1 Introduction
Polyoxometalates (POMs) constitute a diverse class of inorganic oxo-metal clusters composed of early transition metals in their highest oxidation state [1]. They have a great deal of structural diversity and various applications in different areas, including catalysis, material sciences, medicine, and biology. Several types have been characterized, and each of them is defined by the M/X ratio used in the polycondensation under acidic conditions. The two best-known heteropolyanions are the Keggin-type [Xn+M12O40](8–n)– [2] and the Wells–Dawson type [(Xn+)2M18O62](16–2n)– [3] (Fig. 1).
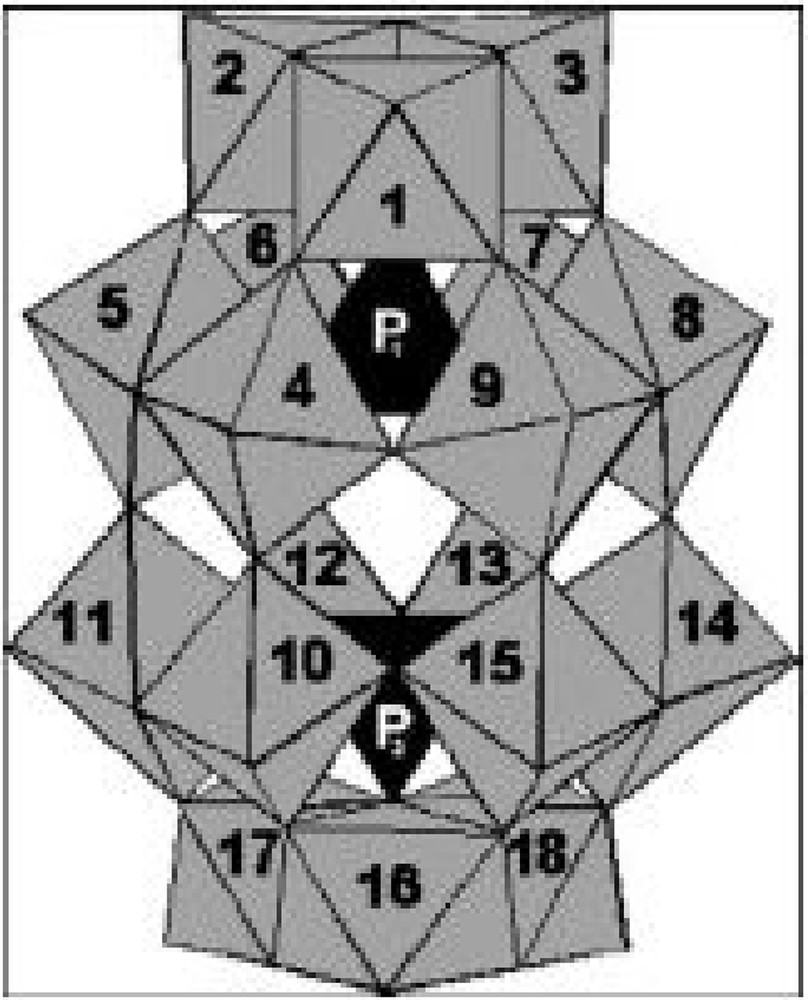
Polyhedral representation of the polyanion Dawson structure.
Polyoxometalltes with Dawson structure may be promising catalysts in homogeneous and heterogeneous systems because their redox and acidic properties can be controlled at atomic and molecular levels. They are used not only in acid-catalyzed [4] reactions, but also in many oxidations of organic compounds [5,6]. Recently, the Dawson-type Fe(III)-substituted heteropolyanion (α2P2W12Mo5O61Fe)7– was successfully used as a catalyst for the oxidation of methyl orange dye (MO) by H2O2 in aqueous solution [7]. This compound was synthesized by addition of iron on the lacunary heteropolyanion (α2P2W12Mo5O62)10– [8]. In this work, we have synthesized and characterized new heteropolyanions that contain iron HFe2.5P2W18O62, 23H2O and HFe2.5P2W12Mo6O62, 22H2O, by addition of Fe3+ ions to the Dawson acid forms H6P2W18O6224H2O or H6P2W12-Mo6O6224H2O.
The catalytic activity of these compounds was evaluated through the oxidation of an aqueous dye, methyl violet (its structure is shown in Fig. 2) [9,10] by hydrogen peroxide.
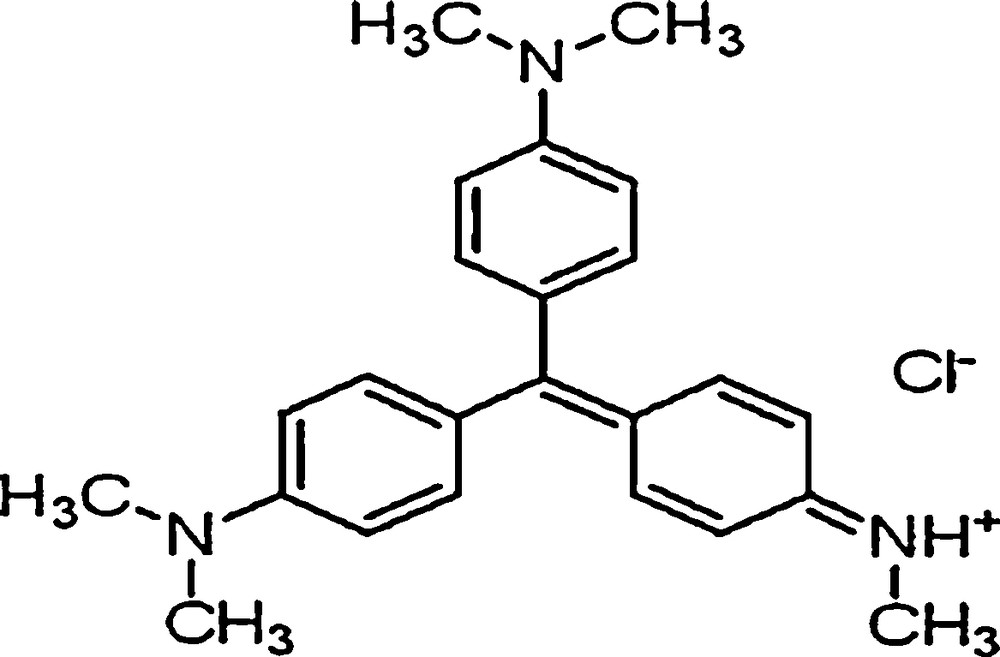
Chemical structure of methyl violet.
Methyl violet (MV), of molecular formula C24H28ClN3, is a triphenylmethane dye, soluble in water, ethanol, methanol, diethylene glycol and dipropylene glycol. It is a dark green powder [11]. Methyl violets are the mixture of tetramethyl, pentamethyl, hexamethyl, and pararosanilines. These dyes are chiefly used in heterogray and printing inks. They impart a deep violet colour in paint and printing ink. They are also used to obtain shades of deep colours that can be applied for the dyeing of cotton, silk, paper, bamboo, weed, straw and leather. Methyl violet dye is widely used in analytical chemistry laboratories as a pH indicator to test pH ranges from 0 to 1.6. The toxic information reveals that the dye may cause hard skin and eye irritation – it means that in case of physical contact with the dye, this causes an irritation with redness and pain. The dye is harmful if it is swallowed. Also, the inhalation of methyl violet may cause an irritation to the respiratory tract, whereas its ingestion causes an irritation to gastrointestinal tract [12]. Therefore, it is necessary to remove it from the waste water. The influence of different parameters such as initial pH, initial H2O2 concentration, catalyst mass, and initial dye concentration has also been studied.
2 Experimental part
2.1 Preparation and characterization of catalysts
The heteropolyanions precursors H6P2W18O62 24H2O and H6P2W12Mo6O62 24H2O, as well as their acids forms, were synthesized according to the published procedures [13,14], and their purity was confirmed by infrared and NMR 31P spectroscopies. IR spectra were recorded on KBr pellets using a spectrophotometer Shimadzu FTIR-8400s. 31P NMR spectra were recorded on a Bruker 2000 apparatus operating at 110 MHz in the Fourier-transform mode. The 31P shifts were measured in a 10−3 M solution of polyanions in a D2O solution and were referenced to H3PO4 85%.
Cyclic voltammetry experiments were performed on an EDAQ e-corder 401 potentiostat. All experiments were carried out using a three-electrode cell configuration with a glassy carbon working electrode, a saturated calomel reference electrode (SCE), and a platinum auxiliary electrode.
All experimental solutions were de-aerated thoroughly by bubbling pure N2 into the solutions for 10 min. All cyclic voltammograms were recorded at a scan rate of 50 mV s−1. All experiments were performed at room temperature.
HFe1,5P2W18O61 23H2O (1): 5 g (1.088 mmol) of H6P2W18O62 were dissolved in 20 ml of water at room temperature and 0.767 g of solid FeCl2, 6H2O (3.26 mmol) was then added. The mixture was then stirred for 10 min. A yellow powder of (1) was obtained after five days by slow evaporation. IR (KBr pellet, cm−1): 1092(s), 1025(w), 960(s), 909(s).NMR of (1): 31P δ = –12.43 ppm. Anal. calcd. (found): P 1.27 (1.20); W 68.10 (62.05); Fe 1.72 (2.01).
HFe1,5P2W12Mo6O6122H2O (2): 5 g (1.2 mmol) of H6P2W12Mo6O62 were dissolved in 20 ml of water at room temperature and 0,541 g (3.56 mmol) of solid FeCl2 6H2O was then added. The mixture was stirred for 10 min. A dark yellow powder of (2) was obtained after five days by slow evaporation. IR (KBr pellet, cm−1): 1084(s), 1025(w), 953(s), 912(s). NMR of (2): 31P δ = –8.82 ppm. Anal. calcd. (found): P 2.13(1.87); W 76.08(74.03); Mo 19.80(18.70); Fe 1.92(3.01).
2.2 Procedure for catalytic oxidations
2.2.1 Reagents
Methyl violet (MV) was supplied by Merck. H2O2 was purchased from Aldrich. All other reagents (NaOH or H2SO4) used in this study were of analytical grade.
2.2.2 Procedure – analysis
The initial concentration of the MV solution was 10 mg/L for all experiments, except for those carried out to examine the effect of the initial dye concentration. In all experiments, 100 mL of MV solution containing the appropriate quantity of catalyst and H2O2 was magnetically stirred at room temperature.
The pH of the reaction was adjusted by using 0.1 N H2SO4 or NaOH aqueous solutions.
The MV concentration was measured by a Jenway UV–vis spectrophotometer. The wavelength that corresponds to the maximum absorbance is λmax = 585 nm. The resolutions of the wavelength and the bandwidth were 1 nm and 0.5 nm. The cell used during the experiments was made of 1-cm-thick quartz.
3 Results and discussion
3.1 Preparation and characterization of catalysts
The addition of Fe3+ ions to Dawson acid forms H6P2W18O62, 24H2O or H6P2W12Mo6O62, 24H2O in an aqueous medium, with a stoichiometric (3:1) amount, leads to a substitution of some protons by Fe3+ ions yielding powder compounds HFe2.5P2W18O62, 22 H2O (1) and HFe2.5P2W12Mo6O62, 23H2O (2).
The IR spectrum of the acid Wells–Dawson compound H6P2W18O6224H2O is characterized by the elongation bands of P–O at 1090 cm−1 and W–O terminal band, inter and intra W–O–W at 960, 914 and 769 cm−1, respectively [15]. However, the incorporation of transition ions in the structure of the two Dawson acids H6P2W18O62, 24H2O and H6P2W12-Mo6O62, 24H2O generates a small displacement of these bands. The selected IR data are presented in Table 1.
Selected IR data of heteropolyanions.
| POM | νas(P–Oa) | νas(W–Od) | M–Ob–M | M–OcM |
| H6P2W18O62 | 1090 | 962 | 914 | 769 |
| HFe2.5P2W18O62 | 1092 | 960 | 909 | 784 |
| H6P2 W12 Mo6O62 | 1084 | 955 | 914 | 789 |
| HFe2.5P2W12Mo6O62 | 1084 | 953 | 912 | 789 |
It is well known that phosphorus NMR is an appropriate and powerful way to check the purity of the product. Phosphorous NMR spectra of (1) and (2) (Fig. 3) reveal a virtually pure product with a single resonance peak at δ = –12.43 ppm and δ = –8.82 ppm, respectively. This means that the two phosphorus atoms are equivalent.

31P NMR spectra of a: compound (1); b: compound (2).
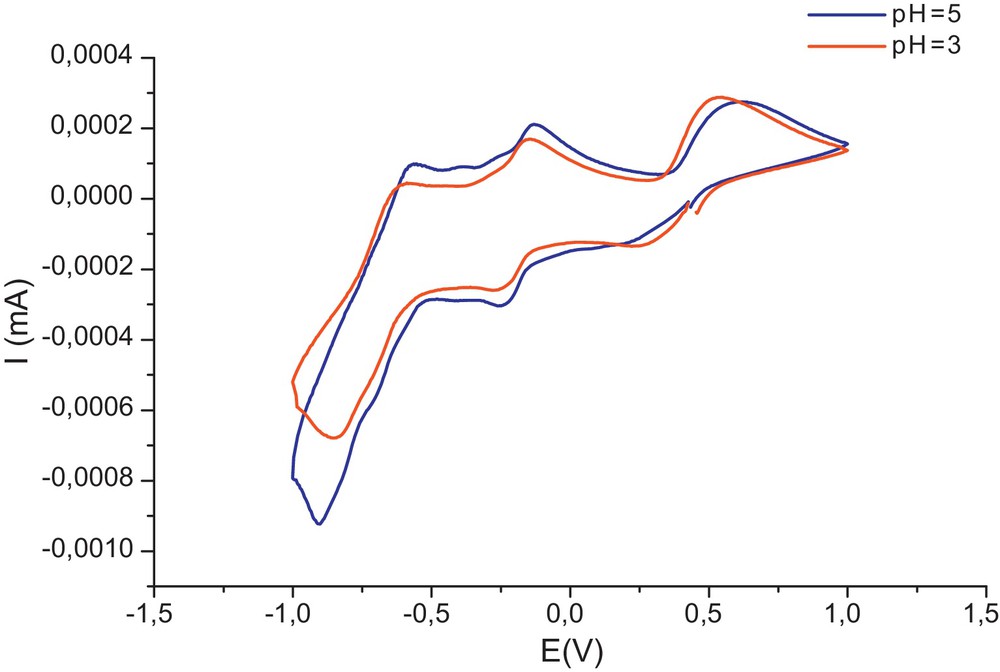
According to the voltammogram of the compound HFe2.5P2W18O62, 22 H2O, we observe five redox systems. The first peak is situated at +0.77 V/ECS; it appears in different voltammograms of heteropolyanionic complexes that contain Fe3+. Example: (α2-P2W17O61Fe)7–and (α1-P2W17O61Fe)7–[16], (Fe3P2W15O59)9–[17]. It is attributed to the reduction of an electron iron (III) into Fe (II): (Fe3+ + 1e– → Fe2+). The other peaks that appeared in 0,1, –0,07, –0,4, and 0,7 V/ECS are attributed to tungsten reduction.
According to the voltammogram of the compound HFe2.5P2W12Mo6O62, 23H2O, we can deduce that the introduction of iron in the compound H6P2W12-Mo6O62, 24H2O makes the last stage of tungsten reduction disappear.
3.2 Catalytic oxidation
Factors that influence the oxidation reaction of methyl violet are:
- • the initial pH of the solution;
- • the mass of the catalyst, Cat 1: (HFe2.5P2W18O61, 23H2O) or Cat 2: (HFe2.5P2W12Mo6O61, 22H2O);
- • the concentration of H2O2;
- • the initial concentration of the solution of MV.
The oxidation efficiency (discolouration), which was determined as shown below [18]:
| (1) |
DE: discolouration efficiency, Ci: initial concentration of MV, Cf: final concentration of MV.
3.2.1 Effect of solution pH
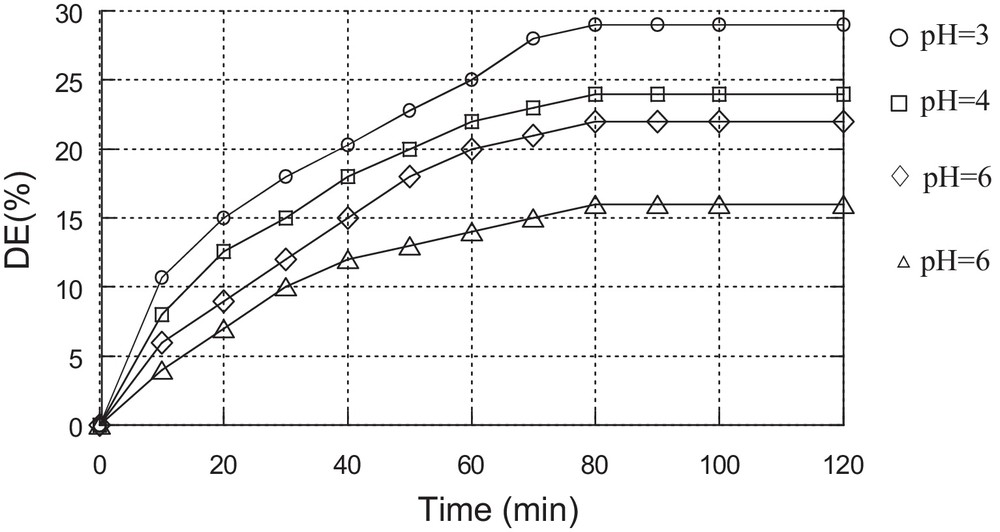
The effect of pH on the discolouration efficiency was investigated by varying the pH from 3 to 10. For more acidic pHs, there is a risk of dimerization of the catalyst, while for pHs above 10, the catalyst is likely to deteriorate. The obtained results are shown in Fig. 4.

Voltammograms of HFe2.5P2W18O62, 22 H2O at pH = 3 and pH = 5.
The optimum results were of found to be obtained at a pH of about 3 using the two catalysts. ED= 40% using catalyst Cat1 and ED = 49% using catalyst Cat2.
This result can be explained by the stability of the catalyst at this pH. Also, it has been shown that the catalytic efficiency of the Fe3+/H2O2 system towards the oxidation of organic dyes is better at pH = 3 than at other pH values [19]. H2O2 molecules are unstable in alkaline solution, and therefore dye degradation decreases in alkaline solution [20]. The stability of heteropolyanions is also affected by a modification of the pH and they can decompose at alkaline pHs [21].
3.2.2 Effect of the mass of the catalyst
The effect of the mass of the catalyst on MV oxidation by H2O2, using Cat1 or Cat2, was investigated. The results are displayed in Fig. 5.
Voltammograms of HFe2.5P2W12Mo6O62, 23H2O at pH = 3 and pH = 5.
These results show that the best discolouration efficiency (ED = 48%) is obtained for a mass of catalyst equal to 0.02 g using catalyst Cat1 and 0.01 g (ED = 61%) using catalyst Cat2. Beyond these masses, discolouration efficiency decreases.
This result is mentioned in the literature [22]. It can be explained by the presence of a secondary reaction consuming the hydroxyl radicals.
3.2.3 Effect of H2O2 concentration
Experiments were conducted to determine the effect of H2O2 concentration on the MV oxidation using Dawson type heteropolyanions as catalysts; the obtained results are displayed in Figs. 6 and 7.
Effect of the solution pH on MV oxidation using Cat1 as the catalyst (C0 = 10 mg/L, mcat = 0.05 g, [H2O2] = 0.25 mM, T = 25 °C).
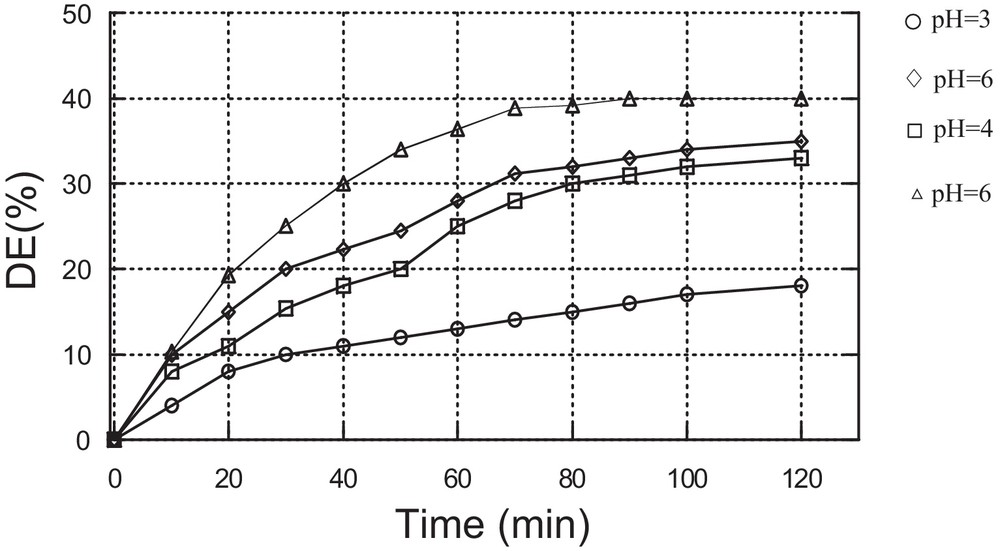
Effect of the solution pH on MV oxidation using Cat2 as the catalyst (C0 = 10 mg/L, mcat = 0.05 g, [H2O2] = 0.25 mM, T = 25 °C).
It was found that the best discolouration efficiency is obtained for a concentration of H2O2 equal to 0.25 mM using catalyst Cat1 (ED = 48%) and 0.4 mM using catalyst Cat2 (ED = 63%).
Generally, the degradation rate of organic compounds increases as H2O2 concentration increases until a critical H2O2 concentration is achieved. However, when a concentration higher than the critical one is used, the degradation rate of organic compounds decreases; this result is called the scavenging effect [23,24]. The reaction of H2O2 and OH• in aqueous solution can be expressed by the following equation [25]:
| (2) |
According to the above results, the critical H2O2 concentration for the degradation of 10 mg/L of MV dye is about 0.25 mM using Cat1 as the catalyst and 0.4 mM using Cat2 as the catalyst.
3.2.4 Effect of methyl violet concentration
The study of the methyl violet dye initial concentration effect on discolouration efficiency was carried out for concentrations ranging from 2 mg/L to 30 mg/L. The results are presented in Figs. 8 and 9. The best result was found for a MV concentration equal to 5 mg/L using catalyst Cat1 (ED = 80%) and equal to 2 mg/L using catalyst Cat2 (ED = 91%) (Figs. 10–13).
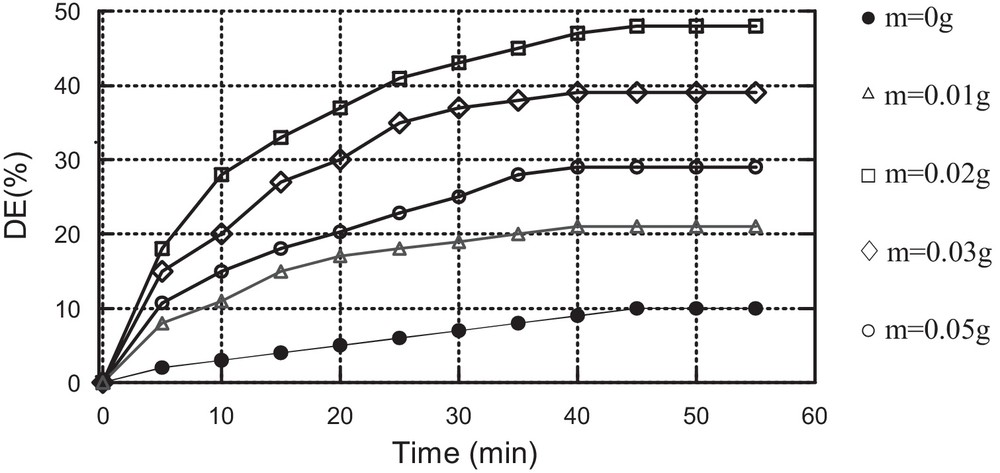
Effect of the mass of the catalyst on MV oxidation using Cat1 as the catalyst (C0 = 10 mg/L, [H2O2] = 0,25 mM, pH = 3, T = 25 °C).
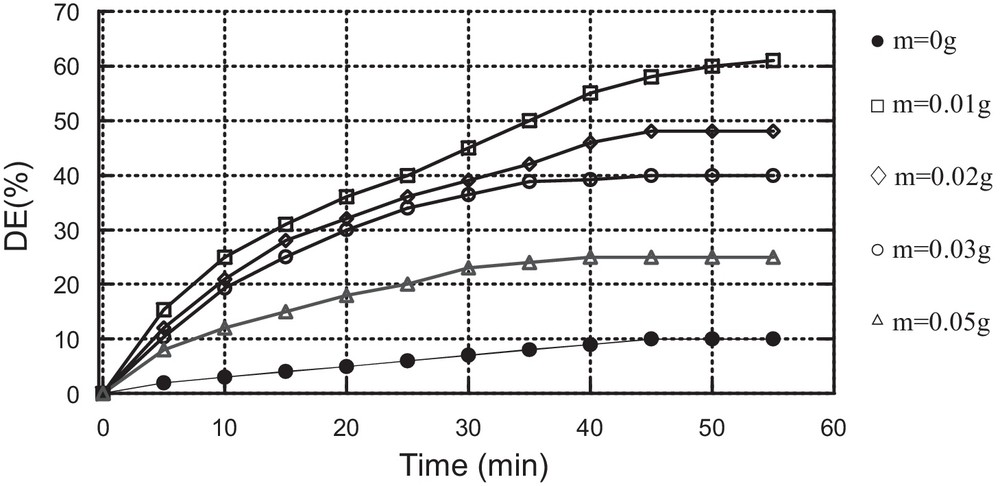
Effect of the mass of the catalyst on MV oxidation using Cat2 as the catalyst (C0 = 10 mg/L, [H2O2] = 0.25 mM, pH = 3, T = 25 °C).

Effect of the initial H2O2 concentration on MV oxidation using Cat1 as the catalyst (C0 = 10 mg/L, mCat1 = 0.02 g, pH = 3, T = 25 °C).
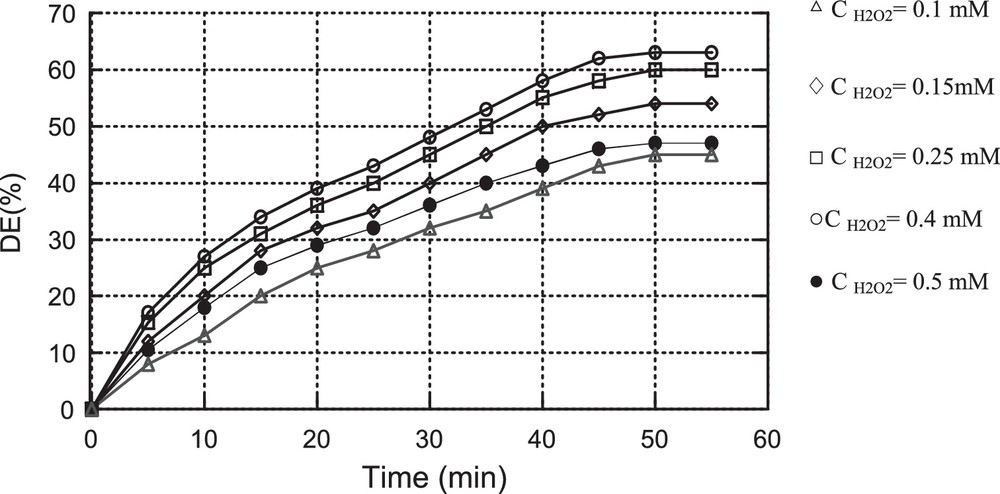
Effect of the initial H2O2 concentration on MV oxidation using Cat2 as the catalyst (C0 = 10 mg/L, mCat2 = 0.01 g, pH = 3, T = 25 °C).

Effect of the initial dye concentration on MV oxidation using Cat1 as the catalyst (mCat1 = 0.02 g, pH = 3, [H2O2] = 0.25 mM, T = 25 °C).

Effect of the initial dye concentration on MV oxidation using Cat2 as the catalyst (mCat2 = 0.01 g, pH = 3, T = 25 °C).
4 Conclusion
A new iron substituted heteropolyanion with general formula [HP2W18–nMonO62]Fe2.5, xH2O, n = 0, 6 have been synthesized and characterized by IR and 31P RMN spectroscopies.
Elementary analysis confirms the mentioned metals’ insertion into the polytunstic and polymolybdotunstic matrix.
The Dawson entity was confirmed by IR spectroscopy for all synthesized heteropolyanions through the appearance of the characteristic peaks.
RMN confirms the synthesized compounds’ purity.
Cyclic voltammetry shows that the iron is present in the Dawson structure.
Approximately 80% of the dye has been eliminated after 40 minutes under the following operating conditions: pH: 3, [MV]0 = 5 mg/L, catalyst (Cat 1) mass: 0.02 g, [H2O2]0 = 0.25 mM. And approximately 91% of the dye has been eliminated after 80 min with the following operating conditions: pH: 3, [MV]0 = 2 mg/L, catalyst (Cat 2) mass: 0.01 g, [H2O2]0 = 0.4 mM.
This system constitutes a simple and effective method compared to those previously reported for the oxidation of methyl violet using a commercial catalyst [26].


