1 Introduction
The development of simple synthetic routes for complex organic molecules from readily available reagents is an important task in organic synthesis [1]. Multicomponent reactions (MCRs) are significant tools for the rapid and efficient synthesis of a wide variety of organic molecules [2]. These reactions have been investigated extensively in organic and diversely oriented synthesis; this is primarily due to their ability to generate complex molecular functionalities from simple starting materials via a one-pot reaction.
In recent years, solvent-free organic reactions [3–6] have captured great interest because of their many advantages such as high efficiency and selectivity, easy separation and purification, mild reaction conditions, reduction in waste, and benefit to the industry as well as to the environment. Solvent-free organic reactions based on grinding two macroscopic particles together mostly involve the formation of a liquid phase prior to the reaction, that is, the formation of an eutectic melt of uniform distribution, where the reacting components, being in proximity, are poised to react in a controlled way [7]. The possibility of performing multicomponent reactions under solvent-free conditions with a heterogeneous catalyst could enhance their efficiency from an economic as well as an ecological point of view [8].
Pyrimidine is an important heterocycle with a variety of biological activities. Azaheterocycles constitute a very important class of compounds. In particular, pyrimidine derivatives include a large number of natural products, pharmaceuticals, and functional materials (Fig. 1) [9–12]. Several examples of pharmaceutically important compounds include trimethoprim [13] and Gleevec (imatinib mesylate) [14]. Natural and unnatural polymers also contain pyrimidine derivatives [15–17]. While development of important methodologies for the synthesis of pyrimidines enjoys a rich history, the discovery of new strategies for the convergent synthesis of pyrimidines remains a vibrant area of chemical research.
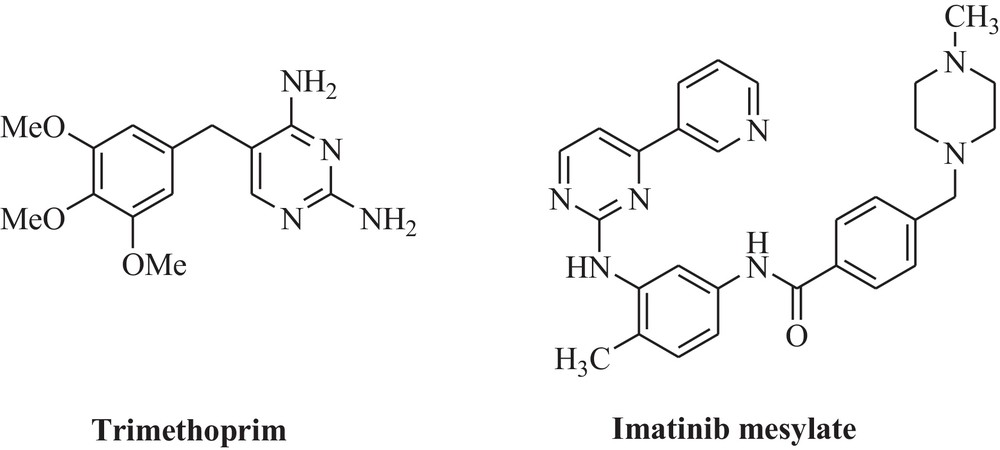
Examples of biologically active pyrimidines.
Many methods are available for the synthesis of the pyrimidine ring system. The most common method used involves the reaction of a 1, 3-dicarbonyl component with a reagent bearing an N–C–N fragment such as urea [18], amidine [19], or guanidine [20]. In recent years, ZnCl2 [21], and TsOH [22] have been utilized for this synthesis. Thus, the synthesis of pyrimidine is an important and useful task in organic chemistry.
2 Experimental
All commercially available chemicals were obtained from Merck and Fluka companies, and used without further purification unless otherwise stated. Nuclear magnetic resonance 1H and 13C NMR spectra (Sharif University and Urmia University) were recorded on Bruker Avance 300 and 500 MHz FT NMR spectrometers. Infrared (IR) spectroscopy was conducted on a PerkinElmer GX FT–IR spectrometer. Mass spectra were recorded on a Shimadzu QP 1100 BX Mass Spectrometer. Elemental analyses (C, H, N) were performed with a Heraeus CHN-O-Rapid analyzer (University of Tarbiatmoallem, Tehran).
2.1 General procedure for synthesis of pyrimidine
A mixture of triethoxymethane (3 mmol), ammonium acetate (2 mmol), ketone (1 mmol), and TBBDA (0.1 g, 18 mol %) was heated at 100–110 °C for the appropriate time. After completion of the reaction (TLC acetone/n-hexane [2:10]), the mixture was cooled, and cold CH2Cl2 (15 mL) was added, then the catalyst was removed by filtration. The organic phase was washed with water (3 × 10 mL), dried and concentrated. The product was purified by TLC (TLC acetone/n-hexane [2:10]).
2.2 Physical and spectroscopic data
2.2.1 Spectra data of 4-phenylpyrimidine
A pale yellow powder (66%); mp 57–60 °C (57.8–58.5 °C) [12]; Rf (17% acetone/n-hexane) 0.26; IR (KBr) (vmax, cm−1) 1603, 1577, 1541; δH (500 MHz, CDCl3) 7.56–7.59 (3H, m), 7.80 (1H, dd, J = 5.34, 1.17 Hz), 8.14–8.16 (2H, m), 8.82 (1H, d, J = 5.35 Hz), 9.33 (1H, s); δC (75 MHz, DMSO); 118.2, 121.9, 126.1, 131.2, 133.7, 159.1, 159.4, 160.7 (Table 2, entry 1).
Synthesis of pyrimidines using TBBDA under solvent-free conditions.
| Entry | Substrate | aProduct | TBBDA | |
| Time (min) | Yield (%) | |||
| 1 | 13 | 66 | ||
| 2 | 12 | 66 | ||
| 3 | 12 | 61 | ||
| 4 | 15 | 54 | ||
| 5 | 12 | 55 | ||
| 6 | 15 | 51 | ||
| 7 | 16 | 52 | ||
| 8 | 15 | 49 | ||
| 9 | 13 | 64 | ||
| 10 | 16 | 55 | ||
| 11 | 7 | 70 | ||
| 12 | 9 | 65 | ||
| 13 | 8 | 58 | ||
| 14 | 8 | 56 |
a Known products were characterized from their physical properties, by comparison with authentic samples, and by spectroscopic methods.
2.2.2 Spectra data of 4-(4-bromophenyl)pyrimidine
A pale yellow powder (66%); mp 71–73 °C [13]; Rf (17% acetone/n-hexane) 0.21; IR (KBr) (vmax, cm−1) 1590, 1574, 1538; δH (500 MHz, CDCl3) 7.68 (2H, d, J = 8.54 Hz), 7.73 (1H, dd, J = 5.36, 1.07 Hz), 8.01 (2H, d, J = 8.54 Hz), 8.82 (1H, d, J = 5.36 Hz), 9.30 (1H, s); δC (125 MHz, CDCl3); 117.1. 126.4, 129.4, 132.7, 135.7, 157.8, 159.4, 163.3 (Table 2, entry 2).
2.2.3 Spectra data of 4-(4-chlorophenyl)pyrimidine
A pale yellow powder (61%); mp72–74 °C (75.9–76.8 °C)[12]; Rf (17% acetone/n-hexane) 0.19; IR (KBr) (vmax, cm−1) 1594, 1578, 1539; δH (500 MHz, DMSO) 7.63 (2H, d, J = 8.57 Hz), 8.13 (1H, d, J = 5.29 Hz), 8.25 (2H, d, J = 8.55 Hz), 8.88 (1H, d, J = 5.36 Hz), 9.26 (1H, s); δC (125 MHz, DMSO); 118.1, 129.6, 130.0, 135.6, 136.9, 159.1, 159.6, 162.2 (Table 2, entry 3).
2.2.4 Spectra data of 4-(4-fluorophenyl)pyrimidine
A colorless crystal (54%); mp 75–76 °C; Rf (17% acetone/n-hexane) 0.21; IR (KBr) (vmax, cm−1) 1601, 1581, 1542; δH (500 MHz, CDCl3) 7.25 (2H, t, J = 11.55 Hz), 7.75 (1H, dd, J = 5.36, 0.93 Hz), 8.15–8.19 (2H, m), 8.83 (1H, d, J = 5.38 Hz), 9.31 (1H, s); δC (125 MHz, CDCl3); 116.5, 116.7, 117.0, 129.7, 129.8, 157.4, 159.0, 163.6, 166.3. (Found: C, 69.08; H, 4.04; N, 16.34. C10H7N2F requires C, 68.96; H, 4.05; N, 16.08%) (Table 2, entry 4).
2.2.5 Spectra data of 4-(4-nitrophenyl)pyrimidine
Yellow powder (55%); mp 100–102 °C; Rf (17% acetone/n-hexane) 0.22; IR (KBr) (vmax, cm−1)1603, 1577, 1546, 1521, 1349; δH (500 MHz, CDCl3) 7.85 (1H, dd, J = 5.26, 0.97 Hz), 8.33 (2H, d, J = 7.13 Hz), 8.42 (2H, d, J = 7.13 Hz), 8.94 (1H, d, J = 5.19 Hz), 9.40 (1H, s); δC (125 MHz, CDCl3); 118.0, 124.6, 128.6, 142.6, 158.4, 159.7, 161.9 (Table 2, entry 5).
2.2.6 Spectra data of 4-p-tolylpyrimidine
A pale yellow powder (51%); mp 62–63 °C; Rf (17% acetone/n-hexane) 0.27; IR (KBr) (vmax, cm−1) 2920, 1611, 1579, 1540; δH (500 MHz, DMSO) 2.39 (3H, s) 7.37 (2H, d, J = 7.8 Hz), 8.06 (1H, d, J = 5.4 Hz), 8.12 (2H, d, J = 7.76 Hz), 8.82 (1H, d, J = 5.36 Hz), 9.2 (1H, s); δC (125 MHz, DMSO); 21.8, 117.6, 127.7, 130.5, 134.0, 142.1, 158.7, 159.5, 163.3. [Found: C, 77.70; H, 5.91; N, 16.29. C13H14N2 requires C, 77.62; H, 5.92; N, 16.46%]; MS, m/z (%): 170 (M+, 100), 115 (55), 91 (30), 79 (25), 39 (40) (Table 2, entry 6).
2.2.7 Spectra data of 4-(4-methoxyphenyl)pyrimidine
A pale yellow powder (52%); mp 77–79 °C (79.7–80.3 °C) [12]; Rf (17% acetone/n-hexane) 0.19; IR (KBr) (vmax, cm−1) 1607, 1579, 1540; δH (500 MHz, CDCl3) 3.91 (3H, s), 7.05 (2H, d, J = 8.7 Hz), 7.67 (1H, d, J = 5.08 Hz), 8.10 (2H, d, J = 8.7 Hz), 8.73 (1H, d, J = 5.11 Hz), 9.24 (1H, s); δC (125 MHz, CDCl3); 55.8, 114.8, 116.5, 129.1, 129.2, 157.5, 159.4, 162.6, 163.8 (Table 2, entry 7).
2.2.8 Spectra data of 4-(4-isopropylphenyl)pyrimidine
A pale yellow powder (49%); mp 45–49 °C; Rf (17% acetone/n-hexane) 0.27; IR (KBr) (vmax, cm−1) 2962, 1609, 1578, 1540; δH (500 MHz, CDCl3) 1.33 (6H, d, J = 6.9 Hz) 3.02 (1H, m, J = 6.9 Hz) 7.41 (2H, d, J = 8.19 Hz), 7.72 (1H, d, J = 5.0 Hz), 8.07 (2H, d, J = 8.19 Hz), 8.77 (1H, d, J = 5.0 Hz), 9.28 (1H, s); δC (125 MHz, CDCl3); 24.2, 34.5, 117.1, 127.6, 127.7, 134.4, 152.8, 157.6, 159.4, 164.3. (Found: C, 78.52; H, 7.28; N, 14.02. C13H14N2 requires C, 78.75; H, 7.12; N, 14.13%); MS, m/z (%): 198 (M+, 50), 183 (100), 168 (50), 103 (10), 79 (6) (Table 2, entry 8).
2.2.9 Spectra data of 5-methyl-4-phenylpyrimidine
A pale yellow powder (64%); mp 29–30 °C (30.2–30.4 °C)[12]; Rf (17% acetone/n-hexane) 0.26; IR (KBr) (vmax, cm−1) 2960, 1602, 1572, 1539; δH (300 MHz, CDCl3) 2.39 (3H, s), 7.47–7.60 (5H, m), 8.62 (1H, s), 9.11 (1H, s); δC (75 MHz, CDCl3); 17.1, 128.2, 128.4, 128.8, 129.4, 137.7, 156.4, 158.5, 165.1 (Table 2, entry 9).
2.2.10 Spectra data of 4-(3, 4-dimethoxyphenyl)pyrimidine
A grey powder (55%); mp 84–86 °C; Rf (17% acetone/n-hexane) 0.22; IR (KBr) (vmax, cm−1) 2937, 1601, 1576, 1542; δH (500 MHz, CDCl3) 3.96 (3H, s), 4.02 (3H, s), 7.01 (1H, d, J = 8.4 Hz), 7.67 (1H, dd, J = 8.4, 1.74 Hz), 7.70 (1H, d, J = 5.32 Hz) 7.80 (1H, d, J = 8.4, 1.64 Hz), 8.74 (1H, d, J = 5.26 Hz), 9.25 (1H, s); δC (125 MHz, CDCl3); 56.4, 110.2, 111.5, 116.6, 120.6 129.5, 149.9, 152.2, 157.5, 159.4, 163.7. (Found: C, 66.25; H, 5.24; N, 13.12. C12H12N2O2 requires C, 66.65; H, 5.59; N, 12.96%); MS, m/z (%): 216 (M+, 100), 173 (25), 130 (25), 103 (18), 79 (16) (Table 2, entry 10).
2.2.11 Spectra data of 4-(pyridin-4-yl)pyrimidine
A colorless powder (70%); mp 79–81 °C; Rf (17% acetone/n-hexane) 0.24; IR (KBr) (vmax, cm−1) 1577, 1536; δH (500 MHz, DMSO) 8.14 (2H, d, J = 5.85 Hz), 8.23 (1H, d, J = 5.26 Hz), 8.79 (2H, d, J = 5.85 Hz), 8.99 (1H, d, J = 5.27 Hz), 9.35 (1H, s); δC (125 MHz, DMSO); 118.8, 121.7, 144.0, 151.1, 159.6, 159.8, 161.3. (Found: C, 68.36; H, 5.01; N, 26.44. C10H7N3 requires C, 68.78; H, 4.49; N, 26.74%); MS, m/z (%): 157 (M+, 100), 130 (20), 103 (40), 76 (25), 53 (20) (Table 2, entry 11).
2.2.12 Spectra data of 4-(4-(pyridin-3-yl)pyrimidine)
A colorless powder (65%); mp 85–86 °C; Rf (17% acetone/n-hexane) 0.20; IR (KBr) (vmax, cm−1) 1579, 1546, 1521; δH (500 MHz, CDCl3) 7.53 (1H, dd, J = 7.89, 3.89 Hz), 7.81 (1H, dd, J = 5.28, 1.26 Hz), 8.50 (1H, d, J = 7.89), 8.80 (1H, d, J = 3.81 Hz) 8.89 (1H, d, J = 5.28 Hz), 9.34 (1H, s), 9.36 (1H, d, J = 0.78 Hz); δC (125 MHz, CDCl3); 117.4, 124.3, 135.3, 148.6, 151.9, 158.3, 159.8, 161.8. (Found: C, 68.14; H, 4.92; N, 26.36. C10H7N3 requires C, 68.78; H, 4.49; N, 26.74%); MS, m/z (%): 157 (M+, 100), 130 (25), 103 (30), 76 (25) (Table 2, entry 12).
2.2.13 Spectra data of 4-(2-methoxybenzyl)pyrimidine
A yellow oil (58%); Rf (17% acetone/n-hexane) 0.31; IR (KBr) (vmax, cm−1) 2962, 1598, 1579, 1540; δH (300 MHz, CDCl3) 2.40 (2H, s), 3.78 (3H, s), 6.98–7.26 (4H, m), 7.43 (1H, s), 8.50 (1H, s), 9.08 (1H, s); δC (125 MHz, CDCl3); 22.5, 55.6, 110.8, 120.7, 130.6, 130.7, 131.8, 132.7, 156.7, 157.0, 170.2. (Found: C, 71.83; H, 5.95; N, 13.98. C12H12N2O requires C, 71.98; H, 6.04; N, 13.99%); MS, m/z (%): 200 (M+, 20), 103 (10), 84 (35), 49 (65), 35 (85) (Table 2, entry 13).
2.2.14 Spectra data of 4-benzyl-5-methylpyrimidine
A yellow oil (56%); Rf (17% acetone/n-hexane) 0.28; IR (KBr) (vmax, cm−1) 2921, 1578, 1552; δH (300 MHz, CDCl3) 2.42 (3H, s), 3.78 (3H, s), 6.98–7.26 (4H, m), 7.43 (1H, s), 8.50 (1H, s), 9.08 (1H, s); δC (125 MHz, CDCl3); 22.1, 35.9, 126.2, 126.7, 128.3, 128.5, 128.8, 132.0, 137.6, 156.7, 166.0. (Found: C, 77.53; H, 6.65; N, 14.80. C12H12N2 requires C, 78.23; H, 6.57; N, 15.21%); MS, m/z (%): 184 (M+, 25), 115 (100), 91 (30), 43 (70), 28 (75) (Table 2, entry 14).
3 Results and discussion
In a continuation of our interest in the application of N,N,N’,N’-tetrabromobenzene-1,3-disulfonamide (TBBDA) [23] in organic synthesis, [23–32], we wish to report here a facile and improved protocol for the preparation of aliphatic, heterocyclic and aromatic pyrimidines from triethoxymethane, ammonium acetate and various ketone derivatives in the presence of TBBDA as a catalyst under solvent-free conditions (Scheme 1).

Three-component synthesis of pyrimidine derivatives.
The advantages of TBBDA are as follows:
- • the preparation of TBBDA is easy;
- • tBBDA is stable under atmospheric conditions for 2 months;
- • after completion of the reaction, the catalyst is recovered and can be reused several times without decreasing the yield.
Initially, we decided to explore the role of our catalyst in ethanol, acetonitrile and toluene as a solvent system for the synthesis of 4-phenylpyrimidine (Table 2, entry 1) used as a model compound. In the absence of a catalyst, no pyrimidine was observed, even after a prolonged reaction time. Since the synthesis of pyrimidine failed in the absence of catalyst, the effect of the catalyst was also investigated in various conditions, and the results are presented in Table 1.
Reaction times and yields in various conditions.
| Entry | Solvent | Amount of catalyst (TBBDA [g]) | Temperature (°C) | Time (h) | Yield (%) |
| 1 | EtOH | 0.05 | 87 | 24 | 21 |
| 2 | PhMe | 0.05 | 110 | 72 | 45 |
| 3 | PhMe | 0.10 | 110 | 72 | 49 |
| 4 | MeCN | 0.05 | r.t. | 24 | – |
| 5 | MeCN | 0.05 | 100 | 24 | 35 |
| 6 | No solvent | 0.03 | 100 | 13 | 45 |
| 7 | No solvent | 0.05 | 110 | 14 | 66 |
| 8 | No solvent | 0.10 | 110 | 13 | 66 |
With respect to the solvent system, the best results were achieved using toluene (Table 1, entry 3). In recent years, synthesis under solvent-free conditions is an important task in heterocyclic synthesis. Therefore, we decided to test this solvent-free reaction with various ratios of the catalyst. We found that the reaction was rapid and gave excellent yield of the product when catalyzed by N,N,N’,N’-tetrabromobenzene-1,3-disulfonamide [TBBDA] (13 h, 66%, entry 8).
These results encouraged us to investigate the scope and generality of this new protocol for various aliphatic, heterocyclic and aromatic ketones under optimized conditions. As shown in Table 2, a series of aliphatic, heterocyclic, and aromatic ketones containing either electron-withdrawing or electron-donating substituents successfully react with triethoxymethane and ammonium acetate to afford good to high yields of high-purity products, at 100–110 °C under solvent-free conditions. The nature and electronic properties of the ketonic substrates did not affect the conversion rate and yield.
We chose 4-phenyl-2-butanone as the reactant and expected to achieve product A, but product B was achieved, which confirms the mechanism of this reaction (Scheme 2).
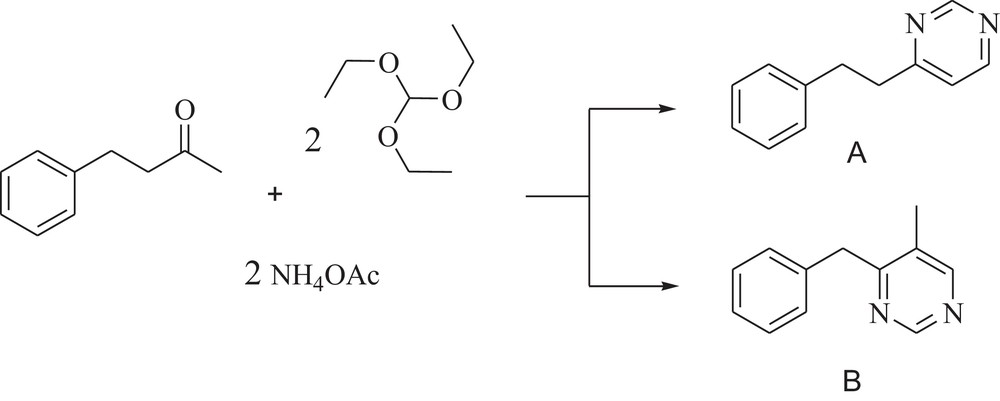
The reaction leading to the synthesis of pyrimidine.
It is likely that these catalysts release Br+ in situ, which can act as an electrophilic species [23–32]. Therefore, the mechanism shown in Scheme 3 can be suggested for the conversion of triethoxymethane, ammonium acetate and various ketone derivatives into pyrimidines [21].
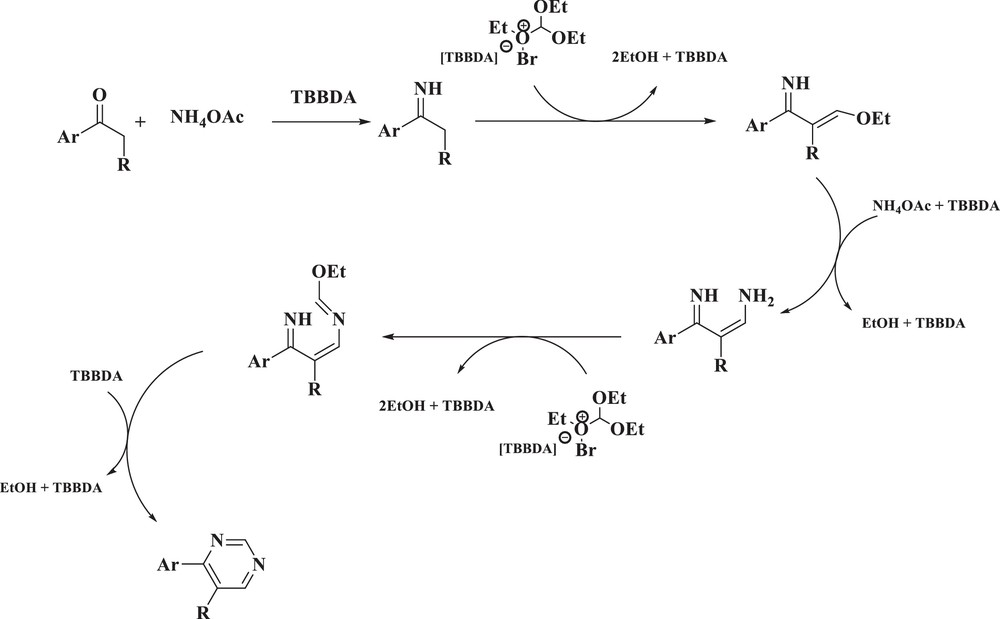
Suggested mechanism for synthesis of pyrimidine derivatives.
4 Conclusions
In summary, we have developed a new facile protocol for the synthesis of new aliphatic, heterocyclic and aromatic pyrimidine derivatives from the reaction of triethoxymethane, ammonium acetate and various ketone derivatives using TBBDA as a catalyst under solvent-free conditions.
Acknowledgement
We are thankful to Bu-Ali Sina University, Center of Excellence in Development of Environmentally Friendly Methods for Chemical Synthesis (CEDEFMCS) for financial support.


