1 Introduction
The use of ultrasound in organic transformation is now well known to enhance reaction rates and yields, selectivity of reaction [1,2]. The imidazole ring system and its derivatives have emerged as an integral part of many biological systems [3,4] viz, histidine, histamine and biotin; active backbones in exciting drugs such as losartan, olmesartan, eprosartan [5], trifenagrel [6] and large classes of imidazole derivatives are also used as ionic liquids [7,8]. Thus, syntheses of this heterocylic nucleus have rekindled our interest in obtaining trisubstituted imidazole. Various methods have been reported [9] for the synthesis of 2,4,5-trisubstituted imidazoles by condensation of benzil, aldehyde and ammonium acetate. Preparation of 2,4,5-triphenyl imidazol 3a (Lophin) [10] was first reported by Oruu [11] and simultaneously by Crouch [12] are synthesized by the reaction of benzyil with aryl aldehyde in an alcoholic ammonia solution. The procedure was later modified by Cook and Jones [13] by refluxing benzil with substituted aldehydes and ammonium acetate in glacial acetic acid. Recently, the synthesis of 2,4,5-trisubstituted imidazoles have been carried out by condensation of benzyl, aldehyde and ammonium acetate in the presence of acetic acid [6], some common Lewis acid catalysts like Yb(OTf)3 [14], NdCl3 [14], LaCl3 [14], FeCl3 [14], AlCl3 [14], Al2O3 [15], NiCl2 [16], [Hbim]BF4 [17], molecular iodine [18], TBAB in isopropanol [19] and polymer-supported ZnCl2 [20], oxalic acid [21], boric acid [22], p-toluenesulfonic acid [23], l-proline [24], l-proline triflate [25], Cu(NO3)2-zeolite [26], zinc (II) [tetra (4-methylphenyl)]porphyrin with ultrasound irradiation [27]. Although these methods were derived from the early success [28–30], the reaction suffered from low yields, harsh conditions, high temperature and side reactions leading to a mixture of products. Additionally, reagents for these procedures are not readily or commercially available. A majority of these procedures also involve microwave-assisted synthesis [31–34]. Moreover, the synthesis of these heterocyclic compounds has been usually carried out in polar solvents such as ethanol, methanol, acetic acid, DMF and DMSO, leading to complex isolation and recovery procedures. Hence, the development of a clean, safe, effective, economical, high yielding and mild environmental benign protocol is still describable and is in demand. We have discovered that MCR protocol for the synthesis of 2,4,5-trisubstituted imidazoles by Selectfluor™ under ultrasound irradiation (Scheme 1).
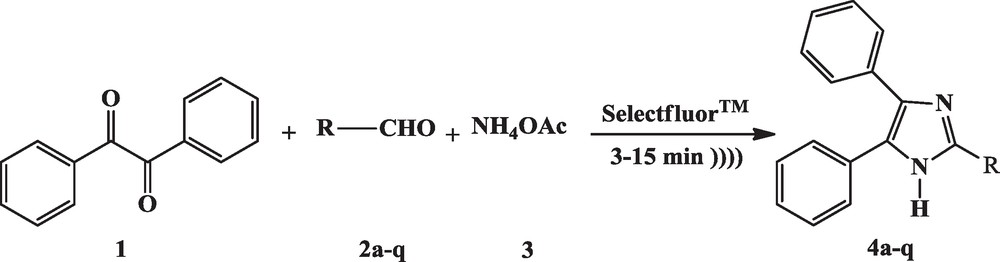
Synthesis of 2,4,5-trisubstituted imidazoles catalysed by Selectfluor™ under ultrasound irradiation.
2 Results and discussion
In terms of the amount of catalyst required for the reaction of benzil 1, benzaldehyde 2a and ammonium acetate 3 to afford 4a with ultrasound irradiation and without ultrasound irradiation, we observed that the concentration of the catalyst played a major role in catalyzing the condensation reaction for the synthesis of 2,4,5-trisubstituted imidazoles. The reaction was performed in the presence of 0, 5, 10, 15 and 20 mol% with and without ultrasound irradiation. In the absence of Selectfluor™ and with ultrasound irradiation we obtained a 0% yield, but in the presence of Selectfluor™ as Lewis acid and without ultrasound irradiation we observed a 4% yield (Table 1, entry 1). The reason may be the phenomenon of cavitations produced by ultrasounds. Cavitations are the origin of sonochemistry, a physical process that creates, enlarges, and implodes gaseous and vaporous cavities in an irradiated liquid, thus enhancing the mass transfer and allowing chemical reactions to occur. Applying ultrasound, compression of the liquid is followed by rarefaction (expansion), in which a sudden pressure drop forms small, oscillating bubbles of gaseous substances. These bubbles are small and rapidly collapse, they can be seen as micro reactors that offer the opportunity of speeding up certain reactions and also allow mechanistically novel reactions to take place in an absolutely safe manner [37,38]. The best results were obtained using 0.036 g (10 mol%) of the catalyst under both conditions (Table 1, entry 3).
Effect of the concentration of catalyst.
| Yielda (%) | Selectfluor™ (mol%) | Entry | |
| Without u.s. | With u.s. | ||
| 4 | 0 | 0 | 1 |
| 23 | 88 | 5 | 2 |
| 32 | 96 | 10 | 3 |
| 31 | 95 | 15 | 4 |
| 31 | 95 | 20 | 5 |
a Isolated yields.
In order to investigate the effect of the ultrasonic intensity power on the reaction, the reaction was also performed at 20%, 40%, 60%, 80% and 100% of the power rate of the ultrasonic bath (40, 80, 120, 160 and 200 W). The results are shown in Table 2. The intensity of sonication is proportional to the amplitude of vibration of the ultrasonic source and, as such, an increment in the amplitude of vibration will lead to an increase in the intensity of vibration and to an increase in the sonochemical effects. Increasing the ultrasound power intensity up to 80% induces relatively higher yields and shorter reaction times; beyond this level, the yield decreases slightly. Generally, the increase in the acoustic power could increase the number of active cavitations bubbles and also the size of the individual bubbles. Both increases can be expected to result in an increase in the maximum collapse temperature [39], and the respective reaction could be accelerated. The results are summarized in Table 2. It can easily be seen that the condensation of a series of aldehydes with benzil and ammonium acetate using Selectfluor™ as a catalyst, leading to 2,4,5-trisubstituted imidazoles, gives good yields under ultrasound irradiation (Table 2).
Effect of ultrasound intensity power on the synthesis of 4aa.
| Max power intensity (W) | 20% (40W) | 40% (80W) | 60% (120W) | 80% (160W) | 100% (200W) |
| Time (min) Yields (%) | 30 (80) | 25 (85) | 10 (90) | 5 (96) | 5 (90) |
a Isolated yields.
As shown in Table 3, in order to introduce pharmacologically relevant substitution patterns, several diversified examples illustrating the present method for the synthesis of 2,4,5-trisubstituted imidazoles 3 were studied. An increase in the reaction rate was observed with aldehyde-bearing electron donating groups (Table 3, entries 7, 9, 10, 15 and 16) in comparison to the unsubstantiated aldehyde (Table 3, entry 1). The electron withdrawing nitro group (Table 3, entry 11) decreased both the rate of reaction and the yield of product. The procedure worked well without the formation of any side products with a variety of structurally and electronically divergent aldehydes. Use of just 10 mol% of Selectfluor™ under ultrasound irradiation is sufficient to push the reaction forward.
Synthesis of 2,4,5-trisubstituted imidazoles catalyzed by Selectfluor™ with ultrasound irradiation.
| Entry | R | Product | Time irradiation (min) | Yielda,b (%) | m.p (°C) | |
| Found | Reported | |||||
| 1 | C6H5 | 3a | 5 | 96 | 272–273 | (271–273 [14,16]) |
| 2 | 4-FC6H4 | 3b | 3 | 97 | 201–202 | (189–190 [27,28]) |
| 3 | 4-CF3C6H4 | 3c | 3 | 95 | 223–224 | – |
| 4 | 4-BrC6H4 | 3d | 10 | 92 | 265–266 | (264–265 [14,16]) |
| 5 | 2-ClC6H4 | 3e | 9 | 91 | 192–193 | (192–193 [16]) |
| 6 | 3-ClC6H4 | 3f | 10 | 89 | 296–297 | (295–296 [19]) |
| 7 | 3,4,5-(CH3O)3C6H2 | 3g | 15 | 95 | 262–263 | (261–262 [19]) |
| 8 | 4-N(CH3)2C6H4 | 3h | 14 | 94 | 262–263 | (263–264 [14]) |
| 9 | 4-OHC6H4 | 3i | 12 | 94 | 242–243 | (243–244 [14,16]) |
| 10 | 3-OHC6H4 | 3j | 5 | 90 | 258–259 | (259–260 [19]) |
| 11 | 3-NO2C6H4 | 3k | 5 | 84 | 298–299 | (297–299 [14]) |
| 12 | 4-NO2C6H4 | 3l | 3 | 82 | 242–243 | (241–242 [14,16]) |
| 12 | 2-Furyl | 3m | 10 | 92 | 241–242 | (242–243 [19]) |
| 13 | 1-Naphthyl | 3n | 11 | 92 | 296–297 | (297–298 [19]) |
| 14 | 2-Naphthyl | 3o | 12 | 93 | 286–287 | (241–242 [35,36]) |
| 15 | 2,4-(OH)2C6H3 | 3p | 8 | 93 | 271–272 | (272–273 [14]) |
| 16 | 4-CH3OC6H4 | 3q | 10 | 99 | 227–228 | (228–229 [14]) |
a Yield of corresponding isolated and purified product.
b All compounds were fully characterized by IR, NMR and mass spectroscopy.
A mechanism explaining the catalytic activity of Selectfluor™ under ultrasound irradiation in the synthesis of trisubstituted imidazoles may be postulated as shown in Scheme 2. The Selectfluor™, as a Lewis, acid probably induces the polarization of the carbonyl group in aldehydes as well as in benzil under ultrasound irradiation. Then nucleophilic attack of the nitrogen of ammonia obtained from ammonium acetate, on activated carbonyl, results in the formation of aryl aldimine and α–imino ketone. Their subsequent reaction followed by intermolecular interaction leads to cyclization, and, eventually, to the formed intermediate dehydrates to give the 2,4,5-trisubstituted imidazoles 4a-q in excellent yields.
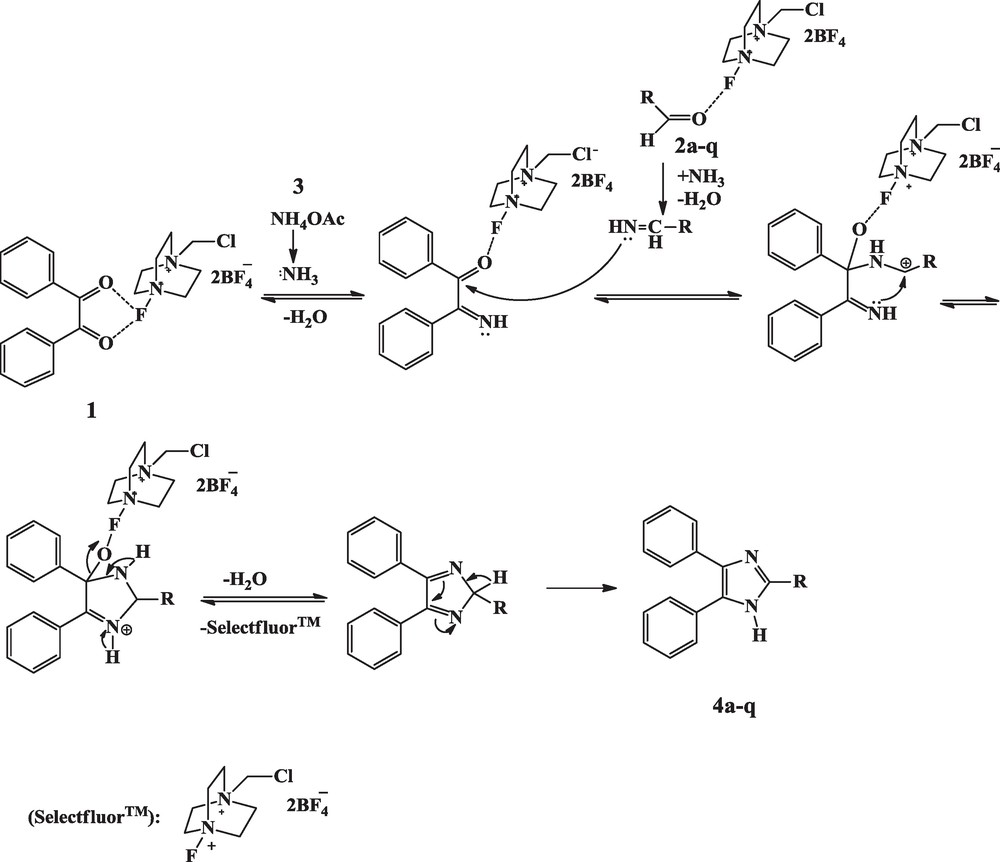
Plausible mechanism for Selectfluor™ catalysis in the synthesis of 2,4,5-trisubstituted imidazoles under ultrasound irradiation.
3 Conclusion
In summary, we have developed an efficient protocol for the selective synthesis of 2,4,5-trisubstituted imidazoles under very mild conditions using Selectfluor™ as a catalyst with ultrasound irradiation. The amount of Selectfluor™ used for this reaction is truly catalytic (10 mol%). Selectfluor™ with ultrasound irradiation is sufficient to push the reaction forward. Because of the high solubility of Selectfluor™ in water, a simple aqueous work-up is enough to obtain most of the 2,4,5-trisubstituted imidazoles in analytically pure form.
4 Experimental method
4.1 Materials and techniques
Selectfluor™ was purchased from Merck Co. Ltd. All the commercial reagents and solvents were used without further purification unless otherwise stated. Melting points were recorded on a Fiesher-John melting point apparatus and are uncorrected. All the reactions were monitored by thin layer chromatography performed on precoated silicagel 60F254 plated (Merck). Compounds were visualized with UV light at 254 nm and 365 nm. FT-IR spectrometer with KBr pellets was used. NMR spectra were recorded on a Brucker 500 MHz using CDCl3 as a solvent. Mass spectra were recorded on a VG micromass 7070H and Finnigan mat 1020B mass spectrometers operating at 70 eV. All sonochemical reactions were carried out in a thermostated (25 ± 1 °C) ultrasonic cleaning bath at 35 kHz. The ultrasonic cleaner had an output power of 200 W. The tank dimensions were 290 × 240 × 150 mm with a liquid holding capacity of 9.5 L. The reactions were carried out in a RB flask of 10 mL capacity, suspended at the center of the cleaning bath, 5 cm below the surface of the liquid.
4.2 General procedure for the synthesis of 2,4,5-trisubstituted imidazoles (4a-q)
Combinations of benzil (2.5 mmol), substituted aldehyde (2.5 mmol) and ammonium acetate (2.5 mmol) and Selectfluor™ (0.036 g, 10 mol%) in ethanol (10 mL) were mixed together on a desk-top. Then, the mixture was irradiated in the water-bath of the ultrasonic cleaner for some time. After completion of the reaction (indicated by TLC), the mixture was dissolved in ethanol and poured into ice-cold water. The resulting precipitate was filtered and recrystallized from ethanol. All products were confirmed by comparing their melting points.
4.3 Spectral data of new compounds
4,5-diphenyl-2-(4-(trifluoromethyl)phenyl)-1H-imidazole (4c). White solid; m.p.: 221–223 °C; IR (KBr) (νmax/cm–1): 3435 (N-H), 1618 (C=C), 1580 (C=N); 1H NMR (CDCl3): δH 7.12–7.76 (10H, m, 2 × C6H55); 7.44 (2H, d, J = 10 Hz), 7.84 (2H, d, 10 Hz), 11.92 (s, NH); LC-MS: 387 [M +23]; Elemental analysis: Found (%): C, 72.63; H, 4.21, N, 7.59; Calcd. for C22H15F3N2 (364.12): C, 72.52, H, 4.15, N, 7.69.
2-(4-fluorophenyl)-4,5-diphenyl-1H-imidazole (4b). White solid; m.p.: 189–190 °C; IR (KBr) (νmax/cm–1): 3451 (N-H), 1624 (C=C), 1578 (C=N); 1H NMR (CDCl3): δH 7.23–7.65 (10H, m, 2 × C6H55); 7.65 (2H, d, J = 8.5 Hz), 7.92 (2H, d, 8.5 Hz), 11.76 (s, NH); LC-MS: 337 [M +23]; Elemental analysis: Found (%): C, 80.18; H, 4.93, N, 8.79; Calcd. for C21H15FN2 (314.12): C, 80.24; H, 4.81; N, 8.91.
Acknowledgments
We gratefully acknowledge the financial support of the Payame Noor University (PNU), Zanjan, Abhar, Iran.


