1 Introduction
The discovery and development of palladium-catalysed cross-coupling reactions, like Suzuki–Miyaura, Heck, Stille, Negishi and Sonogashira couplings, have become one of the hottest areas of research in modern organic chemistry [1,2]. The ability of the Suzuki–Miyaura cross-coupling reaction to create new carbon–carbon bonds is of paramount importance in medicinal and organic chemistry, in the synthesis of the potent natural anti-tumor agent “(+)-dynemicin A” and the anti-viral bromo indole alkaloid “dragmacidin F”, as well as in engineering materials, such as conducting polymers, molecular wires, and liquid crystals [3,4].
In the literature, various Pd sources have been used for the Suzuki–Miyaura cross-coupling reaction. Among them, Pd(dppf)Cl2 is a new catalyst becoming more and more popular in palladium-catalyzed cross-coupling reactions compared to other Pd complexes because it is more stable, less air-sensitive, and has a longer shelf-life [5].
In the Suzuki–Miyaura cross-coupling reactions, the base plays a crucial role in the catalytic cycle, particularly to activate the boronic acid for the trans-metallation step, and is generally used in a homogeneous form [6]. The contamination of the residual metal and other inorganic salts in the final product is a major concern in pharmaceutical industry, which need to be removed after the reaction [7]. Hence, the development and applications of solid-supported reagents and catalysts are attracting more attention in drug development, catalyst field, combinatorial chemistry, biotransformation, etc. [8]. Herein, we suggest the improved alternative to remove these drawbacks by base entrapment in agarose gel, which can be easily removed after completion of the reaction.
The development of a biodegradable and durable polymeric system of agarose became a milestone in biodegradable thermo gel research. The gel-entrapped bases are prepared by immobilizing organic/inorganic bases in aqueous gel matrix of agarose, which is a natural, easily available and biodegradable polymer, composed of repeating units of β-d-galactopyranosyl (G) and 3,6-anhydro-α-l-galactopyranosyl (LA), as shown in Fig. 1a. The three-dimensional structure of agarose in water is a double helix with a three-fold screw axis and the ability of agarose to assemble in complex bundles to form gels in aqueous solution makes it useful in numerous applications [9]. This method of immobilization reduces the amount of bases required and allows easy separation of the product from the reaction mixture. Advantages offered by gel-entrapped bases are that, they are stable in organic solvents, air stable, and can be separated easily by simple filtration from the reaction mixture, which simplifies the product isolation procedure. In addition, the contamination of inorganic salts in the reaction product is also avoided.
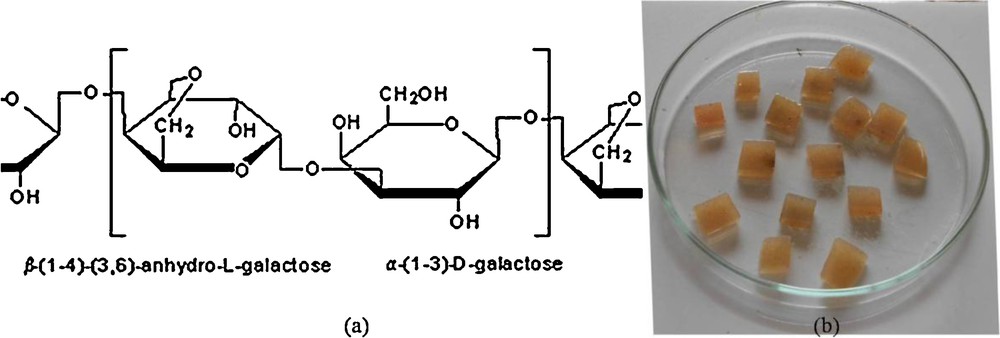
(a) Structure of agarose and (b) photograph of the gel-entrapped base.
Recent work in our group has focused on the development of gel-entrapped bases as efficient and recyclable catalysts for organic transformations [10]. The present work describes the fabrication of an aqueous gel-entrapped base and its application as an improved tool in the ligand-free Suzuki–Miyaura cross-coupling reaction.
2 Results and discussion
The gel-entrapped bases were prepared following the literature procedure [11]. During the preparation of the aqua gel, temperature plays a crucial role; preparation of the gel at low temperatures resulted in a brittle gel, while high temperatures cause coloration of the gel. The gel-entrapped bases were light yellow jelly-like substances that could be cut into cubes (Fig. 1 (b)). The resulting aqueous gels were then solvent exchanged for ethanol as reported in the literature [12]. The resulting ethanol-saturated gels were then used in Suzuki–Miyaura cross-coupling reactions. The pore in the gel, in which the base is entrapped, acts as microreactor, which traps the inorganic salts in the gel, leaving the biaryl products in the reaction solvent. It also removes residual metal from the reaction product.
Initially, 4-bromo benzophenone (1a) and phenyl boronic acid (2a) were chosen as model reaction partners to optimize the various reaction conditions, like selection of solvents, bases and catalyst loading (Scheme 1).
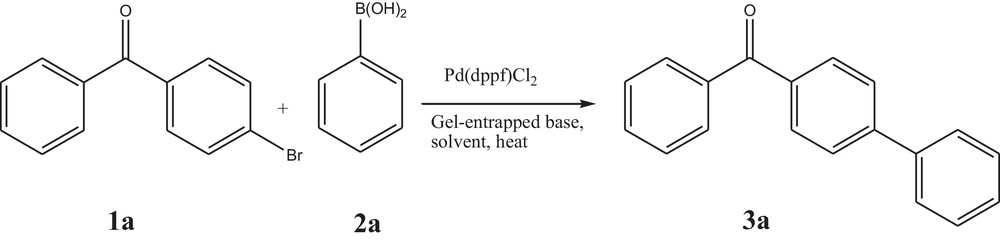
Suzuki–Miyaura cross-coupling reaction using a gel-entrapped base.
It is known that the nature of the base is an important factor for determining the efficiency of the Suzuki–Miyaura cross-coupling reaction [13]. Therefore, the influence of various bases was investigated for the model reaction. Several gel-entrapped organic as well as inorganic bases were screened and the superior reaction efficiency was observed with gel-entrapped NaOH in 95% ethanol at 80 °C (Table 1, entry 1). There are various reports emphasizing the important role played by the solvent in the reaction system, which prompted us to screen various solvents [14,15]. Screening of solvents indicated that 95% ethanol was the best one for this system (Table 1, entry 1), and that its use renders this protocol quite practical and amenable to large-scale synthesis.
Screening of various reaction conditions for the Suzuki–Miyaura cross-coupling reaction.
| Entry | Gel-entrapped base | Solvent | Time (h) | Yielda (%) |
| 1 | NaOH | 95% EtOH | 2 | 95 |
| 2 | KOH | 95% EtOH | 2 | 90 |
| 3 | DABCO | 95% EtOH | 4.5 | 69 |
| 4 | TEA | 95% EtOH | 8 | 5 |
| 5 | NaOH | THF | 3 | 83 |
| 6 | NaOH | CH2Cl2 | 6 | 75 |
| 7 | NaOH | CH3CN | 5 | 78 |
| 8 | NaOH | Toluene | 7 | 50 |
| 9 | NaOH (Solid) | 95% EtOH | 3 | 85 |
a Isolated yields after purification.
To study the effect of entrapped NaOH and compare the activity of non-entrapped NaOH, the model reaction was run under identical condition using solid NaOH, and the results revealed that the gel-entrapped NaOH showed higher activity than the non-entrapped one (Table 1, entry 9). This result demonstrates that the gel matrix may stabilize the active species, enhancing the rate of reaction.
The effect of the concentration of the Pd(dppf)Cl2 complex on the percentage conversion of the model reaction has been studied and the results are shown in Table 2. Thus, among the different loadings tested, 1 mol% proved to be the best one, affording the desired product with 95% yield using gel-entrapped NaOH in 95% EtOH (Table 2, entry 3).
Effect of the amount of Pd(dppf)Cl2 complex on the Suzuki–Miyaura cross-coupling reaction.
| Entry | Amount of catalyst (mol%) | Time (h) | Yielda (%) |
| 1 | 0.1 | 5 | 80 |
| 2 | 0.5 | 3 | 86 |
| 3 | 1.0 | 2 | 95 |
| 4 | 2.0 | 2 | 96 |
a Isolated yields after purification.
To demonstrate the scope of the gel-entrapped base, the optimized protocol [4-bromobenzophenone (1 mmol), phenyboronic acid (1.2 mmol), gel-entrapped NaOH (1 g, 2 mmol), Pd(dppf)Cl2 (1 mol%), stirred in 5 mL 95% EtOH at 80 °C, under aerobic conditions] was applied for reactions of a variety of different aryl bromides and arylboronic acids (Table 3), and all were completely converted to the corresponding products in 88–95% yields within 1–4 h. To further extend the scope of this methodology, we carried out reactions of dibromoarenes with phenyl boronic acid. The results of these reactions were of particular interest, since their outcome involves the formation of products that are extensively used in the synthesis of molecular wires. The reactions of dibromoarenes with aryl boronic acids afforded the desired products in quantitative yields (Table 3, entries 12 and 13). Diphenylanthracene, which is used as a fluorescer in a peroxyoxalate chemiluminescence system [16], was also obtained by coupling 9,10-dibromoanthracene with phenylboronic acid in 80% yield. It was found that aryl chlorides were less reactive as compared to aryl bromides (Table 3, entries 14 and 15).
The Suzuki–Miyaura cross-coupling reaction of various aryl halides and phenyl boronic acids.
| Entry | Aryl halide | Arylboronic acid | Product | Time (h) | Yielda (%) |
| 1 | 1 | 94 | |||
| 2 | 1 | 94 | |||
| 3 | 1 | 90 | |||
| 4 | 2 | 92 | |||
| 5 | 2.5 | 90 | |||
| 6 | 2 | 88 | |||
| 7 | 1.5 | 93 | |||
| 8 | 2 | 92 | |||
| 9 | 2 | 95 | |||
| 10 | 2.5 | 91 | |||
| 11 | 4 | 91 | |||
| 12 | 6 | 80 | |||
| 13 | 3 | 93 | |||
| 14 | 10 | 60 | |||
| 15 | 12 | 62 |
a Isolated yields after purification.
In gel-entrapped bases, the trapped base may diffuse or leach out into the reaction solvent. To check the possibility of leaching, the gel was stirred in ethanol under the reaction conditions, and the alkali leached out in the ethanol was estimated. It was observed that under these reaction conditions, only less than 1% of NaOH was leached out from the gel into ethanol. Using the same quantity of base as that leached out, the reaction could not be carried out, clearly indicating that the reaction was due to the intact base into the gel rather than to the leached one.
3 Conclusion
In summary, this is the first report demonstrating the utility of the gel-entrapped base system in the Suzuki–Miyaura cross-coupling reaction of a variety of mono- and dibromoarenes. The agar-agar biopolymer substrate is easily available, relatively cheap, non-toxic, biodegradable, and non-corrosive. Moreover, an air-stable gel-entrapped base is easy to separate from the reaction mixture by simple filtration, with negligible base leaching, and hence, shows to be a very promising support for the immobilization of the base.
4 Experimental
4.1 General remarks
1H NMR and 13C NMR spectra were recorded on a Bruker AC (300 MHz for 1H NMR and 75 MHz for 13C NMR) spectrometer using CDCl3 as the solvent and tetramethylsilane (TMS) as an internal standard. Melting points were determined with a DBK melting point apparatus and are uncorrected. All the chemicals were obtained from Aldrich, Spectrochem and were used without further purification.
4.2 Preparation of gel-entrapped base
To a boiling mixture of agar-agar (5 g) in H2O (30 mL), a solution of the NaOH (1 g) in H2O (2 mL) was added. The resultant solution was boiled under stirring for 5 min, and cooled at room temperature to form a gel, which was cut into small cubes. The amount of base in 1 g of gel was calculated by titration with standard acid and it was found to be 2 mmol for NaOH.
4.3 General procedure for the Suzuki–Miyaura reaction
An oven-dried Schlenk flask, equipped with a magnetic stir bar, a septum and a condenser was charged with aryl halide (1.0 mmol), arylboronic acid (1.2 mmol), the gel-entrapped base (1 g, 2 mmol), Pd(dppf)Cl2 (0.0085 g, 1 mol%) and 5 mL of 95% ethanol. The flask was immersed and stirred in an oil bath at 80 °C. Upon complete consumption of starting materials as determined by TLC analysis, the gel was separated by filtration and water (10 mL) was added. The filtrate was extracted with diethyl ether (3 × 5 mL). The combined organic layer was collected, dried over anhydrous Na2SO4 and concentrated under vacuum to afford the product, which was purified by silica gel column chromatography (n-hexane:ethyl acetate, 9:1).
4.4 Spectral data for selected compounds
4.4.1 4-Methyl-1,1′,4′.1′′-terphenyl
(White solid, mp 323–324 °C);1H NMR (CDCl3, 300 MHz): δH (ppm) 2.43 (s, 3H,); 7.25–7.28 (m, 2H); 7.30–7.35 (m, 1H); 7.45–7.47 (m, 2H). 7.54–7.55 (m, 1H); 7.60–7.68 (m, 4H), 13C NMR (CDCl3, 75 MHz): δC (ppm) 21.17, 126.80, 127.07, 127.15, 127.72, 127.34, 128.57, 129.57, 136.29, 137.68, 139.48, 140.67, 140.22.
4.4.2 4-(4′-Fluoro-phenyl) benzophenone
(White solid, mp 147–148 °C); 1H NMR (CDCl3, 300 MHz): δH (ppm) 7.00–7.21 (m, 2H); 7.38–7.53 (m, 2H); 7.45–7.67 (m, 5H); 7.72 (d, 2H, J = 6.9 Hz); 7.93 (d, 2H, J = 8.2 Hz),13C NMR (CDCl3,75 MHz): δC (ppm) 114.07, 127.75, 128.27, 128.85, 128.90, 129.94, 130.70, 135.26, 136.14, 136.28, 137.71, 144.10.
4.4.3 2-Phenyl-9H-fluorene
(White solid, mp 296–297 °C); 1H NMR (CDCl3, 300 MHz): δH (ppm) 3.97 (s, 2H), 7.30–7.35 (m, 2H); 7.41–7.46 (m, 2H). 7.54–7.64 (m, 4H); 7.78 (dd, 2H, J = 7.0 Hz) 7.82 (s, 1H); 13C NMR (CDCl3, 75 MHz): δC (ppm) 36.93, 119.91, 120.02, 122.76, 124.93, 125.89, 126.70, 126.78, 127.06, 127.14, 128.64, 139.92, 140.95, 141.34, 141.45, 142.32, 143.66.


