 69b |
a Isolated yield. Conditions: benzaldehyde (1 mmol), malononitrile (2 mmol), thiophenol (1 mmol).
b The desired product (1) plus a mixture of by-products were formed.
c H2O was used as solvent.
As it is summarized in Table 1, 0.5 mL of 2-HEAA efficiently promoted the reaction leading to the desired product 1 in excellent yield (Entry 3). A similar reaction in the presence of [EtNH3+][AcO−] proceeded with a longer reaction time (2.5 h) compared with 2-HEAA (5 min) and produced the desired product in low yield (Entry 4). The obtained results showed the specific role of OH group of 2-HEAA in imparting the catalytic property and indicated that 2-HEAA could act not only as a solvent but also as a functionalized or task-specific ionic liquid in this method. The same reaction was not successful in the absence of 2-HEAA even after 24 h (Entry 5). It was also found that the reaction outcome does not dependent on the anion in [HO(CH2)2NH3+][Y−] or the length of the alkyl chain in [HO(CH2)nNH3+][AcO−] (Entries 6–11). Increasing interest of organic chemists for the use of water as a solvent of choice and its unique properties encouraged us to examine the present reaction in water (Entry 12). Reaction in aqueous media was observed to proceed in a short reaction time (5 min) but produced the desired product in moderate yield (69%) due to the formation of a by-product.
After performing the reaction of benzaldehyde, malononitrile and thiophenol in the presence of 2-HEAA (0.5 mL as the optimal amount), water was added to the reaction mixture, and the product was filtered off and purified by recrystallization in EtOH. The ionic liquid was recovered after evaporation of the filtrate and drying in vacuo at 100 °C for 24 h. Elemental analysis showed high purity of the recovered ionic liquid. A similar reaction in the presence of recovered ionic liquid proceeded well to produce the desired product 1 after 5 min in 95% yield.
This protocol was also easily amenable to scale-up. For example, the reaction of benzaldehyde, malononitrile and thiophenol in a semi scale-up procedure (10 times) in the presence of 2-HEAA was carried out successfully and the desired product was isolated in 92% yield.
To investigate the scope and general applicability of this procedure, the synthesis of 2-amino-3,5-dicarbonitrile-6-thio-pyridines was studied using different aldehydes and thiols under optimized reaction conditions (Table 2).
Synthesis of 2-amino-3,5-dicarbonitrile-6-thio-pyridine derivatives in the presence of 2-HEAA at room temperature.
| Entry | R | R′ | Product | Yielda (%) | MP (°C) Found | MP (°C) Reported [ref] |
| 1 | 1 | 96 | 219–220 | 215–216 [9] | ||
| 2 | 2 | 85 | 222–223 | 222–224 [25] | ||
| 3 | 3 | 86 | 212–213 | 208–211 [25] | ||
| 4 | 4 | 86 | 228–229 | 228–230 [25] | ||
| 5 | 5 | 78 | 228–229 | 228–229 [15] | ||
| 6 | 6 | 76 | 238–239 | 238 [11] | ||
| 7 | 7 | 84 | 238–240 | 236 [11] | ||
| 8 | 8 | 81 | 305–306 | 305–306 [10] | ||
| 9 | 9 | 72 | 218–220 | – | ||
| 10 | Me | 10 | 90 | 224–225 | 225–226 [9] | |
| 11 | n-Bu | 11 | 70 | 178–179 | 182–183 [34] | |
| 12 | 12 | 85b | 310–311 | – |
a Isolated yield. Conditions: aldehyde/malononitrile/thiophenol: 1/2/1.
b Conditions: aldehyde/malononitrile/thiophenol: 1/4/2.
As the results of Table 2 indicates, 2-amino-3,5-dicarbonitrile-6-thio-pyridines 1–6 were produced from a series of various substituted benzaldehyde and thiophenol in good to high yields (Entries 1–6). 1-Naphthaldehyde as a polynuclear aldehyde and pyridine-3-carbaldehyde as a heteroaromatic aldehyde underwent the condensation reaction to afford the corresponding pyridines in good yields (Entries 7 and 8). Interestingly, 2-HEAA efficiently promoted the synthesis of 2-amino-3,5-dicarbonitrile-6-thio-pyridines using aliphatic aldehydes and thiols (Entries 9–11). It is worth to note that both carbonyl groups in terephthalaldehyde reacted with malononitrile and thiophenol and produced the desired product 12 in 85% yield (Entry 12).
In accordance with the mechanism delineated by Evdokinov et al. and Guo et al. [9], the first step of the process involves the Knoevenagel condensation of an aldehyde with malononitrile to form the corresponding Knoevenagel product A. The reaction proceeds through thiolate addition to nitrile of the Knoevenagel product A followed by Michael addition of the second molecule of malononitrile to the adduct and produces dihydropyridine (B). Aromatization of B under the reaction conditions gives pyridine 1–12 (Scheme 2). In this three-component reaction, it is supposed that dual activation of substrates by 2-HEAA has taken place. The Lewis base moiety of the catalyst (OH) activates the malononitrile and thiol, and its Lewis acid moiety (NH3+) activates the aldehyde and nitrile of the Knoevenagel product A. To show the role of the hydroxyl group in the Knoevenagel condensation, the reaction of benzaldehyde and malononitrile in the presence of 2-HEAA and ethylammonium acetate was studied. The reactions proceed with the same yield and rate. These results showed that in the first step of the mechanism (Knoevenagel condensation), hydroxyl group of 2-HEAA does not play any role.
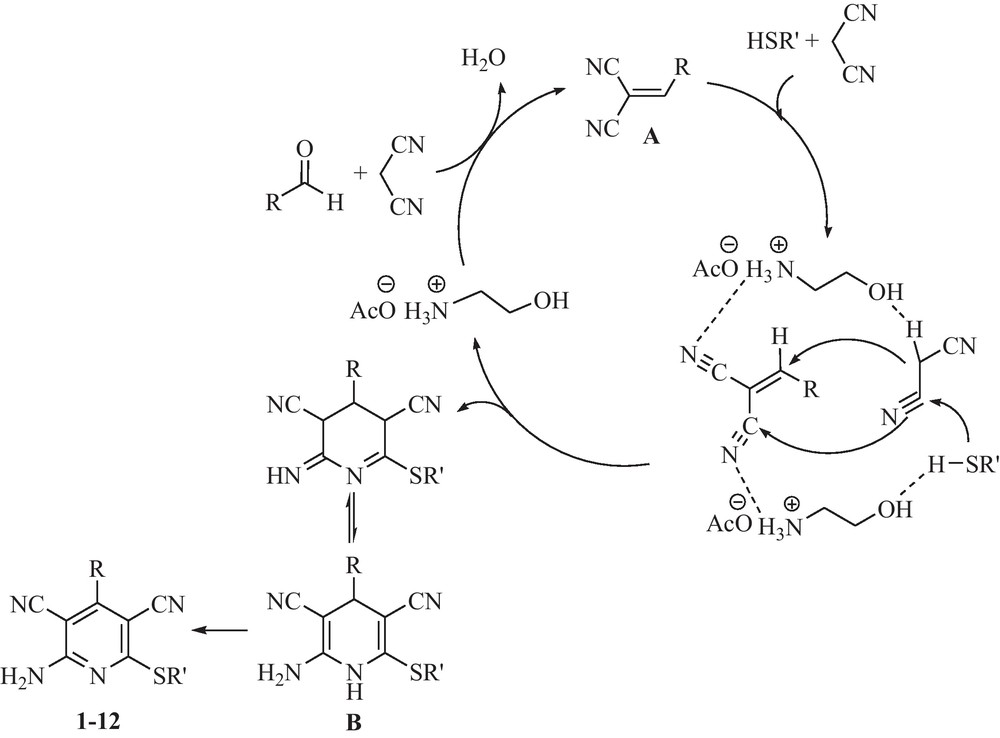
Suggested mechanism for the synthesis of 2-amino-3,5-dicarbonitrile-6-thio-pyridines in the presence of 2-HEAA.
In order to show the merit of the present method for the synthesis of 2-amino-3,5-dicarbonitrile-6-thio-pyridines, we have compared our results obtained using 2-HEAA with some of those reported in the literature for the synthesis of 2-amino-3,5-dicarbonitrile-6-thio-pyridines (Table 3). These results indicate well the superior activity of 2-HEAA than those of other promoters especially for the synthesis of 2-amino-3,5-dicarbonitrile-6-thio-pyridines from aliphatic aldehydes or thiols. Most of the reported methods also suffer from lack of generality for the synthesis of 2-amino-3,5-dicarbonitrile-6-thio-pyridines from arylaldehydes/arylthiols, alkylaldehydes/arylthiols and arylaldehydes/alkylthiols.
Synthesis of 2-amino-3,5-dicarbonitrile-6-thio-pyridines in the presence of reported reagents or catalysts.
| Entry | Catalyst or reagent | Aldehyde/Thiol | Time | Yield (%)a [ref] |
| 1 | Et3N or DABCO | Aryl/Aryl | 2 h [9], 40–90 min [10] | 20–48 [9], 55–96 [10] |
| Alkyl/Aryl | 2 h [9], 40–90 min [10] | 20–29 [9], 39–55 [10] | ||
| Aryl/Alkyl | 2 h [9], 40–90 min [10] | 21–47 [9], 42–92 [10] | ||
| 2 | KF/alumina | Aryl/Aryl | 5–70 min | 56–93 [15] |
| 3 | ZnCl2 | Aryl/Aryl | 2 min–2.5 h | 50–77 [22] |
| Alkyl/Aryl | 3 min–2.5 h | 46 [22] | ||
| 4 | Sodium silicateb | Aryl/Aryl | 1 h | 78–82 [18] |
| 5 | NAP-MgO | Aryl/Aryl | 2–9 h | 48–69 [13] |
| Aryl/Alkyl | 4–8 h | 41–56 [13] | ||
| 6 | Piperidine or TBAH | Aryl/Aryl | 1–24 h | 40–67 [12] |
| Alkyl/Aryl | 1–24 h | 5–6 [12] | ||
| 7 | Microporous molecular sieves | Aryl/Aryl | 30–120 min | 78–91 [21] |
| 8 | H3BO3 | Aryl/Aryl | 8–50 min | 80–94 [24] |
| 9 | DBU | Aryl/Aryl | 10–40 min | 75–91 [11] |
| 10 | [bmlm]OH | Aryl/Aryl | 0.5–1.5 h | 62–92 [25] |
| 11 | [bmlm]Br | Aryl/Aryl | 4–12 min | 75-86 [26] |
| 12 | Nano silica | Aryl/Aryl | 2.5–3 h | 70–85 [14] |
| Alkyl/Aryl | 6 h | 60 [14] | ||
| Aryl/Alkyl | 6 h | 60–65 [14] | ||
| 13 | ZrOCl2.8H2O/NaNH2/[bmim]BF4 | Aryl/Aryl | 2–20 min | 90–98 [21] |
| 14 | Basic alumina | Aryl/Aryl | 50–100 min | 79–90 [16] |
| 15 | TBAF | Aryl/Aryl | 45–120 min | 87–96 [17] |
| Alkyl/Aryl | 420–630 min | 62–64 [17] | ||
| 16 | K2CO3/KMnO4 | Aryl/Aryl | 40–180 min | 70–89 [19] |
| Alkyl/Aryl | 90 min | 80 [19] | ||
| Aryl/Alkyl | 75–90 min | 60–61 [19] | ||
| 17 | IBX | Aryl/Aryl | 1.5–2.5 h | 69–83 [23] |
| 18 | 2-HEAA | Aryl/Aryl | 5 min | 76–96c |
| Alkyl/Aryl | 5 min | 72–90c | ||
| Aryl/Alkyl | 5 min | 70c |
a Isolated yield.
b GC yield.
c This work.
3 Conclusion
In summary, a facile, economical and green protocol for one-pot three-component condensation of aldehydes, malononitrile and thiols in the presence of 2-HEAA as a task-specific ionic liquid has been described. Surprisingly, this ionic liquid efficiently promoted the condensation of both aromatic and aliphatic aldehydes and thiols with malononitrile leading to 2-amino-3,5-dicarbonitrile-6-thio-pyridines in good to high yields in a short reaction time (5 min). All the reactions proceed under nearly neutral conditions reducing the possibility of unwanted side reactions. In addition, the present protocol offers several advantages such as using a reusable and cost-effective ionic liquid, an environmentally benign reaction media avoiding hazardous organic solvents and toxic catalysts, and being amenable to scale-up.
4 Experimental
4.1 Materials and techniques
Chemicals were purchased from Merck and Fluka Chemical Companies. NMR spectra were recorded in ppm in DMSO on a Bruker Avance DPX-400 instrument using TMS as internal standard. Elemental analysis for C, H, N and S were obtained using a Elementar, Vario EL III. IR spectra were run on a Perkin–Elmer 780 instrument. Melting points were determined by Buchi 510 apparatus and are uncorrected. The purification of the products and the progress of the reactions were accomplished by TLC on silica-gel polygram SILG/UV254 plates.
4.2 Typical procedure for the synthesis of 2-HEAA
2-HEAA was prepared according to the previously reported procedure [33]. A solution of acetic acid (50 mmol, 3.00 g) in EtOH (1.5 mL) was added dropwise to a stirring solution of 2-aminoethanol (50 mmol, 3.05 g) in EtOH (1.5 mL) at room temperature within 1 h. The resultant solution was stirred at room temperature for another 20 h. EtOH was removed in vacuo and the oil in residue was dried in vacuo at 50 °C for 48 h to give 2-HEAA as a yellow viscous liquid (pH = 7.96 at 25 °C, b.p. 191 °C, density = 1.11 g cm−3 at 25 °C, electrochemical stability = between −0.6 and 0.7 V, conductivity = 263.3 μs cm−1).
4.3 General procedure for the synthesis of 2-amino-3,5-dicarbonitrile-6-thio-pyridines in the presence of 2-HEAA
A mixture of aldehyde (1 mmol), malononitrile (2 mmol), thiol (1 mmol) and 2-HEAA (0.5 mL) was stirred at room temperature for 5 min. H2O (20 mL) was added to the reaction mixture and the resulting solid was filtered and washed with H2O (2 × 10 mL). Pure product was obtained from the resulting solid by recrystallization in EtOH. Ionic liquid was recovered after evaporation of the filtrate and drying in vacuo at 100 °C for 24 h.
4.4 Spectral data for known 2-amino-3,5-dicarbonitrile-6-thio-pyridines
4.4.1 2-Amino-4-phenyl-6-phenylsulfanyl-pyridine-3,5-dicarbonitrile (1)
314.8 mg, yield 96%; mp 219–220 °C; 1H NMR (400 MHz, DMSO-d6): δH (ppm) 7.84 (2H, br, NH2), 7.63–7.59 (7H, m, Ar), 7.53–7.51 (3H, m, Ar); IR (KBr) (νmax/cm−1): 3471 (NH), 3349 (NH), 2196 (CN).
4.4.2 2-Amino-4-(4-chloro-phenyl)-6-phenylsulfanyl-pyridine-3,5-dicarbonitrile (2)
308.5 mg, 85%; mp 222–223 °C; 1H NMR (400 MHz, DMSO-d6): δH (ppm) 7.88 (2H, br, NH2), 7.68 (2H, d, J = 8.4 Hz, Ar), 7.61 (4H, d, J = 8.4 Hz, Ar), 7.53–7.50 (3H, m, Ar); 13C NMR (100.6 MHz, DMSO-d6): δC (ppm) 166.7, 159.9, 158.0, 135.8, 135.3, 133.2, 130.9, 130.3, 130.0, 129.4, 127.5, 115.7, 115.3, 93.7, 87.6; IR (KBr) (νmax/cm−1): 3470 (NH), 3331 (NH), 2200 (CN).
4.4.3 2-Amino-4-(4-methyl-phenyl)-6-phenylsulfanyl-pyridine-3,5-dicarbonitrile (3)
294.1 mg, 86%; mp 212–213 °C; 1H NMR (400 MHz, DMSO-d6): δH (ppm) 7.81 (2H, br, NH2), 7.62–7.60 (2H, m, Ar), 7.52–7.50 (3H, m, Ar), 7.45 (2H, d, J = 7.6 Hz, Ar), 7.39 (2H, d, J = 8.0 Hz, Ar), 2.42 (3H, s, CH3); 13C NMR (100.6 MHz, DMSO-d6): δC (ppm) 166.6, 160.1, 159.2, 140.8, 135.3, 131.4, 130.2, 129.9, 129.8, 128.9, 127.6, 115.9, 115.6, 93.8, 87.4, 21.4; IR (KBr) (νmax/cm−1): 3460 (NH), 3318 (NH), 2200 (CN).
4.4.4 2-Amino-6-(4-chloro-phenylsulfanyl)-4-phenyl-pyridine-3,5-dicarbonitrile (4)
312.1 mg, 86%; mp 228–229 °C; 1H NMR (400 MHz, DMSO-d6): δH (ppm) 7.89 (2H, br, NH2), 7.63 (3H, d, J = 8.4 Hz, Ar), 7.60–7.56 (6H, m, Ar); 13C NMR (100.6 MHz, DMSO-d6): δC (ppm) 166.3, 160.0, 159.1, 137.2, 135.3, 134.3, 130.9, 129.9, 129.2, 128.9, 126.5, 115.7, 115.5, 93.7, 87.6; IR (KBr) (νmax/cm−1): 3438 (NH), 3315 (NH), 2203 (CN).
4.4.5 2-Amino-6-(4-methoxy-phenylsulfanyl)-4-phenyl-pyridine-3,5-dicarbonitrile (5)
279.2 mg, 78%; mp 228–229 °C; 1H NMR (400 MHz, DMSO-d6): δH (ppm) 7.79 (2H, br, NH2), 7.59–7.55 (5H, m, Ar), 7.52 (2H, d, J = 8.4 Hz, Ar), 7.07 (2H, d, J = 8.4 H, Ar), 3.83 (3H, s, OCH3); IR (KBr) (νmax/cm−1): 3427 (NH), 3322 (NH), 2100 (CN).
4.4.6 2-Amino-6-(4-methyl-phenylsulfanyl)-4-phenyl-pyridine-3,5-dicarbonitrile (6)
259.9 mg, 76%; mp 238–239 °C; 1H NMR (400 MHz, DMSO-d6): δH (ppm) 7.84 (2H, br, NH2), 7.63–7.57 (6H, m, Ar), 7.52–7.50 (3H, m, Ar), 2.51 (3H, s, CH3); IR (KBr) (νmax/cm−1): 3467 (NH), 3348 (NH), 2195 (CN).
4.4.7 2-Amino-4-(2-naphthyl)-6-phenylsulfanyl-pyridine-3,5-dicarbonitrile (7)
317.5 mg, 84%; mp 238–240 °C; 1H NMR (400 MHz, DMSO-d6): δH (ppm) 8.18 (1H, s, Ar), 8.13 (1H, d, J = 8.8 Hz, Ar), 8.09–8.05 (2H, m, Ar), 7.89 (2H, br, NH2), 7.71–7.64 (5H, m, Ar), 7.54–7.52 (3H, m, Ar); 13C NMR (100.6 MHz, DMSO-d6): δC (ppm) 166.7, 160.1, 159.2, 135.4, 133.8, 132.7, 131.9, 130.2, 130.0, 129.0, 128.9, 128.3, 128.2, 127.6, 125.9, 115.9, 115.6, 94.1, 87.7; IR (KBr) (νmax/cm−1): 3431 (NH), 3301 (NH), 2170 (CN).
4.4.8 2-Amino-4-(3-pyridinyl)-6-phenylsulfanyl-pyridine-3,5-dicarbonitrile (8)
266.5 mg, 81%; mp 305–306 °C; 1H NMR (400 MHz, DMSO-d6): δH (ppm) 8.80–8.78 (2H, m, Ar), 8.07 (1H, d, J = 7.6 Hz, Ar), 7.94 (2H, br, NH2), 7.67–7.61 (3H, m, Ar), 7.53–7.52 (3H, m, Ar); IR (KBr) (νmax/cm−1): 3360 (NH), 3290 (NH), 2200 (CN).
4.4.9 2-Amino-4-methyl-6-phenylsulfanyl-pyridine-3,5-dicarbonitrile (10)
239.4 mg, 90%; mp 224–225 °C; 1H NMR (400 MHz, DMSO-d6): δH (ppm) 7.71 (2H, br, NH2), 7.58–7.56 (2H, m, Ar), 7.50–7.47 (3H, m, Ar), 2.46 (3H, s, CH3); IR (KBr) (νmax/cm−1): 3396 (NH), 3310 (NH), 2202 (CN).
4.4.10 2-Amino-4-phenyl-6-(n-butylsulfanyl)-pyridine-3,5-dicarbonitrile (11)
215.6 mg, 70%; mp 178–179 °C; 1H NMR (400 MHz, DMSO-d6): δH (ppm) 8.04 (2H, br, NH2), 7.58–7.53 (5H, m, Ar), 3.25 (2H, t, J = 7.2 Hz, CH2), 1.69–1.62 (2H, m, CH2), 1.48-1.39 (2H, m, CH2), 0.93 (3H, t, J = 7.2 Hz, CH3); 13C NMR (100.6 MHz, DMSO-d6): δC (ppm) 167.7, 160.1, 158.7, 134.5, 130.8, 129.2, 128.9, 115.9, 115.8, 94.0, 86.0, 31.2, 29.7, 21.8, 14.0; IR (KBr) (νmax/cm−1): 3452 (NH), 3313 (NH), 2200 (CN).
4.5 Spectral data for unknown 2-amino-3,5-dicarbonitrile-6-thio-pyridines
4.5.1 2-Amino-4-phenethyl-6-phenylsulfanyl-pyridine-3,5-dicarbonitrile (9)
256.3 mg, 72%; mp 218–220 °C; [Found: C, 70.37; H, 4.51; N, 15.61; S, 8.86. C21H16N4S requires C, 70.76; H, 4.52; N, 15.72; S, 9.00%]; 1H NMR (400 MHz, DMSO-d6): δH (ppm) 7.77 (2H, br, NH2), 7.59–7.57 (2H, m, Ar), 7.50–7.47 (3H, m, Ar), 7.35 (2H, t, J = 7.2 Hz, Ar), 7.28–7.22 (3H, m, Ar), 3.03–2.99 (2H, m, CH2), 2.92-2.89 (2H, m, CH2); 13C NMR (100.6 MHz, DMSO-d6): δC (ppm) 166.3, 160.4, 160.1, 139.9, 135.2, 130.1, 129.9, 129.1, 128.7, 127.6, 127.1, 115.4, 115.0, 93.9, 87.6, 36.1, 35.1; IR (KBr) (νmax/cm−1): 3442 (NH), 3344 (NH), 2215 (CN).
4.5.2 1,4-bis(2-amino-6-phenylsufanyl-4-pyridyl-3,5-dicarbonitrile)benzene (12)
492.1 mg, 85%; mp 310–311 °C; [Found: C, 66.27; H, 3.18; N, 19.29; S, 11.43. C32H18N8S2 requires C, 66.42; H, 3.14; N, 19.36; S, 11.08%]; 1H NMR (400 MHz, DMSO-d6): δH (ppm) 7.90 (4H, br, NH2), 7.80 (4H, s, Ar), 7.64–7.62 (4H, m, Ar), 7.54–7.51 (6H, m, Ar); 13C NMR (100.6 MHz, DMSO-d6): δC (ppm) 166.8, 160.2, 158.2, 139.5, 136.1, 135.3, 129.9, 129.4, 127.5, 115.7, 115.4, 93.7, 87.5; IR (KBr) (νmax/cm−1): 3407 (NH), 3325 (NH), 2216 (CN).
Acknowledgements
We thank University of Birjand Research Council for their support on this work.


