1 Introduction
Nowadays, almost all the devices and technologies are becoming smaller and smaller in size, with improved properties. Among the developments, catalytic technology is an important field. Enhancing the contact between the reactants and catalyst was dramatically observed with the use of nanocatalysts, since the nanoscale increases the exposed surface area of the active component of the catalyst [1–3]. Among nanomaterials, titanium dioxide (TiO2) is a biocompatible and environmentally benign catalyst. TiO2 nanomaterials, including nanoparticles, nanorods, nanowires, and nanotubes, are widely investigated for various applications in photocatalysis, photovoltaics, batteries, smart surface coatings, photonic crystals, sensors, ultraviolet blockers, pigment, and paints [4]. Synthetically, multi-component reactions (MCRs) have emerged as useful methods because the combination of three components to generate new products in a single step is extremely economical [5]. Besides, in medicine, pyranocoumarins are well recognized due to their importance in biological and pharmaceutical researches [6]. Several methods have been reported for the synthesis of pyrano[2,3-c]coumarin as biologically active compounds. In general, pyrano[2,3-c]coumarin derivatives are accessible via the one-pot three-component reaction of hydroxycoumarins, carbonyl compounds and active methylenes in the presence of basic catalysts [7] In recent studies, the synthesis of pyrano[2,3-c]chromenes via a three-component reaction has been described using various reagents, such as sodium dodecyl sulfate (SDS) [8], H6P2W18O62·18H2O [9], hexamethylenetetramine [10] magnesium oxide (MgO) [11], diammonium hydrogen phosphate (DAHP), (S)-proline [12], KF–Al2O3 [13], tetrabutylammonium bromide (TBAB) [14], and triethylbenzylammonium chloride (TEBA) [15]. However, some of these mentioned reports suffer from drawbacks, such as long reaction times, low product yields, harsh reaction conditions, and the use of excessive amounts of catalyst. In this work, a new methodology to obtain novel and known pyrano[2,3-c]chromene under ultrasound irradiation, via a one-pot three-component condensation, is reported. In this paper, we introduce a new application of titanium dioxide nanowires as a novel and safe catalyst for the synthesis of novel and known pyranocoumarin derivatives.
2 Experimental
2.1 General procedure for preparation of TiO2 nanowires
In a typical synthesis, 0.115 g of commercial Degussa P25 powder was mixed with 3.5 mL of 10 M NaOH and 3.5 mL of ethanol. The mixed solution was then transferred into a 35 mL Teflon-lined stainless-steel autoclave. The autoclave was maintained at 180 °C under autogenous pressure for 24 h and then cooled to room temperature naturally. The sample so obtained was filtered off, washed several times with a dilute HCl aqueous solution and water until the pH value of the washing solution was about 7. The TiO2 nanowires so obtained have diameters in the 60–150 nm range and are a few microns in length [16,17].
2.2 General procedure for the synthesis of pyrano[2,3-c]coumarin
Malononitrile (1) (1.1 mmol), aromatic aldehyde 2 (1 mmol), 4-hydroxycoumarin (3) (1 mmol), and TiO2 NWs (0.03 mmol) were added to a 10-mL mixture EtOH/H2O (50/50) in a 25-mL pyrex flask and refluxed for an appropriate time (Table 2). The reaction progress was controlled by thin layer chromatography (TLC) using hexane/EtOAc (1:1). After completion of the reaction, the solvent was removed under vacuum, the crude products 4 were obtained after recrystalization from EtOH.
Synthesis of pyrano[2,3-c]coumarin derivatives using TiO2 NWs (3 mol%).
| Entry | Product | Time (min) | Yielda (%) | Mp. (°C) [lit.] |
| 4a | 60 | 90 | 261–263 [10] | |
| 4b | 45 | 85 | 246–248 [10] | |
| 4c | 60 | 92 | 270–272 [10] | |
| 4d | 45 | 90 | 263–265 [10] | |
| 4e | 40 | 90 | 260–262 [10] | |
| 4f | 65 | 95 | 255–257 [10] | |
| 4g | 60 | 95 | 262–264 [10] | |
| 4h | 70 | 85 | 260–262 [10] | |
| 4i | 45 | 95 | 276–278 [24] | |
| 4j | 60 | 90 | 290–292b | |
| 4k | 55 | 85 | 270–272b | |
| 4l | 70 | 80 | 262–264 [14] |
a Isolated yields.
b Novel compounds.
2.3 Spectral data of some compounds
2.3.1 Compound (4i)
IR (KBr): 3396, 3321, 3195, 2202, 1706, 1671, 1607, 1381, 1053 cm−1. 1H NMR (DMSO, 400 MHz): δ 7.90 (dd, 1H, J = 7.8, 1.2 Hz), 7.75–7.70 (m, 1H), 7.52–7.43 (m, 6H), 7.30–7.28 (m, 2H), 4.50 (s, 1H) ppm. 13C NMR (DMSO, 100 MHz): δ 159.55, 157.94, 153.70, 152.18, 146.02, 132.99, 130.69, 130.39, 130.05, 126.94, 124.65, 122.54, 121.68, 119.05, 116.57, 112.98, 103.15, 57.31, 36.59. Anal. Calcd. for C19H11BrN2O3: C, 57.74; H, 2.81; N, 7.09%. Found: C, 58.05; H, 2.68; N, 7.16%.
2.3.2 Compound (4j)
IR (KBr): 3408, 3280, 3175, 2203, 1705, 1674, 1602, 1381, 1055 cm−1. 1H NMR (DMSO, 400 MHz): δ 7.90 (dd, 1H, J = 7.8, 1.2 Hz), 7.76–7.72 (m, 1H), 7.55–7.48 (m, 4H), 7.55–7.19 (m, 3H), 5.19 (d, 1H, J = 1.6 Hz). 13C NMR (DMSO, 100 MHz): δ 159.38, 158.77, 152.11, 133.18, 133.09, 129.93, 129.83, 124.84, 124.70, 124.66, 122.29, 118.73, 116.68, 115.25, 112.56, 38.83 ppm. Anal. Calcd. for C19H10ClFN2O3: C, 61.89; H, 2.73; N, 7.60%. Found: C, 61.90; H, 2.55; N, 7.72%.
2.3.3 Compound (4k)
IR (KBr): 3391, 3180, 2196, 1712, 1674, 1608, 1381, 1240, 1054 cm−1. 1H NMR (DMSO, 400 MHz): δ 7.90 (dd, 1H, J = 8.0 Hz), 7.73–7.69 (m, 1H), 7.44–7.38 (m, 7H), 7.18 (d, 2H, J = 8.8 Hz), 6.95 (d, 2H, J = 8.8 Hz), 5.06 (s, 2H), 4.40 (s, 1H). 13C NMR (DMSO, 100 MHz): δ 159.51, 157.87, 157.45, 153.09, 152.06, 137.05, 135.60, 132.83, 128.76, 128.40, 127.79, 127.64, 124.63, 122.41, 119.30, 116.53, 114.61, 112.97, 104.19, 69.17, 58.09, 36.13. Anal. Calcd. for C26H18N2O4: C, 73.92; H, 4.29; N, 6.63%. Found: C, 74.15; H, 4.15; N, 6.79%.
2.3.4 Compound (4l)
IR (KBr): 3340, 3312, 2188, 1697, 1669, 1598, 1379, 1066 cm−1. 1H NMR (DMSO, 400 MHz): δ 8.44(d, 1H, J = 6.4 Hz), 7.96 (d, 2H, J = 8.0 Hz), 7.83 (d, 1H, J = 8.0 Hz), 7.76–7.33 (m, 8H), 5.48 (s, 1H). 13C NMR (DMSO, 100 MHz): δ 159.55, 157.83, 153.80, 152.07, 133.26, 132.93, 130.93, 128.47, 127.43, 126.10, 126.00, 125.85, 125.75, 124.74, 123.43, 122.43, 119.15, 116.61, 112.96, 104.65, 58.49 ppm. Anal. Calcd. for C23H14N2O3: C, 75.40; H, 3.85; N, 7.65%. Found: C, 75.65; H, 3.70; N, 7.53%.
3 Results and discussion
In continuation of our efforts toward the development of novel, efficient, and green procedures for the synthesis of organic compounds [18–20] using safe catalysts [21–23], we turned our attention toward the three-component condensation of malononitrile (1), aromatic aldehydes 2, and 4-hydroxycoumarin (3) to produce pyrano[2,3-c]coumarins 4 in the presence of TiO2 nanowires (Scheme 1).

Synthesis of pyrano[2,3-c]coumarins using TiO2 NWs.
The scanning electron microscope (SEM) image of TiO2 nanowires is shown in Fig. 1. In order to optimize the reaction conditions, we used both protic and aprotic solvents in the presence of various amounts of the TiO2 NWs in the reaction of benzaldehyde, 1 and 3 as a model to investigate the effects of the solvent and the catalyst for preparing compounds 4a. In this case, the substrates were mixed together in 10 mL of the solvent.
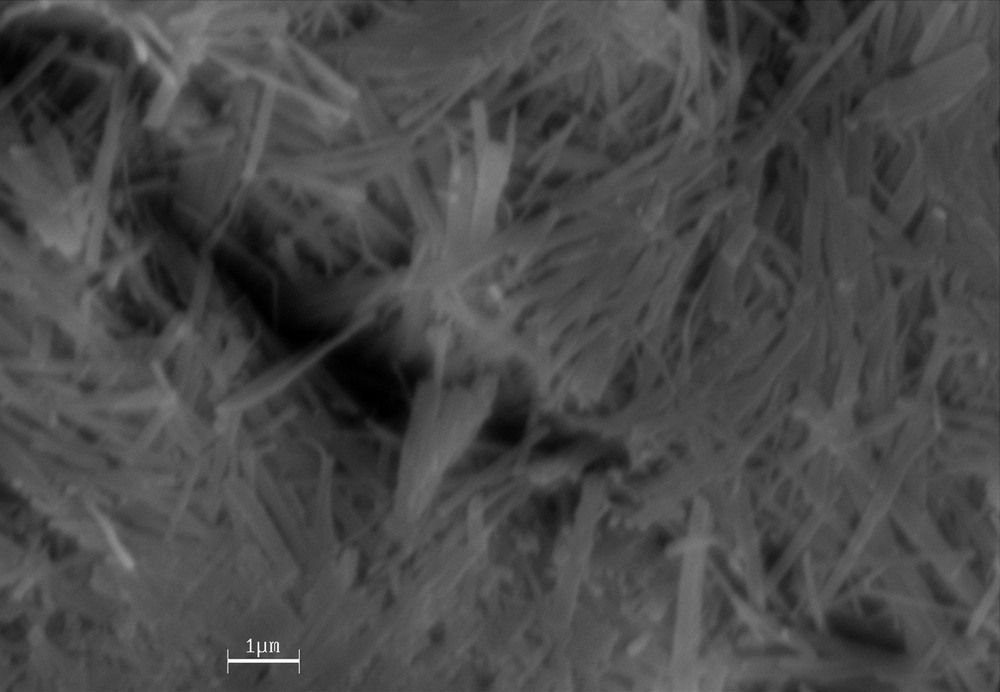
SEM image of TiO2 nanowire.
As shown in Table 1, we found that polar and protic solvents, such as H2O and EtOH or MeOH, afford better yields than aprotic ones and also an equal mixture of H2O and EtOH is the most effective solvent. The effect of temperature was also studied for this reaction and it was found that the reaction would be completed after 180 min at room temperature. However, the thermal-assisted model reaction is efficiently carried out by adding catalytic amounts of TiO2 NWs (3 mol%) in a mixture of EtOH and H2O (50/50).
Effect of solvent and catalyst on the synthesis of 4a under refluxing conditions (90 °C); reaction time: 60 min.
| Entry | Catalyst (mol%) | Solvent | Yield (%) |
| 1 | 5 | CH2Cl2 | 50 |
| 2 | 5 | CH3Cl | 50 |
| 3 | 5 | EtOH | 85 |
| 4 | 5 | MeOH | 85 |
| 5 | 5 | H2O | 65 |
| 6 | – | H2O/EtOH | Trace |
| 7 | 1 | H2O/EtOH | 50 |
| 8 | 3 | H2O/EtOH | 90 |
| 9 | 5 | H2O/EtOH | 90 |
| 10 | 10 | H2O/EtOH | 85 |
After optimization of the reaction conditions, in order to extend the scope of this reaction, a wide range of aromatic aldehydes were used with 1 and 3 (Table 2). All the products were characterized by comparison of their spectra and physical data with those reported in the literature [10,14,24].
Table 3 demonstrates the merit of this method for the synthesis of pyrano[2,3-c]coumarins in comparison with previously reported results. As it can be seen from Table 3, piperidine was employed as a basic catalyst under refluxing EtOH, but this method could not afford good yields (entry 1). In the case of (S)-proline (entry 4), the product was obtained in low yield in a long time. In other variations, such as DAHP (entry 3), KF–Al2O3 (entry 5), and TEBA (entry 6), the methods need too long reaction times. We believe that these reactions can be efficiently carried out under our suggested conditions with respect to reaction times and product yield.
Comparison of present work with other methods reported in the literaturea.
| Entry | Conditions | Time (min) | Yield (%) |
| 1 | Piperidine (0.5 mL), EtOH, reflux | 30 | 70 [7] |
| 2 | SDS (20 mol%), H2O, 60 °C | 120 | 85 [8] |
| 3 | DAHP (10 mol%), H2O:EtOH (1:1), room temperature | 180 | 81 [12] |
| 4 | (S)-Proline (10 mol%), H2O:EtOH (1:1), reflux | 240 | 72 [12] |
| 5 | KF–Al2O3 (0.125 g), EtOH, reflux | 240 | 90 [13] |
| 6 | TEBA (0.07 g), H2O, 90 °C | 420 | 96 [15] |
| 7 | TiO2NWs (3 mol%), H2O:EtOH (1:1), reflux | 60 | 90b |
a Synthesis of 4a.
b Present work.
A mechanistic rationale for the formation of pyranocoumarins 4 is suggested in Scheme 2. It seems that the reaction takes place in three steps. It is reasonable to assume that the initial event involves the generation of the arylmethylene via a Knoevenagel condensation of the aldehyde and malononitrile catalyzed by TiO2 NWs. In the next steps, a Michael-type addition to the arylmethylene and subsequent heterocyclization promoted by TiO2 NWs gives the corresponding products 4.
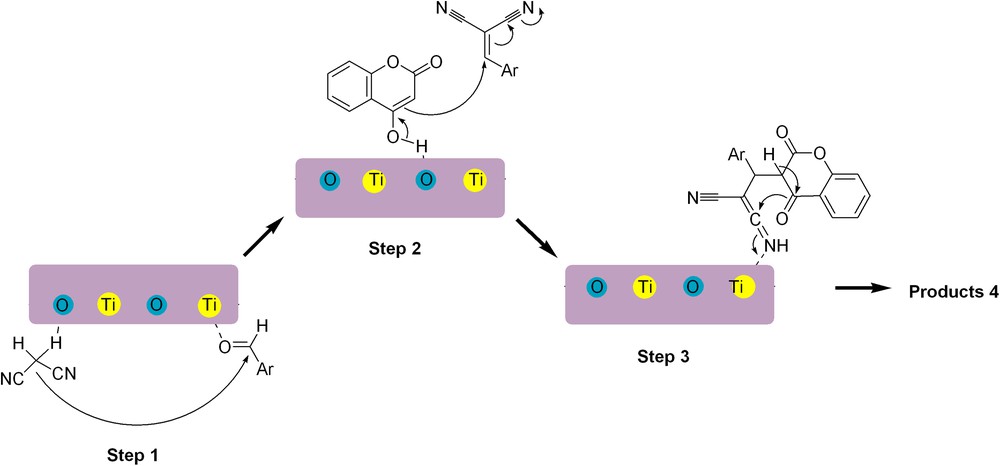
A plausible mechanism for the formation of pyranocoumarins 4 catalyzed by TiO2 NWs.
The main disadvantage of many reported methods for these reactions is that the catalysts are destroyed in the work-up procedure and cannot be recovered or reused. In these processes, as outlined in Fig. 2, the TiO2 NWs showed recyclability up to four cycles, during which there are a little losses in the catalytic activity.
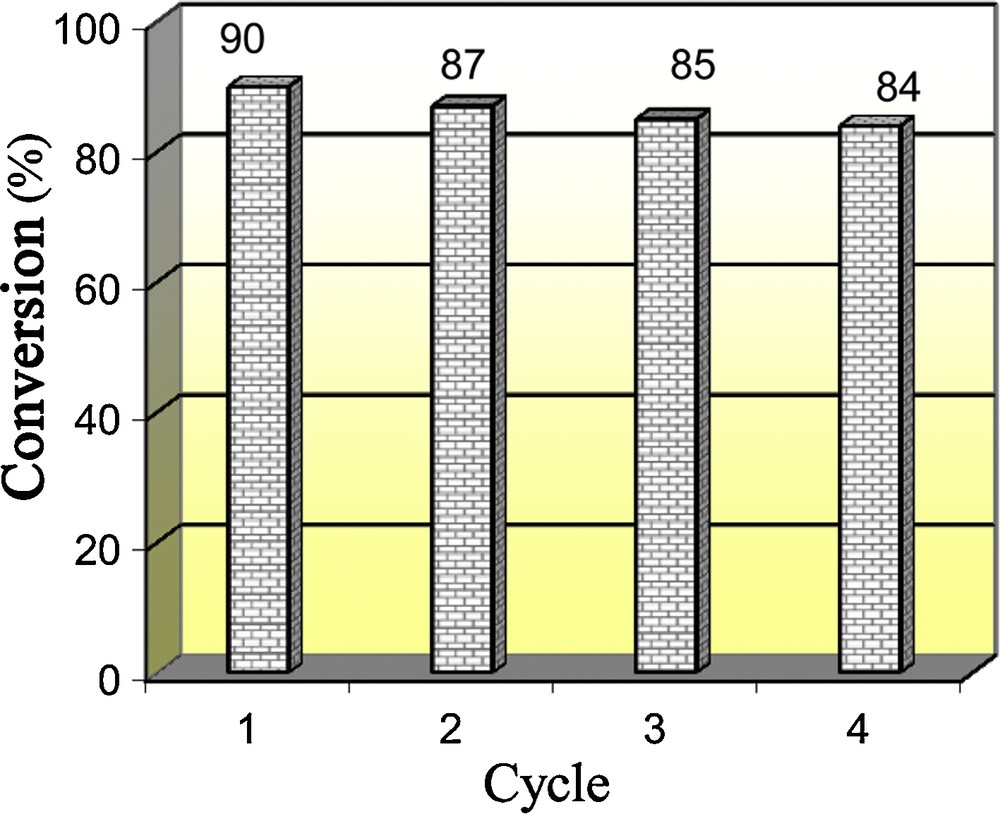
Recyclability of TiO2 NWs for synthesis of 4a. Reaction time: 60 min.
4 Conclusion
In summary, a new application of titanium dioxide nanowires as an efficient and heterogeneous catalyst in the reaction between 4-hydroxycoumarin, various aromatic aldehydes and malononitrile to produce pyrano[2,3-c]chromens of potential synthetic and pharmaceutical interest was presented. This method has unique merits, such as the use of a small amount of a safe catalyst, high product yields, short reaction times, and simple operation and work-up procedure, which make it a valid contribution to the existing methodologies.
Acknowledgements
The authors gratefully acknowledge Yasouj University of Iran for supporting this work.


