1 Introduction
It is well known that all agro-industrial production generates waste. Many by-products of different vegetal sources are now routinely diverted from the waste stream and turned to beneficial use. Agricultural waste is still an ignored source of high-value phytochemicals that can contribute to sustainability objectives [1]. Plants and their products have always played a pivotal role in human health by satisfying various essential needs, ranging from foods to medicines [2].
Located in the northwestern most part of Italy, Piedmont is blessed with plenty of ingredients and a great culinary culture. Even if Piedmont is known as one of the top wine regions in the world, hazelnuts, another typical product, are also held in remarkable esteem. Italy is the second largest hazelnut producer in the world, behind Turkey [3]. Both the bulk of grapevine waste, such as vine pruning, grape stalks, marc (skins and seeds), and hazelnut skins are becoming more and more valued worldwide for their richness in active phytochemicals. Growing interest in the processing of grape seeds comes from cosmetic and food supplements industry. The viability of using solid wastes in animal feed is also being explored. Hitherto, studies were mainly conducted on the phenolic profile of grapevine waste [4], mainly focused on trans-resveratrol, trans-viniferin and ferulic acid [5,6]. Some of these resveratrol derivatives are known to be of high bioactivity. They are hepatoprotective [7] and posses antioxidant properties [8], induce apoptosis of leukemia B cells [9,10] and inhibit human cytochrome P450 enzymes [11], noradrenaline and 5-hydroxytryptamine uptake, and monoamine oxidase activity [12].
Hazelnut skins and other hazelnut processing by-products have mainly been used for livestock feed, but in the past few years, several studies have confirmed that they are a valuable source of natural antioxidants for nutraceutical, cosmeceutical and pharmaceutical applications [13–18].
Power ultrasound (18–40 kHz) is a green and efficient technique that greatly accelerates the extraction process and may reduce energy consumption. The final extract is more concentrated in soluble material which makes it easier to handle and reduces the need for additional process steps. Ultrasound-assisted extraction (UAE) is a clean procedure, and thanks to the low bulk temperature and the rapid execution, usually it does not degrade the extract. It leaves no residue in the extract and uses no moving mechanical parts inside the extract, thus preventing the occurrence of any pollution. It also offers advantages in terms of productivity, yield and selectivity, improves processing time, enhances quality, reduces chemical and physical hazards and is environmentally friendly [19]. Microwave-assisted extraction (MAE) can be carried out in a few minutes with high reproducibility, reducing the consumption of solvent, simplifying manipulation, giving higher final product purity and consuming only a fraction of the energy normally needed for conventional extraction. The volumetric heating generated by microwaves has several advantages which are caused by faster energy transfer, reduced thermal gradients and unique heating selectivity [20,21].
Over the last decade, increasing demand for bioactive natural products for food supplement preparation has exploited the healthy properties of the large polyphenols family. Our investigation is focused on the feasibility of extracting these high-value phytocompounds while reducing the environmental impact and disposal costs of waste by using UAE and MAE techniques. With the aim to compare different methods with classic maceration, among several tests of MAE and UAE we selected in each case the operative conditions that gave the highest yields and highest polyphenols content.
2 Results and discussion
2.1 Extraction yields
The influence on yields of different solvents and their mixture with water as well as different extraction techniques were studied. In the present study, maceration was chosen for comparison with all the non-conventional techniques. For comparison sake, we selected the best conditions found in any extraction technique. Clearly the best results obtained with UAE have operative conditions that differ from the best MAE conditions. It is important to highlight that the experiment carried out at 60 °C under conventional heating (oil bath for 30 min) gave a slightly higher extraction yield compared with maceration at room temperature, but the total polyphenols content was not improved. The total percentage extraction yields for grapevine waste and hazelnut skins are shown in Figs. 1 and 2 respectively. The highest grapevine waste extraction yield was obtained by UAE using a 1.5% β-cyclodextrin solution, while the best yield of hazelnut skins was obtained with a mixture acetone/H2O, under MAE (40 min). In fact, Contini et al. [15] reported that methanol was not the solvent of choice for the extraction of hazelnut skins.

Extraction yield % of grapevine waste under different conditions (statistical errors are presented in bars above columns). GVs: grapevine shoots; GL: grapevine leaves; GM: grapevine marc; MW: microwave; UAE: ultrasound.
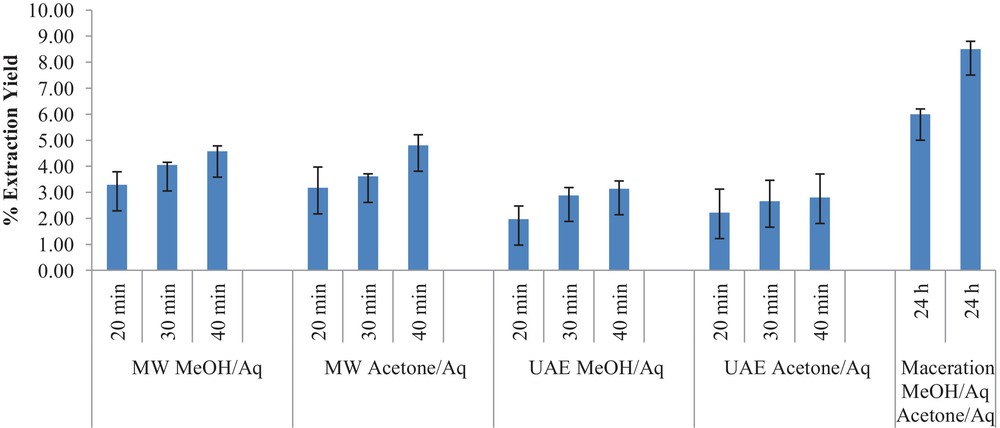
Extraction yield % of hazelnut skins under different conditions (statistical errors are presented in bars above columns).
2.2 Phenolic content and radical scavenging activity
Several studies have reported that polyphenols are more abundant in the outer layers of fruits and their content significantly decreases in fruit pulp or kernels [22]. Generally speaking, no universal solvent or mixture of solvents is ideal for polyphenols extraction. Makkar et al. [23] showed that aqueous acetone is an excellent solvent for phenolics, especially for tannins whose content is almost 65% of the total phenol content in hazelnut skin extracts. Figs. 3 and 4 illustrate total phenolic content averages expressed as gallic acid equivalents (GAE, mg/g dry weight) for both matrices. The highest phenolic contents and the lowest EC50 value for grapevine shoots were obtained by MAE using EtOH. For the hazelnut skins, the greatest phenolic content was recovered by MAE in acetone/H2O. The DPPH• assay was used to characterize antioxidant capacity as it is one of the most accurate and responsive methods for vegetal matrix extracts. The scavenging effect of the various extracts was expressed as the mean of the EC50 values ± standard deviation, as reported in Table 1 (grapevine waste) and Table 2 (hazelnut skins). The higher the EC50 values are, the lower the antioxidant activity is. All the methods used to extract hazelnut skin exhibited a very low EC50 value and this fact is probably due to the tocopherol content in the fat fraction and/or tannins [24,25].

Total phenolic content (GAE mg/g d.w.) of grapevine waste (statistical errors are presented in bars above columns).
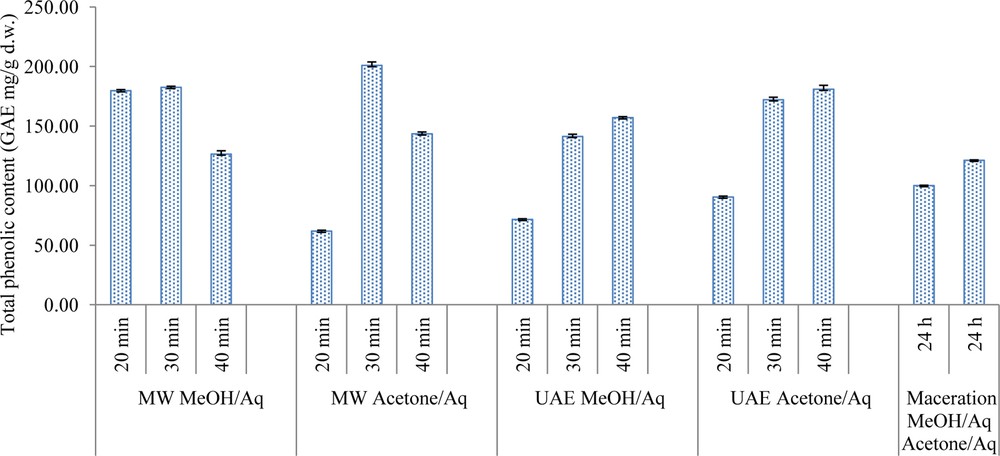
Total phenolic content (GAE mg/g d.w.) of hazelnut skins (statistical errors are presented in bars above columns).
Antioxidant capacity of the different grapevine waste extracts.
| Matrix | Extract | Antioxidant activity (EC50)* ± SD |
| GVs | EtOH maceration | 0.2 ± 0.03 |
| EtOH/Aq MW | 0.027 ± 0.02 | |
| EtOH MW | 0.004 ± 0.002 | |
| Acetone MW | 0.011 ± 0.007 | |
| Butanone MW | 0.085 ± 0.1 | |
| EtOH UAE | 0.09 ± 0.08 | |
| β CD UAE | 0.017 ± 0.0074 | |
| GL | EtOH maceration | 0.017 ± 0.0034 |
| EtOH/Aq MW | 0.016 ± 0.009 | |
| EtOH MW | 0.027 ± 0.01 | |
| acetone MW | 0.007 ± 0.0034 | |
| butanone MW | 0.023 ± 0.02 | |
| EtOH UAE | 0.016 ± 0.01 | |
| β CD UAE | 0.027 ± 0.02 | |
| GM | EtOH maceration | 0.48 ± 0.08 |
| EtOH/Aq MW | 0.014 ± 0.0083 | |
| EtOH MW | 0.06 ± 0.034 | |
| Acetone MW | 0.004 ± 0.002 | |
| Butanone MW | 0.055 ± 0.009 | |
| EtOH UAE | 0.05 ± 0.007 | |
| β CD UAE | 0.004 ± 0.0018 |
* Antiradical activity is expressed as a mean (n = 4) of EC50 values (mg of dry extract/mL of solution).
Antioxidant capacity of the different hazelnut skin extracts.
| Extract | Extraction time | Antioxidant activity (EC50)* ± SD |
| MW MeOH/Aq | 20 min | 0.0004 ± 0.0003 |
| 30 min | 0.0005 ± 0.0001 | |
| 40 min | 0.0002 ± 0.0001 | |
| MW Acetone/Aq | 20 min | 0.0005 ± 0.0002 |
| 30 min | 0.0005 ± 0.0001 | |
| 40 min | 0.0002 ± 0.0001 | |
| UAE MeOH/Aq | 20 min | 0.0009 ± 0.0007 |
| 30 min | 0.0006 ± 0.0002 | |
| 40 min | 0.0021 ± 0.0004 | |
| UAE Acetone/Aq | 20 min | 0.0009 ± 0.0007 |
| 30 min | 0.001 ± 0.0007 | |
| 40 min | 0.0006 ± 0.0004 | |
| Maceration MeOH/Aq | 24 h | 0.0004 ± 0.0001 |
| Maceration Acetone/Aq | 24 h | 0.0015 ± 0.0004 |
* Antiradical activity is expressed as a mean (n = 4) of EC50 values (mg of dry extract/mL of solution).
2.3 Energy consumption within the extraction processes
The UAE of hazelnut skins (20 g/200 mL) consumes 0.155 kWh in acetone/H2O and 0.160 kWh in MeOH/H2O in 30 min. This corresponds to 124–128 g CO2, respectively. The MAE process (25 g/500 mL) consumes 0.179 kWh in acetone/H2O and 0.184 kWh in MeOH/H2O in 30 min. This corresponds to 143–147 g CO2, respectively.
Analogously, but on a much smaller scale (4 g/40 mL), the energy consumption of grapevine waste under UAE was 0.0535 kWh (42.8 g CO2) and 0.075 kWh (60 g CO2) under MAE.
3 Conclusion
Grapevine waste and roasted hazelnuts skins are an abundant and costless source of natural phenolic antioxidants. Both UAE and MAE strongly improved polyphenols extraction compared to maceration. The relationship between phenolic content and antioxidant capacity can be influenced by the presence of other non-phenolic reducing agents. MAE of polyphenols from grapevine shoots and leaves in EtOH yielded the highest content, while the extraction from grape marc gave comparable results by UAE and MAE. Both techniques gave excellent results with hazelnut skins, whose outstanding antioxidant power is noteworthy. Undoubtedly, the use of UAE and MAE methods can enhance polyphenols recovery and antioxidant capacity with respect to maceration.
4 Experimental
4.1 Plant material
Grapevine shoots, leaves and marc were obtained from a Nebbiolo variety cultivated in Castiglione Falletto (Cuneo, Italy) and directly harvested from the Rocche vineyard on the 1st of October 2012 (Cantina Terre del Barolo). The samples were kept in sealed plastic bags and frozen at −20 °C until their extraction. The skins of roasted hazelnut (120 °C for 30 min) were kindly supplied by Tecnogranda (Dronero, Cuneo) from a crop of August 2012 in Corneliano d’Alba (Cuneo).
4.2 Instruments
All UAE procedures were performed using a high-power probe system equipped with a thermostated cooling bath and an immersion titanium horn, frequency 21.1 kHz in a power range of 80–100 W. The sonochemical device was developed in collaboration with Danacamerini sas (Torino, Italy).
MW-assisted extractions were carried out in a SynthWAVE reactor (Milestone, Bergamo, Italy) in a 1 L pressure-resistant PTFE cavity (up to 200 bar) equipped with a 5-position vial rack. This device enables high power density (1.5 kW/L) and inert atmosphere and the possibility of simultaneously carrying out multiple extraction tests.
4.3 Chemicals
All chemicals and solvents were of analytical grade and were purchased from Sigma Aldrich (Milan, Italy).
4.4 Extraction of phenolic compounds
With the aim of enhancing the polyphenols content in crude extracts, a full plan of experiments was designed and carried out using extraction conditions described in previous studies [4,15,25–28]. Classic maceration and non-conventional techniques (UAE, MAE) were performed using a plant/solvent ratio of 1:10 (w/v). Samples of grapevine wastes (GVs: grapevine shoots; GL: grapevine leaves; GM: grapevine marc) were extracted as follows:
- • maceration with ethanol for 24 hours at room temperature;
- • MAE with ethanol, ethanol/water 50:50 (v/v %), acetone and butanone at 60 °C for 30 min, power (1.5 kW), under nitrogen pressure (5 bar);
- • UAE with ethanol, 1.5% β-cyclodextrin (β CD) solution for 5 min at 100 W and 30 min at 80 W.
Samples of hazelnut skins (HS) were extracted as follows:
- • maceration with methanol/water and acetone/water 80/20 (v/v %) for 24 hours at room temperature;
- • MAE with methanol/water and acetone/water 80/20 (v/v %) for 20, 30 and 40 min at 60 °C, power (1.5 kW), under nitrogen pressure (5 bar);
- • UAE with methanol/water and acetone/water 80/20 (v/v %) for 5 min at 100 W, 20, 30 and 40 min at 80 W. All extractions were performed in triplicate and expressed as averages ± standard deviation.
4.5 Determination of total phenolic content
The phenolic content was determined according to the method developed by Cicco et al. [29] on the crude extracts obtained from the maceration, UAE and MAE procedures. The proposed method is a variation on the classic Folin–Ciocalteau method as it uses a new combination of time, temperature, alkali and alcohol for the spectrophotometric evaluation of low-concentration phenolic compounds in methanol extracts. The absorption of the final mixtures was measured at 740 nm, in a 1 cm cuvette, on a Cary 60 UV-Vis spectrophotometer (Agilent Technologies, Santa Clara, CA, USA). These conditions provided the assay with high accuracy and reproducibility. Quantification was carried out on the basis of a standard curve using appropriate dilutions of a solution of gallic acid. Total phenolic content is expressed as gallic acid equivalents (GAE, mg/g dry weight d.w.). All analyses were performed in triplicate and expressed as averages ± standard deviation.
4.6 Determination of antioxidant activity by the DPPH• radical scavenging method
The radical scavenging ability of the extracts was monitored using the stable free radical DPPH, following the method described by Brand-Williams et al. [30]. In order to obtain an absorbance of between 0.45–0.55 at 517 nm, a standard solution of DPPH• (0.1 mM) was prepared. For each extract or pure antioxidant, at least five different concentrations were tested. 700 μL of DPPH• standard solution were placed in five cuvettes for the UV-Vis colorimetric assay.
The reaction started when 700 μL of the diluted solutions were added to the cuvette containing the DPPH• standard solution. Mixtures were shaken vigorously and left to stand in the dark at room temperature for 20 min (time required to reach the steady state). The bleaching rate of DPPH• was monitored in presence of different sample concentrations. The UV absorbance of each test was monitored at 517 nm. At this time, the decrease in absorbance was measured at 517 nm against a pure methanol blank, with a Cary 60 UV-Vis spectrophotometer (Agilent Technologies, Santa Clara, CA, USA).
Data analysis, the computation of EC50 (amount of either extract necessary to decrease the initial concentration of DPPH• to 50% in the steady state) and the Probit Regressions were performed using an algorithm implemented in Microsoft Visual Basic 6.0 (Microsoft Corporation, Redmond, WA, USA). All samples were prepared in quadruplicate and the DPPH• radical scavenging activity was expressed as mg dry extract/mL solution ± standard deviation.
Acknowledgements
The present work was supported by the ALCOTRA project “Eco extraction transfrontaliere”. Cantina Terre del Barolo is kindly acknowledged for the grapevine material. We are also grateful to Dr. D. Vallauri and Dr. G. Reita (Tecnogranda, Dronero – Italy) for the useful technical suggestions.


