1 Introduction
Conventional, toxic and polluting BrØnsted and Lewis acid catalysts, such as H2SO4, HCl, CF3CO2H, AlCl3, FeCl3, TiCl4, ZrCl4 are very effective catalysts for a wide variety of organic reactions [1], but one of the major problems associated with these homogeneous catalysts is the recovery of catalyst from the reaction medium. In recent years, there have been intense efforts to replace these catalysts with eco-friendly reusable heterogeneous catalysts. The use of polymer-supported catalysts in organic transformations has been receiving extraordinary attention and the design of functionalized polymers carrying catalytically active metal species has attracted considerable interest [2–5]. Immobilization of catalysts on solid support improves the available active sites, stability, hygroscopic properties, handling, and reusability of catalysts, all factors that are important in industry [6]. A large number of polymer-supported Lewis acid catalysts have been prepared by immobilization of the catalysts on polymer via coordination or covalent bonds [7]. Such polymeric catalysts are usually as active and selective as their homogeneous or solution-phase counterparts while having the distinguishing characteristics of being easily separable from the reaction mixture, recyclability, easier handling, non-toxicity, enhanced stability, and improved selectivity in various organic reactions. Polystyrene is one of the most widely studied heterogeneous and polymeric supports due to its environmental stability and hydrophobic nature which protects water-sensitive Lewis acids from hydrolysis by atmospheric moisture until it is suspended in an appropriate solvent where it can be used in a chemical reaction [8]. It is well known that gallium trichloride is a strong Lewis acid and an important catalyst in organic transformations. However, it easily hydrolyzes in air, so that its use, storage, and separation from the reaction mixture are inconvenient and difficult. Polystyrene-supported gallium chloride, PS–GaCl3, which is a tightly bound and stable complex between anhydrous GaCl3 and polystyrene-divinylbenzene copolymer beads, has been described for the first time by Ruicheng et al. [9]. The use of PS–GaCl3 complex catalyst has several advantages over conventional Lewis acid catalysts like its cost-effectiveness, ease of handling, recyclability, and tunable Lewis acidity.
Coumarin and its derivatives are the important class of naturally occurring oxygen heterocyclic compounds having a distinct and important place in the realm of natural and synthetic organic chemistry as these compounds display useful and diverse biological properties, viz. antibacterial, antiviral, anticancer, anti-HIV [10–12], and also have been used as additives in food, cosmetics, optical brightening agents, and laser dyes [13–15]. Due to these applications and properties, a variety of methods have been developed to synthesize coumarins [16–20]. Despite these developments, the Pechman reaction, a two-component (activated phenols and β-ketoesters) coupling under homogeneous acid catalysis, is a very well established method for the preparation of substituted coumarin rings [21–32]. However, this reaction usually involves the use of non-reusable homogeneous BrØnsted, Lewis, and mineral acids in an excess amount are necessary in the classical preparations, resulting in environmental pollution. The present drive therefore is towards the development of more effective, non-stoichiometric, preferably recyclable heterogeneous catalysts. For eco-friendly reasons, some solid-phase catalysts such as a microporous polymer based on Nafion resin/silica [33], Well–Dawson heteropolyacid [34], montmorillonite KSF [35], calcined Mg–Al hydrotalcite [36], sulfated zirconia [37], ion exchange resins [38], polyaniline sulfate salt [39], zeolites [40], etc. [41] as alternatives to homogeneous acids have been employed for the preparation of coumarins. Nevertheless, substituent diversity in the synthesis of 4-substituted coumarins remains a challenge, and restricted reaction conditions are often involved in the preparation. Therefore, there is still demand for the introduction of mild, selective and environmentally benign methods, especially using recyclable heterogeneous catalysts for this transformation. The search continues for finding a better catalyst in terms of operational simplicity, economic viability, and greater selectivity.
In continuation of our recent works on the use of polymeric Lewis acid catalysts in synthetic transformations [42–45], herein we wish to report the preparation of polystyrene-supported gallium trichloride as a stable, highly active and reusable heterogeneous Lewis acid catalyst in the Pechman coumarin synthesis (Scheme 1), and the investigation of its catalytic activity. To the best of our knowledge, this is only the second example of PS–GaCl3 as a catalyst for any synthetic transformation.
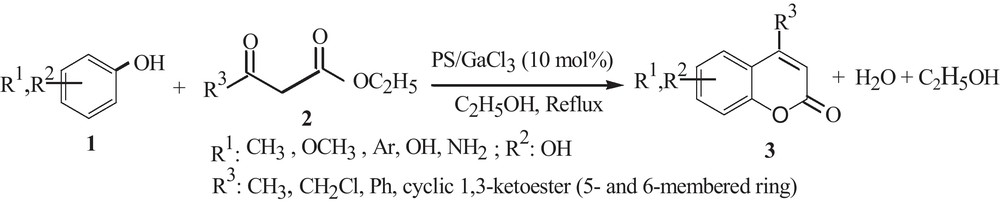
PS–GaCl3-catalyzed Pechman coumarin synthesis.
2 Experimental
2.1 Materials and instruments
All chemical reagents were obtained from Fluka and Merck chemical companies and were used without further purification. Cross-linked polystyrene (7–8% divinylbenzene, mesh size: 20–80) was prepared via suspension polymerization as reported in the literature [42,43]. PS/AlCl3 was also prepared as previously reported [42]. The products were isolated and identified by comparing their physical and spectral data with those in the literature [21–32]. 1H and 13C NMR spectra were recorded on a Bruker DPX-250 Avance spectrometer at 250.13 MHz. FT-IR spectra of the samples were recorded from 400 to 4000 cm−1 on a Unicam Matteson 1000 spectrophotometer. UV spectra were taken using a Pharmacia Biotech Ultraspec 3000 model 80-2106-20 spectrometer. Mass spectra were recorded on a Fisons instrument. Elemental analyses were performed using a Heraeus CHN-O-Rapid analyzer by RIPI and the results agreed favorably with calculated values. The capacity of the catalyst was determined by the Mohr titration method and atomic absorption technique using a Philips atomic absorption instrument. Reaction monitoring and purity determination of the products were accomplished by TLC on silica gel polygram SILG/UV254 plates or by a Shimadzu Gas Chromatograph GC-16A instrument with a flame ionization detector.
2.2 Preparation of PS–GaCl3
Anhydrous GaCl3 (4.50 g) was added to polystyrene (7–8% divinylbenzene, mesh size: 20–80, 40 g) in carbon disulfide (5 mL) as the reaction medium. The mixture was stirred using a magnetic stirrer under reflux condition for 1 h, cooled, and then water (50 mL) was cautiously added to hydrolyze the excess GaCl3. The mixture was stirred until the bright red color disappeared, and the polymer became yellow. The polymer beads were collected by filtration and washed with water (300 mL) and then with ether (30 mL) and chloroform (30 mL). The catalyst was dried in a vacuum oven overnight at 50 °C before use. The chlorine content of PS–GaCl3 was 4.17% analyzed by the Mohr titration method [46] and the loading capacity of GaCl3 on the polymeric catalyst or the amount of GaCl3 complexed with polystyrene was calculated to be 0.391 mmol/g [47].
2.3 General experimental procedure for the synthesis of coumarins (3)
In a round-bottom flask (25 mL) equipped with a condenser and a magnetic stirrer, a stirring mixture of the phenolic substrate (1,1 mmol), β-ketoester (2,1 mmol) and PS–GaCl3 (2.55 g, 1 mmol of GaCl3) in ethanol (5 mL) was heated under reflux for an appropriate time as indicated by TLC (Tables 1 and 2). After completion of the reaction, the reaction mixture was cooled to room temperature and then hot ethanol (5 mL) was added to the mixture and the catalyst was collected by filtration and then washed with chloroform and ether (2 × 5 mL). The filtrate was concentrated on a rotary evaporator under reduced pressure and the crude product obtained was washed with water (10 mL × 2), and purified by recrystallization from ethanol to afford the pure coumarin derivative. The spent catalyst from different experiments was washed with chloroform and ether, dried and used again.
Optimization of reaction conditions and comparison of the condensation reaction of resorcinol (1, 1 mmol) with ethyl acetoacetate (2, 1 mmol) catalyzed by PS–GaCl3 with the other catalysts used for this reaction.
| Entry | Solventa | Catalyst (mol %) | Time (h) | Yieldb (%) | Ref. |
| 1 | Toluene | PS–GaCl3 (10) | 2 h | 26 | This work |
| 2 | Cyclohexane | PS–GaCl3 (10) | 2 h | 20 | This work |
| 3 | CH2Cl2 | PS–GaCl3 (10) | 2 h | 14 | This work |
| 4 | CH3Cl | PS–GaCl3 (10) | 2 h | 15 | This work |
| 5 | CH3CN | PS–GaCl3 (10) | 2 h | 77 | This work |
| 6 | H2O | PS–GaCl3 (10) | 2 h | NRe | This work |
| 7 | C2H5OH | None | 2 h | NR | This work |
| 8 | C2H5OH | PS–GaCl3 (5) | 50 min | 62 | This work |
| 9 | C2H5OH | PS–GaCl3 (10) | 50 min | 94 | This work |
| 10 | C2H5OH | PS–GaCl3 (15) | 50 min | 94 | This work |
| 11c | C2H5OH | PS (10) | 2 h | NR | This work |
| 12c | C2H5OH | Toluene-GaCl3 complex (10) | 75 min | 61 | This work |
| 13d | C2H5OH | PS–GaCl3 (10) | 50 min | 51 | This work |
| 14e | C2H5OH | PS–GaCl3 (10) | 50 min | 77 | This work |
| 15f | C2H5OH | PS–GaCl3 (10) | 20 min | NR | This work |
| 16 | C2H5OH | PS/AlCl3 (10) | 2 h | 58 | This work |
| 17 | C2H5OH | SiO2/AlCl3 (10) | 2 h | 59 | This work |
| 18 | C2H5OH | AlCl3 (10) | 2 h | 54 | This work |
| 19 | C2H5OH | BiCl3 (10) | 2 h | 58 | This work |
| 20 | C2H5OH | ZnCl2 (10) | 2 h | 67 | This work |
| 21 | C2H5OH | FeCl3 (10) | 2 h | 36 | This work |
| 22 | C2H5OH | I2 (10) | 2 h | 41 | This work |
| 23 | C2H5OH | SnCl4 (10) | 2 h | 51 | This work |
| 24 | C2H5OH | KAl(SO4)2.12H2O (10) | 2 h | 47 | This work |
| 25 | C2H5OH | TiCl4 (10) | 2 h | 71 | This work |
| 26 | Toluene, reflux | Amberlyst | 2 h | 95 | [38] |
| 27 | Toluene, reflux | Zeolite | 4 h | 77 | [40] |
| 28 | Toluene, reflux | Montmorillonite K-10 (30 wt%) | 8 h | 94 | [35b] |
| 29 | Toluene, reflux | Montmorillonite KSF (30 wt%) | 10 h | 90 | [35b] |
| 30 | None, 170 °C | SO4/ZrO2 | 3 h | 91 | [37] |
| 31 | Toluene, reflux | W/ZrO2 | 6 h | 80 | [51] |
| 32 | Toluene, reflux | Nafion/silica | 2 h | 92 | [33] |
| 33 | None, 170 °C | Polyaniline sulfate salt (95 mg) | 6 h | 72 | [39] |
| 34 | n-Hexadecane, 160 °C | SiO2/MeSO3H (0.25 g) | 2 h | 99 | [52] |
| 35 | None, 175 °C | Metal ion-exchanged | 6 h | 90 | [53] |
| ZAPO-5 |
a All reactions were carried out under reflux conditions with 10 mol% of catalyst in 5 mL of solvent.
b Isolated yield.
c PS and the toluene–GaCl3 complex were used as catalysts, respectively.
d Two reactions were carried out in ethanol at 45, 60 °C, respectively.
e NR: no reaction.
f Catalyst was removed by filtration after 20 min.
PS–GaCl3-catalyzed synthesisa of coumarins via Pechman condensation of phenol substrates with β-ketoesters.
| Entry | Phenol substrate | β-Ketoester | Product | Time (min) | Yieldb (%) | m.p.(°C) | Ref. |
| 1 | CH3COCH2CO2 C2H5 | (3a) | 50 | 9 | 184–186 | [31] | |
| 2 | PhCOCH2CO2C2H5 | (3b) | 85 | 87 | 253–255 | [25] | |
| 3 | ClCH2COCH2CO2C2H5 | (3c) | 55 | 90 | 180–182 | [25] | |
| 4 | CH3COCH2CO2C2H5 | (3d) | 60 | 90 | 258–260 | [25] | |
| 5 | PhCOCH2CO2C2H5 | (3e) | 90 | 82 | 210–213 | – | |
| 6 | CH3COCH2CO2C2H5 | (3f) | 60 | 84 | 137–139 | [28] | |
| 7 | PhCOCH2CO2C2H5 | (3g) | 90 | 83 | 284–285 | [28] | |
| 8 | CH3COCH2CO2C2H5 | (3h) | 45 | 96 | 283–285 | [31] | |
| 9 | PhCOCH2CO2C2H5 | (3i) | 70 | 90 | 242–244 | [23b] | |
| 10 | ClCH2COCH2CO2C2H5 | (3j) | 55 | 91 | 186–188 | [23c] | |
| 11 | CH3COCH2CO2C2H5 | (3k) | 65 | 82 | 241–243 | [25] | |
| 12 | CH3COCH2CO2C2H5 | (3l) | 100 | 78 | 131–133 | [35] | |
| 13 | CH3COCH2CO2C2H5 | (3m) | 60 | 81 | 149–151 | [35] | |
| 14 | CH3COCH2CO2C2H5 | (3n) | 70 | 85 | 160–162 | [23c] | |
| 15 | CH3COCH2CO2C2H5 | (3o) | 85 | 84 | 165–166 | [23c] | |
| 16c | CH3COCH2CO2C2H5 | (3p) | 300 | 45 | 80–82 | [21] | |
| 17 | CH3COCH2CO2C2H5 | (3q) | 60 | 84 | 222–224 | [24] | |
| 18c | CH3COCH2CO2C2H5 | (3r) | 100 | 71 | 154–156 | [23b] | |
| 19 | (3s) | 70 | 81 | 243–245 | [54] | ||
| 20 | (3t) | 60 | 82 | 264–265 | – |
a Reaction conditions: phenol (1 mmol), β-ketoester (1 mmol), PS–GaCl3 (0.1 mmol), C2H5OH (5 mL), Reflux.
b Yields refer to isolated pure products which were characterized by comparison of their m.p., IR, 1H and 13C NMR spectra with those of authentic samples prepared by reported procedures.
c The reaction was performed using 20 mol% PS–GaCl3.
2.4 The spectral data for new compounds
(Table 2, 3e, entry 5): White solid; m.p.: 210–213 °C; IR (KBr): ν = 3180 (OH), 1680 (CO) cm−1; 1H NMR (250 MHz, DMSO-d6): δ = 10.15 (s, 1H), 7.32–7.36 (m, 5H), 6.72 (s, 1H), 6.46 (s, 1H), 5.95 (s, 1H), 3.02 (s, 3H); [Elemental analysis, Found: C, 76.08, H, 4.78, C16H12O3 requires C, 76.19, H, 4.76%].
(Table 2, 3t, entry 20): White solid; m.p.: 264–265 °C; IR (KBr): ν = 3185 (OH), 2925, 2726, 2360, 1637 (CO), 1460, 1376, 1301, 1160, 820, 722 cm−1; 1H NMR (250 MHz, DMSO-d6): δ 1.64–1.66 (m, 4H, CH2), 2.33–2.36 (m, 2H, CH2), 3.03–3.05 (m, 2H, CH2), 6.15 (s, 1H, Ar-H), 6.24 (s, 1H, Ar-H), 10.12 (s, OH), 10.37 (s, OH); [Elemental analysis, Found: C, 67.33, H, 5.16, C13H12O4 requires C, 67.24, H, 5.18%].
3 Results and discussion
By considering the excellence of polystyrene-divinylbenzene (7–8%) copolymer beads (PS) as an effective supporting material for immobilization of Lewis acid previously reported [42,43,45] by our group, we decided to use PS beads as a support for the heterogenization of gallium trichloride. PS–GaCl3 was prepared by addition of anhydrous gallium trichloride to polystyrene (7–8% divinylbenzene) in carbon disulfide under reflux conditions. The loading capacity of the polymeric catalyst obtained by gravimetric method and checked by atomic absorption technique was 0.391 mmol GaCl3/g of complex beads catalyst [46]. The data obtained by these two techniques showed, within the limits of experimental error, that the catalyzing species are in the form of GaCl3 supported on the polymeric support. The UV spectrum of the solution of the PS–GaCl3 complex in CS2 showed a new strong band at 470 nm, which is due to the formation of a stable π→p type coordination complex between the benzene rings in the polystyrene carrier with gallium trichloride. The IR spectrum of PS–GaCl3 showed new absorption peaks due to the C–C stretching vibration and the C–H bending vibration of the benzene ring at 1500–1560 and 400–800 cm−1, by which complex formation was demonstrated. The structure of the PS–GaCl3 complex is similar to that of the PS–AlCl3 complex as suggested by Neckers et al. [48], because the Lewis acid GaCl3 is complexed with the benzene rings of the polystyrene and the GaCl3 is stabilized due to the decreased mobility of the benzene rings hindered by the long polystyrene chain. The PS–GaCl3 complex is a non-hygroscopic, water tolerant, and especially stable species. In addition, this polymeric catalyst is easy to prepare, stable in air for a long time (over 2 years) without any change, easily recycled and reused without appreciable loss of its activity.
To study the feasibility of the PS–GaCl3-catalyzed Pechman condensation, the reaction of resorcinol (1,3-dihydroxybenzene) with ethyl acetoacetate was selected as a model to screen the best conditions. The swelling property of cross-linked resin (PS) in organic solvents is an important factor for effective solid-phase reactions [49]. Our initial experiments focused on the selection of solvent. The results are summarized in Table 1. It was found that among the tested solvents, polar solvents such as CH3CN and C2H5OH were better than non-polar solvents. Ethanol was chosen as the solvent system for this conversion, because of its swellability with the catalyst and miscibility with the substrates. No reaction occurred when H2O was used as a solvent (Table 1, entry 6), maybe because of the aggregation of the catalyst caused by its hydrophobic nature, leading to inadequate access of substrates to the active sites of the catalyst [50]. Further studies showed that the reaction temperature has a great influence on the model reaction and the best results were observed when the reaction temperature was 80 °C (Table 1, entries 9, 13, 14). Consequently, we chose 80 °C as the optimal temperature for the model reaction. Next, the amount of the catalyst was examined and we found that the yields were obviously affected with different amount of catalyst. No reaction was observed in the absence of catalyst, while 10 mol% of PS–GaCl3 was sufficient to drive the reaction completely and excessive amount of catalyst did not alter either reaction time or yield of the product significantly (Table 1, entries 7–10). The key role played by the Lewis acidity of the heterogeneous catalyst PS–GaCl3 was proved by employing the cross-linked polystyrene beads (PS) (Table 1, entry 11) and the GaCl3–toluene complex as catalysts under the same experimental conditions. In fact, while in the former case no reaction occurred, which indicated that polystyrene itself did not promote the reaction, in the latter, the desired product was isolated in low yield 61% (Table 1, entry 12). Also, PS–GaCl3 was found to be a more effective catalyst than PS/AlCl3 and SiO2/AlCl3 in terms of reaction time and the yield of the product 3a under identical conditions (Table 1, entries 16, 17). We also tested the catalytic activity of different homogeneous catalysts for comparing the efficiency of PS–GaCl3, and we obtained low to moderate yields in ethanol (Table 1, entries 18–25). All the reactions were run in the same reaction conditions, and similar amounts of catalysts (10 mol%) were used. As shown in Table 1, PS–GaCl3 catalyzes the reaction better than other catalysts, and satisfactory results were obtained only with PS–GaCl3 (Table 1, entry 9). In addition, the catalytic efficiency of PS–GaCl3 was compared with that of the reported heterogeneous catalytic systems in similar reactions as shown in Table 1, entries 26–35. It is worth mentioning that our method is more efficient and simpler than the existing methods.
The lifetime and leaching of active metal species into the solution are important issues to consider when heterogeneous catalysts are used, particularly for practical applications of the reaction. Our preliminary investigations demonstrated that PS–GaCl3 catalyst (a stable π complex) is very stable to air and moisture. Moreover, in a separate experiment the catalyst PS–GaCl3 was heated under reflux in ethanol for 20 min and then isolated by filtration. When reactants were added to the filtrate (the catalyst-free liquid) and heated under reflux for 2 h, no reaction took place (Table 1, entry 15). On the other hand, after each run the filtrates were used for determination of catalyst leaching (gallium content), which showed a negligible release of GaCl3 by atomic absorption or ICP measurement. The capacity of the catalyst after six uses was 0.378 mmol of GaCl3 per gram. Therefore, we may conclude that PS–GaCl3 is stable, that no significant leaching of Lewis acid moieties is operating under our reaction conditions, that any gallium species that leached into the reaction mixture is not an active homogeneous catalyst, and that the observed catalysis is truly heterogeneous in nature.
With the optimal conditions in hand, the substrate scope was investigated. We subjected a series of monohydric and polyhydric phenols to react with a variety of β-ketoesters to obtain the corresponding 4-substituted coumarins (Table 2). A wide range of structurally varied phenols reacted smoothly to give the corresponding coumarins in good yields. The remarkable feature of this improved protocol is the wide stability of a variety of functional groups, such as ether, hydroxyl, amino, etc., under the present reaction conditions. It is worth mentioning that substrates like phenol and cresols, which failed to react in many of the protocols reported in the literature, showed better reactivity, giving moderate to good yields, under these reaction conditions (Table 2, entries 12, 13, 16). From the experimental results, substrates having electron-donating groups in the para position to the site of electrophilic substitution (the meta position to the phenolic–OH) facilitated the condensation and gave maximum yields in a short period of time. The resonance effects of these substituents support formation of the reactive polarized carbocation in the ortho position. An alkyl group is not strong enough to furnish the activation needed and thus gives a moderate yield (Table 2, entries 12, 13). In contrast, electron-withdrawing groups, such as salicylaldehyde and 2-chloro phenol inhibit the reaction. Phenol required a higher amount of catalyst and longer reaction duration, as no electron-donating group is present. Another important feature is that no detectable demethylation was observed in the case of 3-methoxy and 4-methoxy phenol (Table 2, entries 14, 15). In the case of 1-naphthol, a higher amount of catalyst and longer reaction time was required to obtain the corresponding coumarin derivative due to presence of another phenyl moiety (Table 2, entry 18). Finally, to generalize the protocol, we also attempted the condensation reaction using a further variety of β-ketoesters such as 4-chloroethyl acetoacetate, benzoyl acetoacetate, 2-carbethoxy cyclo pentanone, and also 2-carbethoxy cyclohexanone. It was found that R3 can be different groups and in all these cases, good yields of the corresponding coumarin derivatives were obtained (Table 2, entries 2, 3, 5, 7, 9, 10, 19, 20). Nevertheless, the steric effect of R3 had a negative influence on this reaction.
The experimental procedure with this polymeric catalyst is very simple and the catalyst can be removed easily by filtration. Hence, there will not be any unnecessary acidic waste streams to create environmentally hazardous pollution and in all the cases, no chromatographic separation or cumbersome reaction work-up is necessary to get the pure compounds.
The main objective and potential benefits of supporting a homogeneous Lewis acid on to a polymer support include enhancing the life and facilitating the separation of the resulting heterogeneous catalyst from the reagents and reaction products, and its recyclability for repeated use. It is critical that catalyst recovery should be simple and efficient, and that the recovered catalyst should retain its original reactivity through multiple cycles. To test this, we have examined the reusability of PS–GaCl3. After the reaction of resorcinol with ethyl acetoacetate was complete, the catalyst was recovered by filtration and thoroughly washed with chloroform, ether, and dried carefully for consecutive runs. The results are shown in Table 3. The recovered catalyst was used in a new reaction batch of the reactants. The catalyst showed at least six cycle reusability without showing noticeable deterioration in catalytic activity.
| Run | 1 | 2 | 3 | 4 | 5 | 6 |
| Yieldc (%) | 94 | 92 | 90 | 88 | 86 | 84 |
| Time (min) | 50 | 50 | 50 | 60 | 60 | 60 |
a The capacity of the catalyst after six uses was 0.378 mmol GaCl3 per gram.
b Reaction conditions: resorcinol (1 mmol), ethyl acetoacetate (1 mmol), PS–GaCl3 (0.1 mmol), C2H5OH (5 mL), reflux.
c Isolated yield.
Selectivity of the catalysts for chemical transformations is important, especially, when the catalyst is used in multi-step synthesis. The difference in reactivity of the PS–GaCl3 catalyst towards phenols gave us the impetus to study chemoselective reactions. When an equimolar mixture of resorcinol and hydroquinone or phenol or p-hydroxybenzaldehyde was reacted with ethyl acetoacetate under the same reaction conditions, only the resorcinol converted predominantly to coumarin products (Scheme 2).
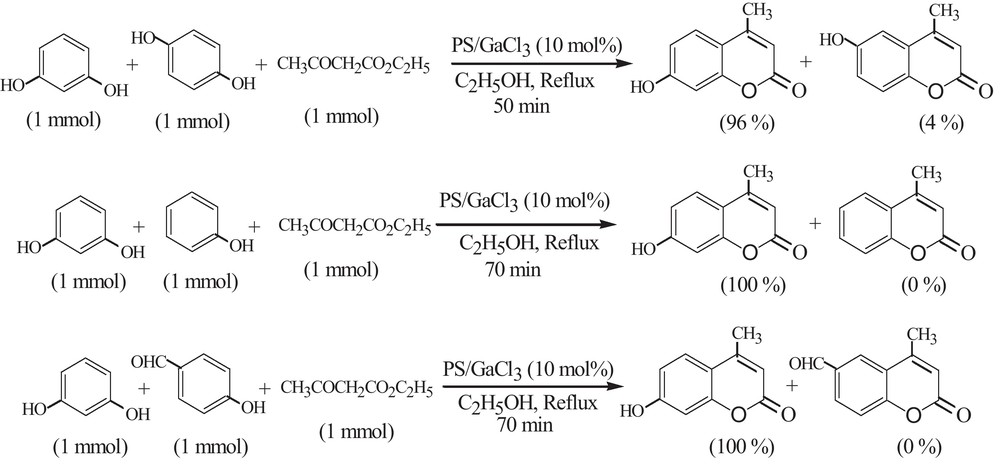
Competitive reaction in Pechman coumarin synthesis catalyzed by PS–GaCl3.
4 Conclusion
In summary, we have developed an environmentally benign and chemoselective method for the synthesis of 4-substituted coumarins by PS–GaCl3-catalyzed condensation of phenol and β-ketoesters. The mild reaction conditions, short reaction times, high yields, low cost and easy preparation and handling (as a bench top catalyst) of the polymeric catalyst, its ability to tolerate a wide variety of substitutions in both components, and the simple experimental and product isolation procedures are the significant advantages of the present method. In addition, the use of PS–GaCl3 has resulted in a reduction in the unwanted and hazardous waste that is produced during conventional homogeneous processes. Finally, this polymeric catalyst behaves in a truly heterogeneous manner and can be recycled and reused at least five times with negligible loss in its activity.
Acknowledgments
The authors are grateful to TPC for providing polystyrene.


