1 Introduction
Imidazoles, because of the N-heterocycles [1], played a significant role in many application fields, such as drug cores [2], natural products [3], conjugated and functional polymers [4], coordination complexes [5], important ligands in metalloenzymes [6], precursors of stable carbene ligands [7], photosensitive material [8,9], non-linear optical material [10], ionic liquids [11] and so on. Hence, 1,2,4,5-tetrasubstituted imidazoles were understandably suggested to hold an important position in imidazole derivatives. Tetrasubstituted imidazole compounds have been reported to possess good biological and pharmacological activities [12]. Especially, many of the substituted imidazoles were known as inhibitors of p38 MAP kinase, herbicides, fungicides, antibacterial, antitumor, therapeutic agents, pesticides and plant growth regulators [13,14]. Therefore, the synthesis and properties of these compounds have attracted much attention from the synthetic organic chemists and biologists.
Common methods for synthesizing 1,2,4,5-tetrasubstituted imidazoles included the cyclocondensation of the 1,2-dicarbonyl compound [15] or 2-hydroxy-1,2-diphenylethanone [16], aldehyde and ammonium acetate or amine, as well as between 1,2-dicarbonyl compounds, aromatic amine and aromatic cyanide [17], and the nucleophilic substitution reaction of a trisubstituted imidazole derivative and benzyl chloride [18]. However, the aromatic compounds mentioned here were correlated with benzene rings. The compounds bearing furan rings were rarely synthesized in the previous literature [15–18], because the furan ring possesses inherent chemical properties and disadvantages, such as electron richness, lower conjugation energy, easily ring-opening in Brønsted acid, the fact they easily become yellow in oxygen, and so on [19]. In addition, although the biological and pharmacological activity of tetrasubstituted imidazoles have been widely studied, their luminescence properties were seldom investigated, except for 1-R1-2-R-4,5-dialkyl-1H-imidazoles [20]. Our work focused on the synthesis and luminescence properties of a series of novel tetrasubstituted imidazoles with furan rings. Herein, a series of novel tetrasubstituted imidazoles with furan rings were synthesized by the reaction of 2-R-4,5-di(furan-2-yl)imidazoles and benzyl chlorides or allyl chloride according to the reported procedure [18] (Scheme 1). Besides, the comparison of the fluorescence spectra of compounds 4,5-di(furan-2-yl)-1-(4-methylbenzyl)-2-phenyl-1H-imidazole (3a) and (E)-4,5-di(furan-2-yl)-1-(4-methylbenzyl)-2-styryl-1H-imidazole (3h) in different solutions suggested that long conjugated chain played a key role in the red shift of the fluorescence emission of compound 3h in the same solution.
2 Experimental
2-R-4,5-di(furan-2-yl)imidazoles were synthesized according to the reported procedure [19]. All reagents were of analytical-reagent grade and used without further purification. All reported yields were isolated yields. All melting points were determined with a XT–4 melting point apparatus and were uncorrected. HRMS were an Agilent1290-micrOTOF Q II spectrometer. 1H and 13C NMR spectra were measured using a Bruker AVANCE-500 NMR spectrometer with TMS as an internal standard. FT-IR spectra were obtained as KBr pellets using an IRAffinity-1 instrument in the range between 500 and 3500 cm−1. Fluorescence spectra were recorded with a Shimadzu RF-5301 spectrofluorimeter. The absorption spectra were recorded on an Australian GBC Cintra 10e UV–vis spectrometer within the wavelength range from 200 to 800 nm.
2.1 Experimental procedures for the synthesized compounds (3a–3o)
A mixture of compounds 2-R-4,5-di(furan-2-yl) imidazoles (1 mmol) and NaH (36 mg, 1.5 mmol) in 20 mL of anhydrous THF was heated until the reaction temperature was about 60 °C. An amount of 1.5 mmol of benzyl chloride or allyl chloride dissolved in 3 mL of anhydrous THF was dropped into the mixture, and the resulting mixture was refluxed for 13–37.5 h. The reaction progress was monitored by TLC on Silufol-254 plates. After the reaction was over, the reaction mixture was filtered while cooled to room temperature, and washed with anhydrous THF (2 × 5 mL). The combined filtration was poured into 20 mL of water ice water. The organic phase dried by anhydrous Na2SO4 was concentrated. The residue was purified by column chromatography on Chemapol (200–300 mesh) silica gel using petroleum ether/ethyl acetate (3:1) as an eluent to afford the compound.
2.2 Physical and spectroscopic data
2.2.1 4,5-Di(furan-2-yl)-1-(4-methylbenzyl)-2-phenyl-1H-imidazole (3a)
Mp: 127–129 °C. Yield: 69.7%. IR (KBr) ν (cm−1): 3090, 3020, 2920, 1600, 1520, 1470, 1440, 1400, 1360, 1310, 1210, 1170, 1070, 1020, 968, 887, 773, 733, 592. 1H NMR (500 MHz, DMSO): 1H NMR (500 MHz, DMSO) δ (ppm): 7.80 (dd, J = 1.7, 0.9 Hz, 1H), 7.64–7.58 (m, 3H), 7.50–7.44 (m, 3H), 7.05 (d, J = 7.8 Hz, 2H), 6.71 (d, J = 8.1 Hz, 2H), 6.61–6.55 (m, 2H), 6.51 (dd, J = 3.3, 1.8 Hz, 1H), 6.47 (dd, J = 3.3, 0.8 Hz, 1H), 5.22 (s, 2H), 2.21 (s, 3H). 13C NMR (125 MHz, DMSO): δ (ppm) 149.3, 149.1, 144.6, 142.7, 142.6, 136.9, 134.5, 133.1, 130.5, 129.8, 129.7, 129.1, 126.0, 120.3, 113.0, 111.9, 111.7, 107.04, 48.6, 21.0. HRMS, m/z: (M + H)+ calcd for C25H21N2O2 381.1603, found 381.1605.
2.2.2 1-(4-Chlorobenzyl)-4,5-di(furan-2-yl)-2-phenyl-1H-imidazole (3b)
Mp: 144–145 °C. Yield: 89.8%. IR (KBr) ν (cm−1): 3090, 2930, 1890, 1600, 1490, 1440, 1400, 1350, 1320, 1210, 1160, 1090, 1020, 968, 887, 773, 733, 700, 592. 1H NMR (500 MHz, DMSO) δ (ppm): 7.79 (dd, J = 1.8, 0.7 Hz, 1H), 7.63–7.59 (m, 3H), 7.50–7.46 (m, 3H), 7.32–7.29 (m, 2H), 6.87–6.83 (m, 2H), 6.60 (dd, J = 3.4, 0.7 Hz, 1H), 6.58 (dd, J = 3.4, 1.9 Hz, 1H), 6.51 (dd, J = 3.3, 1.8 Hz, 1H), 6.49 (dd, J = 3.3, 0.8 Hz, 1H), 5.25 (s, 2H). 13C NMR (125 MHz, DMSO) δ (ppm): 149.2, 149.2, 144.7 142.7, 142.4, 136.5, 133.2, 132.4, 130.3, 129.9, 129.2, 128.1, 120.2, 113.3, 111.9, 111.8, 107.1, 48.3. HRMS, m/z: (M + H)+ calcd for C24H18ClN2O2 401.1057, found 401.1067.
2.2.3 1-Benzyl-4,5-di(furan-2-yl)-2-phenyl-1H-imidazole (3c)
Mp: 68–70 °C. Yield: 78.6%. (KBr) ν (cm−1): 3100, 3060, 3020, 2370, 1970, 1600, 1500, 1470, 1440, 1400, 1360, 1320, 1210, 1160, 1070, 1020, 964, 887, 777, 733, 698, 592, 503. 1H NMR (500 MHz, DMSO) δ (ppm): 7.79 (t, J = 1.3 Hz, 1H), 7.63–7.59 (m, 3H), 7.49–7.45 (m, 3H), 7.26–7.18 (m, 3H), 6.82 (d, J = 7.1 Hz, 2H), 6.59–6.54 (m, 2H), 6.51 (dd, J = 3.3, 1.8 Hz, 1H), 6.48 (dd, J = 3.4, 0.6 Hz, 1H), 5.27 (s, 2H). 13C NMR (125 MHz, DMSO) δ (ppm): 149.3, 149.2, 142.6, 137.5, 133.1, 130.5, 129.8, 129.2, 129.0, 127.8, 126.2, 120.3, 113.1, 111.9, 111.8, 107.1, 48.8. HRMS, m/z: (M + H)+ calcd for C24H19N2O2 367.1447, found 367.1444.
2.2.4 2,4,5-Tri(furan-2-yl)-1-(4-methylbenzyl)-1H-imidazole (3d)
Mp: 114–116 °C. Yield: 82.4%. IR (KBr) ν (cm−1): 3100, 3020, 2920, 2860, 1600, 1520, 1460, 1400, 1360, 1320, 1220, 1170, 1120, 1070, 1020, 916, 885, 812, 735, 660, 592, 523. 1H NMR (500 MHz, DMSO) δ (ppm): 7.83 (d, J = 9.0 Hz, 2H), 7.63 (s, 1H), 7.08 (d, J = 7.5 Hz, 2H), 6.84 (dd, J = 14.7, 5.4 Hz, 3H), 6.63 (dd, J = 6.0, 4.4 Hz, 3H), 6.50 (dd, J = 13.7, 2.0 Hz, 2H), 5.36 (s, 2H), 2.22 (s, 3H). 13C NMR (125 MHz, DMSO) δ (ppm): 148.9, 144.9, 144.6, 142.7, 142.0, 140.2, 137.1, 134.4, 133.4, 129.7, 126.2, 120.3, 113.6, 112.3, 112.0, 111.8, 111.3, 107.3, 48.4, 21.0. HRMS, m/z: (M + H)+ calcd for C23H19N2O3 371.1396, found 371.1395.
2.2.5 1-(4-Chlorobenzyl)-2,4,5-tri(furan-2-yl)-1H-imidazole (3e)
Mp: 90–92 °C. Yield: 85.1%. IR (KBr) ν (cm−1): 3110, 2920, 1600, 1490, 1410, 1370, 1220, 1170, 1090, 1010, 885, 816, 739, 644, 594. 1H NMR (500 MHz, DMSO) δ (ppm): 7.82 (d, J = 6.3 Hz, 2H), 7.64 (s, 1H), 7.35 (d, J = 8.3 Hz, 2H), 6.97 (d, J = 8.2 Hz, 2H), 6.89 (d, J = 3.1 Hz, 1H), 6.64 (ddd, J = 10.6, 4.9, 2.4 Hz, 3H), 6.51 (dd, J = 11.0, 2.2 Hz, 2H), 5.40 (s, 2H). 13C NMR (125 MHz, DMSO) δ (ppm): 148.8, 144.5, 142.8, 141.8, 140.2, 136.5, 133.5, 132.5, 129.1, 128.2, 120.1, 113.8, 112.3, 112.0, 111.8, 111.4, 107.4, 48.2. HRMS, m/z: (M + H)+ calcd for C22H16ClN2O3 391.0850, found 391.0849.
2.2.6 1-Benzyl-2,4,5-tri(furan-2-yl)-1H-imidazole (3f)
Mp: 58–60 °C. Yield: 76.1%. IR (KBr) ν (cm−1): 3120, 3030, 2930, 1600, 1500, 1450, 1400, 1360, 1320, 1220, 1170, 1080, 1010, 920, 887, 822, 735, 594. 1H NMR (500 MHz, DMSO) δ (ppm): 7.83 (dd, J = 5.8, 1.2 Hz, 2H), 7.63 (d, J = 0.8 Hz, 1H), 7.28 (t, J = 7.4 Hz, 2H), 7.22 (t, J = 7.2 Hz, 1H), 6.94 (d, J = 7.4 Hz, 2H), 6.86 (d, J = 3.4 Hz, 1H), 6.68–6.60 (m, 3H), 6.52 (dd, J = 3.2, 1.8 Hz, 1H), 6.48 (d, J = 3.2 Hz, 1H), 5.41 (s, 2H). 13C NMR (125 MHz, DMSO) δ (ppm): 148.9, 144.9, 144.6, 144.6, 142.8, 142.0, 140.3, 137.4, 133.4, 132.5, 129.1, 127.9, 126.3, 120.3, 113.6, 112.3, 112.0, 111.8, 111.34, 107.3, 48.7. HRMS, m/z: (M + H)+ calcd for C22H17N2O3 357.1239, found 357.1241.
2.2.7 1-Allyl-2,4,5-tri(furan-2-yl)-1H-imidazole (3g)
Mp: 86–88 °C. Yield: 59.0%. IR (KBr) ν (cm−1): 3120, 2920, 2350, 1650, 1560, 1460, 1400, 1360 1320, 1220, 1170, 1080, 1010, 920, 887, 812, 739, 594. 1H NMR (500 MHz, DMSO) δ (ppm): 7.88 (m, 2H), 7.62 (s, 1H), 6.96 (d, J = 2.8 Hz, 1H), 6.77 (d, J = 2.7 Hz, 1H), 6.68 (m, 2H), 6.49 (m, 2H), 5.94 (m, 1H), 5.11 (d, J = 10.3 Hz, 1H), 4.77 (dd, J = 23.0, 9.5 Hz, 3H). 13C NMR (125 MHz, DMSO) δ (ppm): 149.0, 144.6, 142.0, 140.0, 134.0, 133.1, 120.0, 116.8, 113.5, 112.3, 112.0, 111.8, 111.2, 107.1, 47.7. HRMS, m/z: (M + H)+ calcd for C18H15N2O3 307.1083, found 307.1084.
2.2.8 (E)-4,5-Di(furan-2-yl)-1-(4-methylbenzyl)-2-styryl-1H-imidazole (3h)
Mp: 112–114 °C. Yield: 71.6%. IR (KBr) ν (cm−1): 3120, 3030, 2920, 2850, 1600, 1520, 1460, 1420, 1350, 1320, 1210, 1010, 891, 800, 742, 688, 594. 1H NMR (500 MHz, CDCl3) δ (ppm): 1H NMR (500 MHz, CDCl3) δ 7.81 (d, J = 11.5 Hz, 1H), 7.54 (dd, J = 1.7, 0.8 Hz, 1H), 7.48–7.41 (m, 3H), 7.34–7.30 (m, 2H), 7.29–7.26 (m, 1H), 7.11 (d, J = 7.9 Hz, 2H), 6.97 (d, J = 8.1 Hz, 2H), 6.82 (d, J = 15.8 Hz, 1H), 6.56–6.43 (m, 3H), 6.40 (dd, J = 3.3, 1.8 Hz, 1H), 5.19 (s, 2H), 2.31 (s, 3H). 13C NMR (125 MHz, DMSO) δ (ppm): 148.9, 146.8, 142.8, 141.7, 137.6, 136.4, 134.5, 133.6, 129.6, 128.7, 128.4, 126.9, 126.0, 119.7, 113.0, 112.6, 111.3, 111.1, 106.7, 47.4, 21.1. HRMS, m/z: (M + H)+ calcd for C27H23N2O2 407.1760, found 407.1736.
2.2.9 (E)-1-(4-Chlorobenzyl)-4,5-di(furan-2-yl)-2-styryl-1H-imidazole (3i)
Mp: 74–76 °C. Yield: 77.5%. IR (KBr) ν (cm−1): 3110, 3050, 1630, 1600, 1490, 1410, 1370, 1270, 1210, 1010, 891, 804, 742, 680, 594. 1H NMR (500 MHz, DMSO): δ (ppm): 7.82 (dd, J = 1.9, 0.8 Hz, 1H), 7.70–7.67 (m, 2H), 7.64–7.58 (m, 2H), 7.39–7.36 (m, 4H), 7.31 (t, J = 7.3 Hz, 2H), 7.01 (d, J = 8.5 Hz, 2H), 6.65 (dd, J = 3.4, 0.7 Hz, 1H), 6.62 (dd, J = 3.4, 1.9 Hz, 1H), 6.51 (dd, J = 3.3, 1.8 Hz, 1H), 6.47 (dd, J = 3.3, 0.8 Hz, 1H), 5.42 (s, 2H). 13C NMR (125 MHz, DMSO) δ (ppm): 148.7, 146.3, 142.2, 141.9, 136.4, 136.0, 133.3, 133.1, 132.0, 128.7, 128.7, 128.5, 128.0, 127.1, 119.2, 113.8, 112.7, 111.5, 111.3, 106.7, 45.9. HRMS, m/z: (M + H)+ calcd for C26H20ClN2O2 427.1213, found 427.1204.
2.2.10 (E)-4-((4,5-Di(furan-2-yl)-2-styryl-1H-imidazol-1-yl)methyl)benzonitrile (3j)
Mp: 148–150 °C. Yield: 86.5%. IR (KBr) ν (cm−1): 3090, 3020, 2240, 1600, 1500, 1460, 1410, 1360, 1250, 1220, 1010, 891, 831, 741, 688, 592. 1H NMR (500 MHz, DMSO) δ (ppm): 7.81–7.76 (m, 3H), 7.68 (d, J = 7.4 Hz, 2H), 7.65–7.60 (m, 2H), 7.38 (t, J = 7.5 Hz, 2H), 7.34–7.29 (m, 2H), 7.17 (d, J = 8.4 Hz, 2H), 6.63 (d, J = 3.4 Hz, 1H), 6.60 (dd, J = 3.4, 1.9 Hz, 1H), 6.52 (dd, J = 3.3, 1.8 Hz, 1H), 6.49 (dd, J = 3.3, 0.6 Hz, 1H), 5.54 (s, 2H). 13C NMR (125 MHz, DMSO) δ (ppm): 146.4, 143.1, 142.2, 141.4, 136.2, 133.5, 133.2, 132.6, 128.7, 128.5, 127.1, 126.9, 119.2, 118.5, 113.6, 112.8, 111.5, 111.3, 110.2, 106.8, 46.4. HRMS, m/z: (M + H)+ calcd for C27H20N3O2 418.1556, found 418.1558.
2.2.11 (E)-1-Benzyl-4,5-di(furan-2-yl)-2-styryl-1H-imidazole (3k)
Mp: 117–118 °C. Yield: 74.2%. IR (KBr) ν (cm−1): 3110, 3010, 1600, 1500, 1450, 1410, 1350, 1310, 1210, 1010, 887, 808, 735, 588. 1H NMR (500 MHz, CDCl3) δ (ppm): 1H NMR (500 MHz, CDCl3) δ 7.81 (d, J = 15.8 Hz, 1H), 7.53 (dd, J = 1.6, 1.0 Hz, 1H), 7.46–7.42 (m, 3H), 7.32 (ddd, J = 7.7, 4.9, 1.8 Hz, 4H), 7.28–7.26 (m, 2H), 7.10–7.05 (m, 2H), 6.82 (d, J = 15.8 Hz, 1H), 6.53–6.45 (m, 3H), 6.40 (dd, J = 3.4, 1.8 Hz, 1H), 5.23 (s, 2H). 13C NMR (125 MHz, DMSO) δ (ppm): 148.8, 146.8, 142.8, 136.6, 136.3, 134.6, 133.7, 128.9, 128.7, 128.5, 127.8, 126.9, 126.1, 119.7, 112.9, 112.7, 111.3, 111.1, 106.8, 47.6. HRMS, m/z: (M + H)+ calcd for C26H21N2O2 393.1603, found 393.1575.
2.2.12 (E)-4,5-Di(furan-2-yl)-2-(2-(furan-2-yl)vinyl)-1-(4-methylbenzyl)-1H-imidazole (3l)
Mp: 94–95 °C. Yield: 79.7%. IR (KBr) ν (cm−1): 3110, 3020, 2920, 1740, 1640, 1520, 1460, 1350, 1320, 1260, 1210, 1150, 1070, 1010, 960, 883, 802, 737, 594, 538. 1H NMR (500 MHz, CDCl3) δ (ppm):7.58 (d, J = 15.6 Hz, 1H), 7.52 (dd, J = 1.6, 1.0 Hz, 1H), 7.43 (dd, J = 1.7, 0.8 Hz, 1H), 7.36 (s, 1H), 7.10 (d, J = 7.9 Hz, 2H), 6.93 (d, J = 8.1 Hz, 2H), 6.75 (d, J = 15.6 Hz, 1H), 6.46 (m, 3H), 6.40 (d, J = 1.3 Hz, 2H), 6.38 (m, 1H), 5.17 (s, 2H), 2.30 (s, 3H). 13C NMR (125 MHz, DMSO) δ (ppm): 152.3, 149.3, 146.4, 144.3, 142.6, 142.5, 137.2, 134.7, 133.4, 129.8, 126.4, 121.1, 112.0, 113.2, 112.9, 112.3, 112.0, 111.8, 111.8, 107.1, 46.8, 21.1. HRMS, m/z: (M + H)+ calcd for C25H21N2O3 397.1552, found 397.1534.
2.2.13 (E)-1-(4-Chlorobenzyl)-4,5-di(furan-2-yl)-2-(2-(furan-2-yl)vinyl)-1H-imidazole (3m)
Mp: 87–89 °C. Yield: 84.1%. IR (KBr) ν (cm−1): 3110, 2950, 1640, 1490, 1410, 1370, 1220, 1160, 1090, 1010, 955, 885, 804, 739, 669, 594. 1H NMR (500 MHz, DMSO) δ (ppm): 7.82 (d, J = 1.1 Hz, 1H), 7.72 (d, J = 1.2 Hz, 1H), 7.63 (d, J = 0.9 Hz, 1H), 7.44 (d, J = 15.6 Hz, 1H), 7.39 (d, J = 8.5 Hz, 2H), 7.01 (d, J = 8.5 Hz, 2H), 6.90 (d, J = 15.6 Hz, 1H), 6.74 (d, J = 3.3 Hz, 1H), 6.65 (d, J = 3.3 Hz, 1H), 6.62 (dd, J = 3.3, 1.9 Hz, 1H), 6.57 (dd, J = 3.3, 1.8 Hz, 1H), 6.51 (dd, J = 3.3, 1.8 Hz, 1H), 6.48 (d, J = 2.7 Hz, 1H), 5.34 (s, 2H). 13C NMR (125 MHz, DMSO) δ (ppm): 152.3, 149.2, 146.5, 144.8, 144.4, 142.7, 142.3, 136.7, 133.5, 132.6, 129.2, 128.3, 121.3, 119.8, 113.3, 112.9, 112.4, 112.0, 111.8, 111.6, 107.2, 46.5. HRMS, m/z: (M + H)+ calcd for C24H18ClN2O3 417.1006, found 417.0999.
2.2.14 (E)-4-((4,5-Di(furan-2-yl)-2-(2-(furan-2-yl)vinyl)-1H-imidazol-1-yl)methyl)benzonitrile (3n)
Mp: 99–101 °C. Yield: 88.9%. IR (KBr) ν (cm−1): 3120, 2930, 2230, 1740, 1610, 1510, 1470, 1410, 1370, 1260, 1220, 1160, 1070, 1020, 953, 885, 816, 741, 594, 548. 1H NMR (500 MHz, DMSO) δ (ppm): 7.80 (d, J = 8.0 Hz, 3H), 7.71 (s, 1H), 7.63 (s, 1H), 7.45 (d, J = 15.5 Hz, 1H), 7.17 (d, J = 7.6 Hz, 2H), 6.89 (d, J = 15.5 Hz, 1H), 6.74 (s, 1H), 6.64 (s, 1H), 6.62–6.58 (m, 1H), 6.57 (d, J = 1.5 Hz, 1H), 6.51 (d, J = 4.5 Hz, 2H), 5.46 (s, 2H). 13C NMR (125 MHz, DMSO) δ (ppm):152.2, 149.1, 146.6, 144.4, 143.4, 142.8, 142.2, 133.6, 133.2, 127.4, 121.6, 119.8, 119.0, 113.3, 112.9, 112.5, 112.0, 111.8, 111.4, 110.8, 107.3, 46.9. HRMS, m/z: (M + H)+ calcd for C25H18N3O3 408.1348, found 408.1345.
2.2.15 (E)-1-Benzyl-4,5-di(furan-2-yl)-2-(2-(furan-2-yl)vinyl)-1H-imidazole (3o)
Mp: 62–64 °C. Yield: 81.6%. IR (KBr) ν (cm−1): 3120, 3030, 2930, 1740, 1640, 1500, 1450, 1360, 1260, 1220, 1160, 1070, 1010, 955, 885, 808, 735, 594. 1H NMR (500 MHz, DMSO) δ (ppm): 7.83 (d, J = 1.2 Hz, 1H), 7.71 (d, J = 1.4 Hz, 1H), 7.63 (d, J = 1.0 Hz, 1H), 7.43 (d, J = 15.6 Hz, 1H), 7.32 (t, J = 7.5 Hz, 2H), 7.25 (t, J = 7.3 Hz, 1H), 7.00 (d, J = 7.4 Hz, 2H), 6.91 (d, J = 15.6 Hz, 1H), 6.73 (d, J = 3.3 Hz, 1H), 6.64 (d, J = 3.3 Hz, 1H), 6.62 (dd, J = 3.3, 1.9 Hz, 1H), 6.57 (dd, J = 3.3, 1.8 Hz, 1H), 6.52 (dd, J = 3.3, 1.8 Hz, 1H), 6.50–6.46 (m, 1H), 5.35 (s, 2H). 13C NMR (125 MHz, DMSO) δ (ppm): 152.3, 149.2, 146.5, 144.7, 144.3, 142.6, 142.5, 137.7, 133.5, 129.2, 128.0, 126.4, 121.2, 112.0, 113.2, 112.9, 112.3, 112.0, 111.8, 107.2, 47.1. HRMS, m/z: (M + H)+ calcd for C24H19N2O3 383.1396, found 383.1380.
2.3 Determination of the fluorescence quantum yield
The fluorescent quantum yields were measured against quinine sulfate (ϕs = 0.55 in 0.1 N sulfuric acid, 200 nm ≤ λ ≤ 400 nm) as a standard [21,22] and were calculated following Eq. (1) improved according to the literature [23]. The crossing wavelength of UV–vis absorption (λ ≥ 300 nm) was chosen to excite the sample and the standard. If not, 320 nm was confirmed as the excitation wavelength.
| (1) |
3 Results and discussion
3.1 Synthesis of the compounds
In the present work, a wide range of benzyl chloride and allyl chloride were employed and fifteen new 1-R1-2-R-4,5-di(furan-2-yl)-1H-imidazoles were obtained in better yield from 59.0% to 89.8%. Herein, the nucleophilic substitution reaction of 2-R-4,5-(di-furan-yl)-1H-imidazole with 1.5 equiv of benzyl chloride or allyl chloride was readily carried out using NaH as a base under mild reaction conditions referring to the reported method [18] (Table 1). The structures of 1,2,4,5-tetrasubstituted imidazoles including furan rings were confirmed by FT-IR, 1H and 13C NMR and HRMS analysis. The aryl–CH2–N–RC = N–protons of the compounds exhibited resonances at 5.17–5.55 ppm when using DMSO as the solvent, while the resonances for the corresponding aryl–CH2–N–RCN–carbon atom were observed between 45.9 and 48.8 ppm. The resonances for the – N–RCN – carbon atom on the imidazole ring were observed at 141.4–144.7 ppm.
Synthesis of 1,2,4,5-tetrasubstituted imidazoles containing furan rings under anhydrous THFa and their luminescence properties in a 0.1 N H2SO4 aqueous solution with 0.5 mL of dissolved CH3OH.
| Entry | R | R1 | Product | Reaction time (h) | Yield (%)b | λmax(nm) | PL/λ (nm) | ϕuc |
| 1 | 3a | 37.5 | 69.7 | 285 | 473 | 0.151 | ||
| 2 | 3b | 18 | 89.8 | 287 | 471 | 0.118 | ||
| 3 | 3c | 29 | 78.6 | 287 | 467 | 0.101 | ||
| 4 | 3d | 35 | 82.4 | 312 | 466 | 0.069 | ||
| 5 | 3e | 19 | 85.1 | 307 | 464 | 0.056 | ||
| 6 | 3f | 25 | 76.1 | 306 | 467 | 0.070 | ||
| 7 | 3g | 29.5 | 59.0 | 311 | 448 | 0.084 | ||
| 8 | 3h | 37 | 71.6 | 350 | 506 | 0.132 | ||
| 9 | 3i | 20 | 77.5 | 347 | 505 | 0.078 | ||
| 10 | 3j | 14 | 86.5 | 351 | 506 | 0.112 | ||
| 11 | 3k | 25 | 74.2 | 345 | 507 | 0.161 | ||
| 12 | 3l | 36 | 79.7 | 342 | 501 | 0.010 | ||
| 13 | 3m | 19 | 84.1 | 351 | 502 | 0.009 | ||
| 14 | 3n | 13 | 88.9 | 346 | 503 | 0.004 | ||
| 15 | 3o | 27 | 81.6 | 346 | 506 | 0.008 |
a Reaction condition: 1 mmol 2-R-4,5-di(furan-2-yl) imidazoles, 1.5 mmol NaH, and 1.5 mmol benzyl chloride or allyl chloride dropped in 20 mL anhydrous THF were refluxed.
b Isolated product yield
c Quinine sulfate as standards, calculated by means of Eq. (1).
As shown in Table 1, for clarity, the results indicated that good yields were obtained when 4-substituted benzyl chlorides containing electron–withdrawing groups (–CN,–Cl) were used as starting materials (entries 2, 5, 9, 10, 13 and 14), whereas the better yields were obtained when benzyl chloride and 4-substituted benzyl chlorides containing electron-donating groups were employed (entries 1, 3, 6, 8, 11, 12 and 15). Moreover, the yields of the synthesized compounds were enhanced and the reaction time was shortened with increasing electron-withdrawing effects. For example, the reaction time was 13 h and 36 h, respectively when 4-cyanobenzyl chloride (entry 14) and 4-methyl benzyl chloride (entry 12) were employed in the reaction. Besides, the desired compound was not obtained when 4-methoxy benzyl chloride was employed. The reason might be that the interactions between the conjugation of the benzene ring and the inductive effect of electron-withdrawing–CN or–Cl made the electron atmosphere of cyanobenzyl and chlorobenzyl reduce and be facilely attacked by nucleophilic C–N—–C of the imidazole ring. Conversely, the electron-donating inductive effect was contrary to the benzene ring conjugation when the 4-substituent on benzyl chloride is –CH3. Therefore, the yield was low, the reaction time was long, and even the desired product could not be obtained.
3.2 UV-vis and photoluminescence spectra analysis
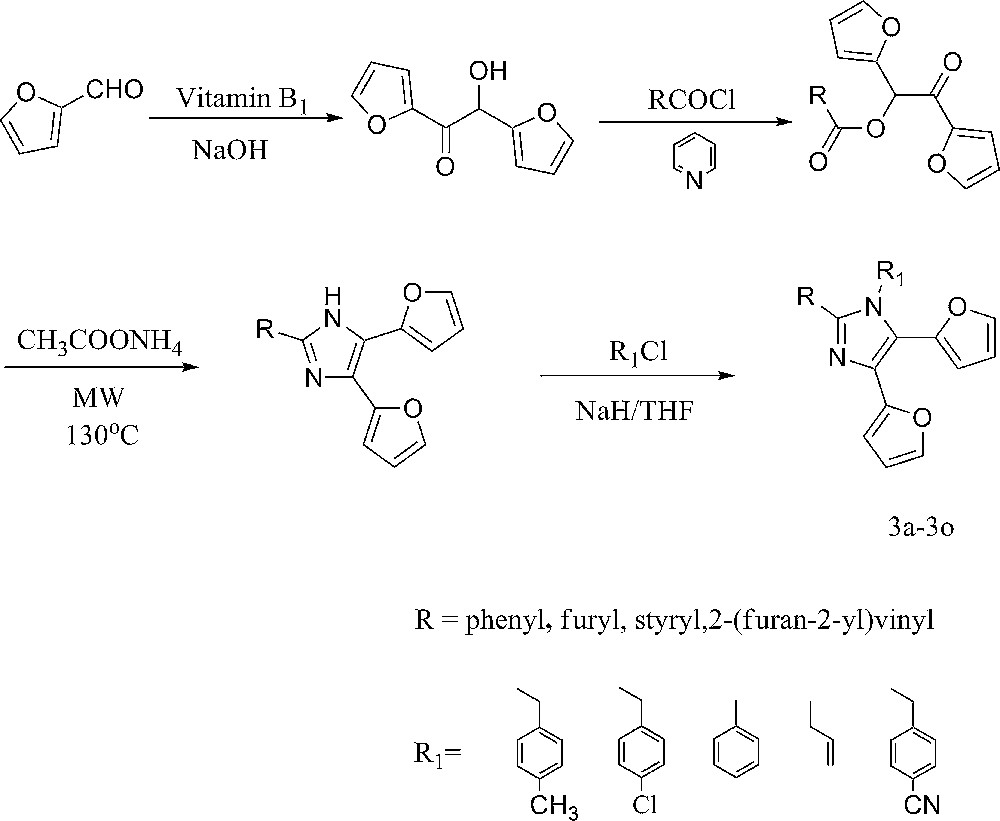
The UV-vis absorption spectra of compounds 3a–3o (4.0 × 10−6 mol/L) in a 0.1 N H2SO4 aqueous solution in which 0.5 mL of CH3OH has been dissolved are shown in Fig. 1. Two main absorption peaks of 3a–3c were around 220–231 nm and 281–293 nm, respectively. Two main absorption peaks of 3d–3g were around 217–236 nm and 302–318 nm, respectively. Such a red-shift at 280–320 nm compared with that of 2-phenyl (entries 1, 2, and 3) was attributed to the new n–π conjugated structure of 2-furyl (entries 4, 5, and 6). Three absorption peaks of 3h–3k were around 220–234 nm, 278–289 nm and 337–371 nm, respectively. Three absorption peaks of 3l–3o were around 220–238 nm, 284–295 nm and 340–380 nm, respectively. Such a red-shift compared with 2-phenyl (entries 1, 2 and 3) or 2-furyl (entries 4, 5 and 6) substituted group on imidazole was attributed to 2-styryl (entries 8, 9 and 11) or (2-furan-2-yl)vinyl (entries 12, 13 and 15). The substituted groups on imidazole possess a long conjugated chain lead to the π→π* transition energy decrease [24]. However, we can see that the different N-substituted groups slightly affected the absorption of the synthesized compound.
UV-vis spectra of 3a–3o.
In general, the emission spectra of the compounds did not change in a given solvent with varying the excitation wavelengths in the lower wavelength range. However, the intensity of the emission spectra changed as the excitation wavelength [25]. The fluorescence emission spectra of 3a–3o (4.0 × 10−6 mol/L) in the 0.1 N H2SO4 aqueous solution containing 0.5 mL of dissolved CH3OH are shown in Fig. 2. The emission spectra of 3a–3o were in the blue and green regions, around 450–550 nm. The fluorescence quantum yields (ϕu) of products 3a−3o were calculated according to Eq. (1). The calculated fluorescence quantum yield of each compound is summarized in Table 1. In general, the fluorescence quantum yields of the imidazole derivatives bearing the conjugated units furan rings and substituted furan rings on the 2-position of imidazole (entries 4, 5, 6, 7, 12, 13, 14 and 15) were lower than that of bearing the conjugated units of the benzene ring and the substituted benzene ring on the 2-position of imidazole (entries 1, 2, 3, 8, 9, 10 and 11) against quinine sulfate as a standard [21–23,26,27]. The reason for the higher fluorescence quantum yields of the synthesized compounds (entries 1, 2, 3, 8, 9, 10 and 11) might be that the conjugated effect of the benzene ring made the plane of the trisubstituted imidazole twist a less angle [20,28]. Besides, benzyl groups or allyl group introduced to the 1-position of trisubstituted imidazole increase the chance of a rotational degree of freedom and result in a non-planar conformation. Therefore, the fluorescence quantum yields of the synthesized tetrasubstituted imidazole derivatives were generally lower (Table 1). The reason for the fluorescence quenching of the synthesized compounds (entries 12, 13, 14 and 15) might be the interaction of ground-state molecules and excimers of (2-furan-2-yl)vinyl to form a dimer under light irradiation.

Fluorescence emission spectra of 3a–3o.
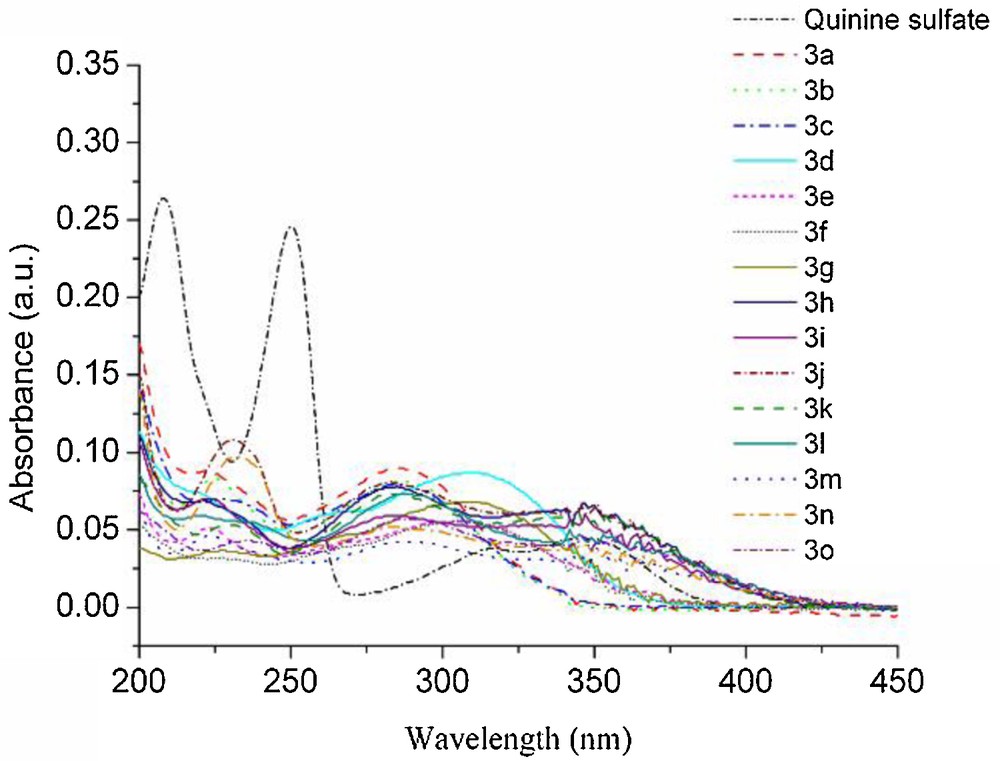
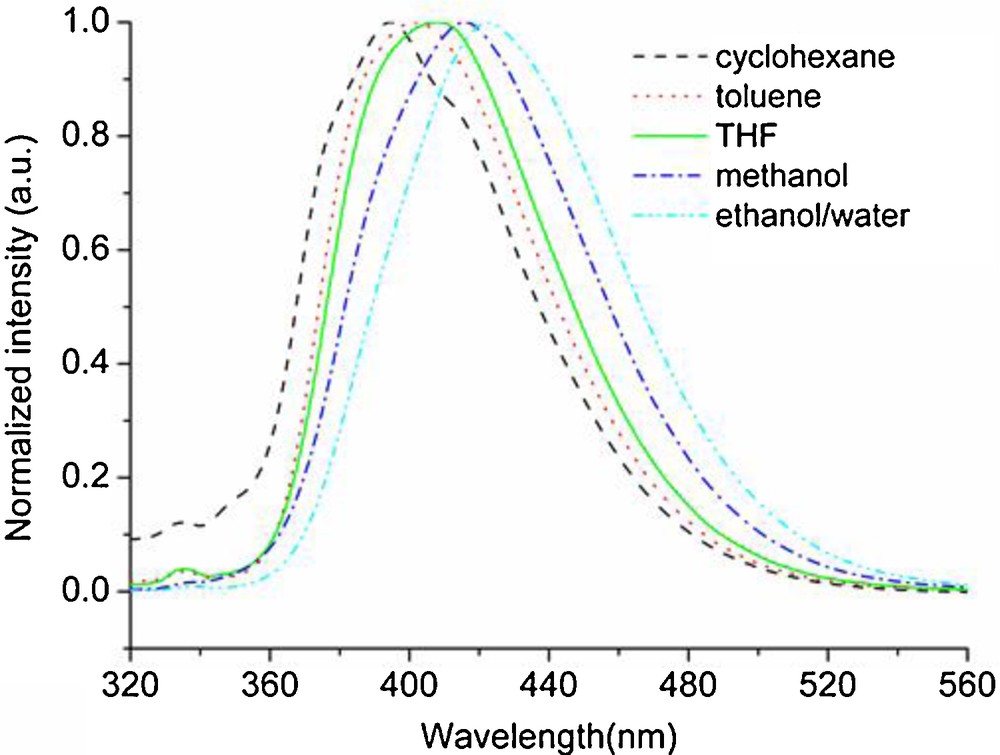
In addition, the fluorescence emission spectra of 3a and 3h (4.0 × 10−6 mol/L) in solvents with different polarity (cyclohexane, toluene, THF, methanol and ethanol/water v/v = 1/1) are shown in Figs. 3 and 4, respectively. The corresponding data are summarized in Table 2.
Normalized emission spectra of 3a in the solvents with different polarities (λex = 300 nm).
Normalized emission spectra of 3h in the solvents with different polarities (λex = 300 nm).
The emission wavelength of 3a and 3h in the solvents with different polarities (λex = 300 nm).
| Solvent | Emission wavelength (nm) | Shift (nm) | |
| 3a | 3h | ||
| Cyclohexane | 394 | 450 | 56 |
| Toluene | 402 | 459 | 57 |
| THF | 409 | 461 | 52 |
| Methanol | 416 | 465 | 49 |
| Ethanol/water | 423 | 473 | 50 |
Notably, the emission bands of compounds 3a and 3h were significantly red-shifted with an increase of the solvent polarity. In cyclohexane, the emissions of 3a and 3h were at 394 nm (Fig. 3) and 450 nm (Fig. 4), respectively. However, in ethanol/water (ethanol/water, v/v = 1/1), they were red-shifted to 423 nm (3a) and 473 nm (3h), respectively. The red-shift of the emission band might be due to the stronger interaction between the solvent and the excited state molecules. The excited state of 3a was more stabilized in polar solvents than in non-polar solvents. This led to a red shift of the emission with increasing solvent polarity [10,29]. Therefore, both compounds 3a and 3h exhibited a broad shape of the emission spectra and a solvent dependence of the emission [30,31]. The emission of 3a was blue-shifted to 394 nm compared with the emission at 423 nm in the solution, so that the emitting color was purple (Fig. 3 and Table 2). However, the emission of 3h, blue-shifted to 450 nm, can be compared to the emission at 473 nm in the solution, which was nicely in the range of blue light when excited at 300 nm (Fig. 4 and Table 2). Moreover, in the solvents with different polarities, the red-shift was about 49–57 nm when comparing the emission of 3h with that of 3a (Figs. 3 and 4, Table 2). The reason was that 3h with styryl exhibited a longer conjugated chain than 3a with the benzene ring. However, 3a and 3h were respectively red-shifted to 473 nm and 506 nm in the 0.1 N H2SO4 aqueous solution containing 0.5 mL of dissolved CH3OH (Table 1). The reason might be that the 0.1 N H2SO4 aqueous solution with 0.5 mL of dissolved CH3OH exhibited higher polarity and lower pH than the ethanol/water solvent (v/v = 1/1).
4 Conclusion
A series of novel 1,2,4,5-tetrasubstituted imidazoles containing furan rings were successfully synthesized using 2-R-4,5-di(furan-2-yl)imidazoles and a series of benzyl chloride and allyl chloride. The luminescent properties of the synthesized compounds were studied. In the 0.1 N H2SO4 aqueous solution with 0.5 mL of dissolved CH3OH, the absorption bands exhibited a red shift with increasing the long conjugation or introducing a n-electron on the 2-position of imidazole. Their maximum emission was 440–510 nm, which basically emits blue or green light with fluorescence quantum yields of 0.004–0.161 against quinine sulfate as a standard. Meanwhile, the emission of compounds 3a and 3h in solution could be tuned by varying the polarity of the solvents. Such functional compounds might possess potential applications in the detection of environments and the material science fields.


