1 Introduction
One of the most fundamental and useful reactions in organic chemistry is the reduction reaction. Reduction by alkali metal borohydrides and their modified forms has been widely used in organic synthesis [1–4]. Sodium borohydride is a mild, inexpensive and invaluable reagent for the reduction of aldehydes and ketones [4]. However, certain limitations are observed using this reagent, such as the use of polar and protic solvents, its instability, the reduction of a few functional groups, the use of a large excess of sodium borohydride, sometimes long reaction times, and low selectivity [4]. The reduction with sodium borohydride in aqueous or alcoholic solvents can be accompanied by side reactions [4,5]. In order to change the reactivity of NaBH4, various structural modifications of NaBH4 are reported in the literature [6]. There are few reports in the literature on the use of transition-metal borohydride complexes as reducing agents, because these complexes are volatile unstable solids [7]. Modified stable forms of such compounds were reported [8–10]; however, because of their instability, they should be used in freshly prepared solutions [11].
The immobilization of reagents on polymeric backbone has acquired popularity extensively because insoluble polymeric reagents increase the range of applicable solvents, and work-up as well as product purification and isolation is easy. Also, in most cases, recovery and regeneration of the supported reagent is possible [12,13]. Polymer-supported borohydride resins (PSBR) have been used as a polymer-supported reducing agent for the reduction of carbonyl groups [12]. They possess greater selectivity and stability, and a simple filtration of the reagent affords the pure product [14].
Very recently, in continuation of our studies on the development of new catalysts and methods for organic transformations [15], we wish herein to report the fact that sodium borohydride can be stabilized on poly(n-butyl-4-vinylpyridinium) chloride and can be used as an efficient polymeric reducing agent.
2 Experimental
2.1 General
Chemicals were purchased from Fluka AG, Merck and Synthetic Chemicals Ltd. Poly(4-vinylpyridine) cross-linked with 2% divinylbenzene was purchased from Fluka AG. All the reduction products were known compounds and they were identified by comparison of their spectra and physical data with those of the authentic samples [16]. Reaction monitoring and purity determination of the products were accomplished by TLC or GC–MS on an Agilent GC-Mass-6890 instrument under 70 eV conditions. IR and FT–IR spectra were obtained using a PerkinElmer spectrometer 781 and Bruker Equinox 55 using KBr pellets for solids and neat samples for liquids in the 4000–400 cm−1 range. In all cases, 1H NMR spectra were recorded with a Bruker Avance 400 or 300 MHz instrument. 13C NMR data were collected on a Bruker Avance 100 or 75 MHz instrument. All chemical shifts are quoted in parts per million (ppm) relative to TMS using a deuterated solvent. Microanalyses were performed on a PerkinElmer 240-B microanalyzer. Melting points were recorded on a Büchi B-545 apparatus in open capillary tubes.
2.2 Preparation of poly(n-butyl-4-vinylpyridinium) chloride
Cross-linked poly(4-vinylpyridine) (5.0 g) was suspended in sulpholane (50 mL) and allowed to swell for about 24 h at room temperature. To this suspension, freshly distilled n-butyl chloride (20 mL) was slowly added and the reaction mixture was stirred at 40–50 °C for 48 h. An additional portion of n-butyl chloride (5 mL) was added and stirred at the same temperature for 24 h. The precipitate was then filtered, washed with distilled water (5 × 10 mL) and diethyl ether (3 × 5 mL). The cream-colored solid, P(BVP)Cl, was dried in the presence of P4O10 under vacuum at 50 °C overnight to give 7.22 g of the product. The amount of chloride was determined by gravimetric and potentiometric titration methods [17], which was 4.2 mmol of Cl– per gram of the polymer (Scheme 1).

Preparation, use, and regeneration of poly(n-butyl-4-vinylpyridinium) borohydride.
To a solution of NaBH4 (2.12 g, 0.056 mol) in water (100 mL), P(BVP)Cl (1.0 g) was added portion wise at room temperature and stirred for 48 h. The resulting material was separated and washed with distilled water (5 × 10 mL) and diethyl ether (2 × 10 mL), then dried under a vacuum desiccator in the presence of P4O10 at 50 °C overnight to obtain a white-cream colored powder, P(BVP)BH4 (1.34 g). The capacity of the reagent, determined by hydrogen evolution on acidification with 1.0 M hydrochloric acid in a suitable hermetic apparatus and the average capacity, was found to be 2.3 mmol of BH4– per gram of the polymeric reagent. The dried resin was stored at 20 °C and the hydride content was constant over 6 weeks.
2.3 General procedure for the reduction of carbonyl compounds with P(BVP)BH4
To a solution of the substrate (1 mmol) in ethanol as a solvent (5 mL) in a round-bottomed flask (25 mL) equipped with a magnetic stirrer, P(BVP)BH4 (100 mg) was added and stirred at room temperature. The progress of the reaction was monitored by TLC. On completion of the reaction, the mixture was filtered and the used reagent was washed successively with HCl (1.0 M, 2 × 10 mL) and ethanol (2 × 5 mL). The combined filtrates were evaporated and the pure product was obtained in moderate to excellent yields. In a few cases in which the reaction was not complete, the crude product was purified on silica gel with an appropriate eluent (Scheme 1).
2.4 Regeneration of cross-linked P(BVP)BH4
The utilized polymeric reagent (5.0 g) collected from different experiments was washed with HCl (1.0 M, 2 × 10 mL), NaHCO3 (2.0 M, 4 × 10 mL) and ether (2 × 10 mL). The solid was dried under vacuum at room temperature to give the original polymer (4.94 g) as a fine precipitate, which was stirred with sodium borohydride to provide the initial polymeric reducing reagent (Scheme 1).
3 Results and discussion
3.1 Characterization of P(BVP)BH4
Fig. 1 shows the FT–IR spectra of poly(4-vinylpyridine) and poly(n-butyl-4-vinylpyridinium) borohydride. The absorbance of the pyridine ring vibration after the modification is much lower than that of the starting material, which indicates a high degree of quaternization [18]. The strong band at 1650 cm−1 due to the quaternization of the pyridine moieties appeared and the C–H aromatic bands at 2858–3030 cm−1 became very weak. The FT–IR spectra showed characteristic bands at 2400–1600 cm−1 and 1700–1600 cm−1, 1415–1300 cm−1, and 1126 cm−1, due to stretching, bridge stretching, asymmetric bridge stretching and BH2 deformation, respectively [19].
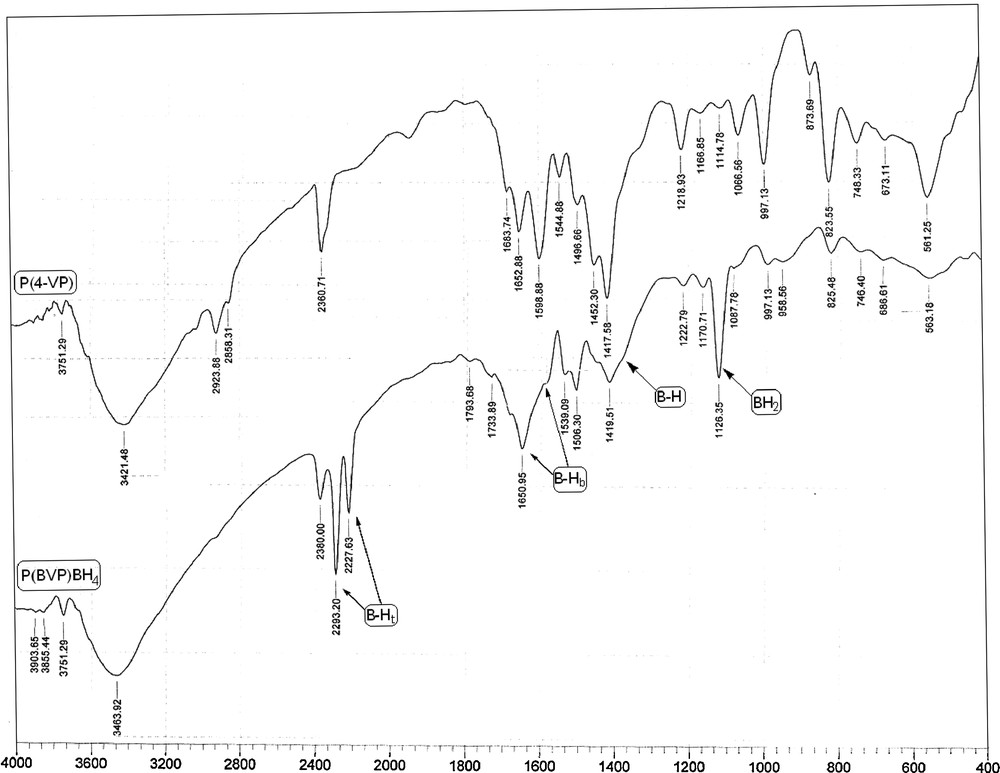
FT–IR spectra for poly(4-vinylpyridine) (top) and poly(n-butyl-4-vinylpyridinium) borohydride (bottom).
The swelling behavior of poly(n-butyl-4-vinylpyridinium) borohydride was investigated (Table 1). Polymer swelling is a thermodynamic phenomenon, and it gives a measure of the amount of liquid sorbed by the polymer under equilibrium conditions. Dry poly(n-butyl-4-vinylpyridinium) borohydride was weighed (Wd) and immersed in deionized water or 100% ethanol. P(BVP)BH4 was left in the liquids for 24 h. After that, the swollen P(BVP)BH4 was taken out and the superfluous liquid was carefully wiped with a filter paper to remove the excess of liquid on the polymer surface and then weighed (Ws). The swelling degree of the reagent was calculated using the following equation:
| S (%) = [(Ws − Wd)/Wd] × 100 |
P(BVP)BH4 swelling degree S in different solvents.
| Solvent | H2O | MeOH | EtOH | Et2O |
| S (%) | 7.5 | 2.0 | 1.6 | 0.08 |
The high degree of swelling in polar solvent, such as water, shortened the time of ion exchange for the preparation and the regeneration of P(BVP)BH4. Due to the different degree of swelling in the solvents, the solvent or mixture of solvents can be selected to control the reaction rate and to increase chemoselectivity.
The solvolytic stability of P(BVP)BH4 was investigated in 100% ethanol (pH = 5.1) and in water (pH = 6.9) (Tables 2 and 3). Blank studies for sodium borohydride were conducted. Stability measurements were obtained by observing the volume of hydrogen gas evolved due to solvolytic decomposition as a function of time. The percentage of hydride lost as a function of time was calculated from the weight of P(BVP)BH4 and the initial hydride content. The P(BVP)BH4 resin was substantially more stable solvolytically than sodium borohydride.
Stability of P(BVP)BH4 and NaBH4 in 100% ethanol at room temperature.
| Time (min) | 0 | 50 | 100 | 150 | 200 |
| Hydride loss (%) | |||||
| NaBH4 | 0.0 | 3.8 | 8.4 | 12.0 | 16.4 |
| P(BVP)BH4 | 0.0 | 1.4 | 2.5 | 3.6 | 4.4 |
Stability of P(BVP)BH4 and NaBH4 in water (pH = 6.9) at room temperature.
| Time (min) | 0 | 50 | 100 | 150 | 200 |
| Loss hydride (%) | |||||
| NaBH4 | 0.0 | 7.2 | 8.1 | 9.5 | 9.8 |
| P(BVP)BH4 | 0.0 | 0.4 | 0.6 | 0.6 | 0.6 |
3.2 Reductive activity of P(BVP)BH4
The preparation and characterization of some of the coordination complexes of poly(4-vinylpyridine) with a large number of metal salts have been reported in the literature [20]. These complexes are easily formed when alcoholic solutions of the metal salts are added to a solution or a suspension of the polymer. n-Butyl chloride was supported on cross-linked poly(4-vinylpyridine) by adding n-butyl chloride to a suspension of the polymer in sulpholane. The polymeric reagent was then obtained by an exchange reaction between the polymer-supported poly(n-butyl-4-vinylpyridinium) chloride and sodium borohydride in water. This PSBR could be stored without appreciable change in its capacity over 6 weeks. Most importantly, no new contaminants, such as Na+ and BO2–, were added to the environment, since the borate ion remains bonded to the reagent.
It is known that solvents can play an important role in the stability, reducing power, and selectivity of borohydride reagents in reduction reactions [4,20]. It is also known that for using a polymeric reagent in an organic reaction, a solvent should be chosen in which it could swell to a considerable extent [12]. Considering these facts, solvent optimization reactions for the reduction of carbonyl groups was performed using different solvents, and the best solvent was found to be ethanol. This solvent is a greener solvent than methanol. In ethanol, the polymeric reagent was completely insoluble and boron moieties before and after the reduction remained firmly bound to the insoluble polymeric support. Product isolation and purification were performed simply by filtration of the reaction mixture, evaporation of the solvent, and, if necessary, by further separation of the starting material by column chromatography.
We initially examined the reduction of benzaldehyde, acetophenone, benzophenone and cyclohexanone using NaBH4 itself, PVP·BH3 and P(BVP)BH4 (Table 4). The reductions were carried out by mixing a mixture of the aldehyde or ketones (1 mmol) and NaBH4 (1 mmol), PVP·BH3 (1 mmol) and P(BVP)BH4 (0.32–0.64) in the presence of 5 mL of ethanol at room temperature in air. The produced alcohols were isolated and the yields were determined by GC–MS analysis. As shown in Table 4, benzaldehyde was rapidly reduced to benzyl alcohol, even by NaBH4 alone. Unlike benzaldehyde, the reduction of ketones using sodium borohydride itself was not completed. For example, the reduction of acetophenone provided 1-phenylethanol in only 32% yield in 240 min, with recovery of the unreacted starting ketone in 68% yield. However, in the presence of P(BVP)BH4, reduction gives the produced alcohol in 71% yield in 240 min. This methodology was successfully applied to the reduction of benzophenone, but 1 mmol of benzophenone required 0.64 mmol of the reducing agent for complete reduction. It seems to be due to the fact that the solution of NaBH4 in ethanol is rapidly decomposed into borates, which reduce only very reactive substrates.
Reduction of benzaldehyde and acetophenone and benzophenone to their corresponding hydroxyl compounds with NaBH4, (PVP)·BH3 and P(BVP)BH4.
| Substrate | NaBH4 | PVP·BH3 | P(BVP)BH4 |
| Benzaldehyde | 99 | 24 | 92 |
| Acetophenone | 32 | 32 | 71 |
| Benzophenone | 18 | 40 | 68 |
Obviously, some functional groups were more resistant to reduction than others and, therefore, higher amounts of the reducing agent were used to obtain reasonable yields of the products in acceptable times. The chemoselective reduction of aldehydes in the presence of ketones is one of the most important transformations in organic synthesis [4]. Table 5 shows the reduction of various carbonyl compounds with P(BVP)BH4. This reagent reduced aldehydes and ketones into the primary and secondary alcohols in good to high yields, respectively (Table 5, entries 1–13). But, ketones were reduced to their corresponding alcohols using a higher reducing agent:substrate molar ratio and longer reaction times (Table 5, entries 14–18).
| Entry | Substrate | Product | Time (min) | Yield (%)c |
| 1 | 160 | 86 | ||
| 2 | 154 | 88 | ||
| 3 | 150 | 88 | ||
| 4 | 152 | 78 | ||
| 5 | 150 | 84 | ||
| 6 | 145 | 85 | ||
| 7 | 142 | 84 | ||
| 8 | 140 | 85 | ||
| 9 | 170 | 78 | ||
| 10 | 172 | 84 | ||
| 10 | 176 | 82 | ||
| 11 | 180 | 80 | ||
| 12 | 168 | 86 | ||
| 13 | 164 | 82 | ||
| 14 | 155 | 84 | ||
| 15 | 264 | 89 | ||
| 16 | 262 | 81 | ||
| 17 | 245 | 85 | ||
| 18 | 261 | 78 | ||
| 19 | 268 | 76 | ||
| 20 | 260 | 88 | ||
| 21 | 234 | 86 | ||
| 22 | 238 | 81 | ||
| 23 | 237 | 87 | ||
| 24 | 234 | 85 | ||
| 25 | 234 | 88 | ||
| 26 | 223 | 87 | ||
| 27 | 223 | 86 | ||
| 28 | 120 | 100d + 0d |
a Reaction conditions: substrate, 1.0 mmol; P(BVP)BH4, 0.32 mmol (entries 1–3, 7–14 and 28) and 0.64 mmol (entries 4–6 and 15–27); ethanol 5 mL; room temperature.
b Products were identified by comparison of their IR, NMR spectra, and physical data with those of the authentic samples.
c Isolated yield.
d GC–MS yield.
Due to the importance of synthetic precursors of allylic alcohols, the regioselective 1,2-reduction of α,β-unsaturated carbonyl compounds without disturbing the carbon–carbon double bond is synthetically very important. [4]. Generally, the 1,4-addition to α,β-unsaturated carbonyl compounds becomes equally important than the 1,2-addition in the presence of NaBH4 itself, which leads usually to a mixture of 1,2- and 1,4-product with 59% and 41% yields, respectively (Scheme 2) [21]. The polymeric reagent shows a satisfying regioselectivity in the reduction of α,β-unsaturated aldehydes as well as of ketones (Table 5, entries 12,13 and 20–22). On the other hand, in alcoholic media, NaBH4 reduces halides, while P(BVP)BH4 did not affect chlorides (Table 5, entry 25).

The regioselectivity in the reduction of 2-cyclohexen-1-one in the presence of NaBH4 and P(BVP)BH4.
It may be of interest to note that selective reduction of aldehydes in the presence of ketones is ordinarily impracticable using sodium borohydrides [22]. The chemoselectivity of this method was checked by the competitive reduction of benzaldehyde and acetophenone together. The results showed that benzaldehyde was reduced selectively in the presence of acetophenone (Table 4, entry 28). This may be considered as a useful practical achievement in the reduction of aldehydes in the presence of ketones.
Regeneration of the polymeric reagent was accomplished by a simple washing reactivation procedure in which the spent reagent was first washed with hydrochloric acid and then with NaHCO3, distilled water, and ether, to obtain the original polymer followed by an activation reaction. No change in the capacity of the regenerated reagent was observed and the loss of the original polymer during this process was negligible.
Table 6 shows the comparison of P(BVP)BH4 with the other reported polymer-supported borohydrides in the reduction of various organic functional groups [19,23,24]. As shown in many reduction reactions, the efficiency of P(BVP)BH4 is higher than those of poly(4-vinylpyridine)-supported chloroaluminum borohydride [PVP–Al(BH4)Cl2], poly(4-vinylpyridine)-supported zinc borohydride [PVP–Zn(BH4)2], and poly(4-vinylpyridine)-supported zirconium borohydride [PVP–Zr(BH4)4].
Comparison of polymer-supported poly(n-butyl-4-vinylpyridinium), chloroaluminum, zinc and zirconium borohydrides in the reduction of different organic functional groups.
| Reducing agent | Reagent:Substrate (molar ratio) | Time (min) | Yield (%)a | Reference |
| PVP–Al(BH4)Cl2 | 2.7 | 150 | 90 | [16] |
| PVP–Zn(BH4)2 | 1.0 | 900 | 75 | [17,18] |
| PVP–Zr(BH4)4 | 1.0 | 360 | 84 | [19] |
| PVP BH3 | 2.0 | 22 | 99 | [25] |
| P(BVP)BH4 | 0.32 | 172 | 84 | This work |
| PVP–Al(BH4)Cl2 | 5.00 | 480 | 75 | [16] |
| PVP–Zn(BH4)2 | 2.00 | 900 | 5 | [17,18] |
| PVP–Zr(BH4)4 | 2.00 | 720 | 80 | [19] |
| PVP·BH3 | 2.0 | 120 | 18 | [25] |
| P(BVP)BH4 | 0.64 | 261 | 88 | This work |
| PVP–Al(BH4)Cl2 | 5.00 | 1200 | 90 | [16] |
| PVP–Zn(BH4)2 | 2.00 | 2700 | 0.0 | [17,18] |
| PVP–Zr(BH4)4 | 2.00 | 1080 | 75 | [19] |
| PVP·BH3 | 2.0 | 120 | 1 | [25] |
| P(BVP)BH4 | 0.64 | 268 | 86 | This work |
| PVP–Al(BH4)Cl2 | 5.00 | 360 | 98 | [16] |
| PVP–Zn(BH4)2 | 1.00 | 720 | 90 | [17,18] |
| PVP–Zr(BH4)4 | 1.00 | 360 | 95 | [19] |
| P(BVP)BH4 | 0.32 | 168 | 96 | This work |
| PVP–Al(BH4)Cl2 | 5.00 | 360 | 96 | [16] |
| PVP–Zn(BH4)2 | 2.00 | 900 | 10 | [17,18] |
| PVP–Zr(BH4)4 | 2.00 | 900 | 85 | [19] |
| P(BVP)BH4 | 0.64 | 260 | 98 | This work |
a Isolated yields.
We found that passing the ethanol to be purified through a column containing the P(BVP)BH4 resin removes any impurity that can be reduced with borohydride ion. We observed that a contact time of 30 min is usually sufficient to remove most impurities. This procedure can be generally effective for the purification of other solvents. However, depending on the solvent or on specific impurities, it may be necessary to alter the contact time. The most important advantage of this method over NaBH4 is that the P(BVP)BH4 resin is remarkably stable in ethanol. Also, since the hydride capacity is on the order of 3.2 mmol of hydride per gram of dry resin, a small amount of P(BVP)BH4 resin will remove traces of carbonyl impurities from a substantial volume of ethanol.
4 Conclusions
Sodium borohydride stabilized on poly(n-butyl-4-vinylpyridinium) chloride is used as an efficient and regenerable polymer-supported borohydride reagent for the reduction of a variety of carbonyl compounds. In conclusion, regioselectivity, easy reaction work-up with no boron moiety in the final product solution, and good to high reaction yields make this new polymer-supported borohydride reagent useful in the field of organic synthesis. Based on the results of this study, we feel that poly(n-butyl-4-vinylpyridinium) borohydride offer several advantages in the treatment of systems where introducing ionic species is undesirable. We are continuing more detailed studies on the use of poly(n-butyl-4-vinylpyridinium) borohydride for solvent and ethanol purification.


