1 Introduction
Pyrazoles are an important class of compounds for new drugs’ development, as they are the core structure of numerous biologically active compounds, including blockbuster drugs such as celecoxib, Viagra, pyrazofurine, and many others [1–4]. Furthermore, heterocycles containing a phthalazine moiety are of current interest due to their pharmacological and biological activities [5–7]. For example, 1H-pyrazolo[1,2-b]phthalazine-dione is described as an anti-inflammatory, analgesic, anti-hypoxic, and anti-pyretic agent [6]. Phthalazine derivatives are also found to possess anti-convulsant [8], cardiotonic [9], and vasorelaxant [10] activities.
To our knowledge, there are only a few multicomponent reports for the synthesis of 1H-pyrazolo[1,2-b]phthalazine-5,10-diones [11]. However, in spite of their potential utility, some of the reported synthetic methods suffer from limitations such as the use of an expensive catalyst, long reaction times, difficult work-up and drastic reaction conditions. Therefore, any new facile and highly efficient synthetic approach to corresponding heterocycles containing a phthalazine ring fragment is highly desirable.
Recently, it was found that chemical bases could be replaced with an electrogenerated base (EGB) to promote reactions in higher yields [12]. Electroorganic reactions proceed generally smoothly and take place with good to excellent yields with easy work-up and do not require the use of harsh conditions such as high temperatures and expensive reagents. Also, to date, no reports have been published on the electrosynthesis of 1H-pyrazolo[1,2-b]phthalazine-5,10-diones.
All these facts have prompted us to design a convenient and facile multicomponent synthesis of 1H-pyrazolo[1,2-b]phthalazine-5,10-diones based on the electrocatalytic transformation of phthalhydrazide 1, aromatic aldehydes 2a–k, and malononitrile 3 in an undivided cell (Scheme 1).
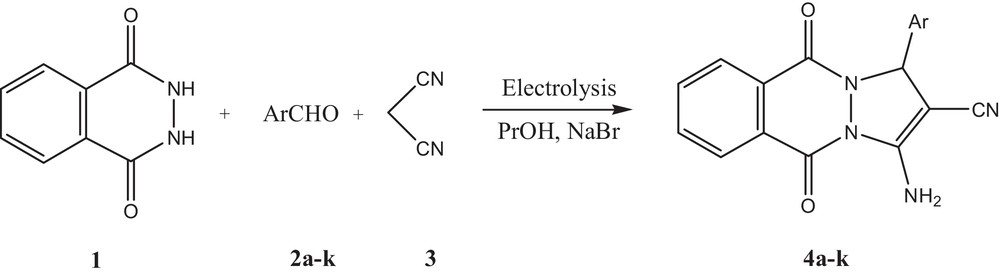
Electrosynthesis of 1H-pyrazolo[1,2-b]phthalazine-5,10-diones.
2 Results and discussion
First, to evaluate the synthetic potential of the procedure proposed and to optimize the electrolysis conditions, the electrocatalytic multicomponent transformation of phthalhydrazide 1, 3-nitrobenzaldehydes 2a, and malononitrile 3 into the corresponding 1H-pyrazolo[1,2-b]phthalazine-5,10-dione 4a in n-PrOH in an undivided cell containing an iron electrode as cathode and a Pt electrode as anode at constant current in the presence of sodium bromide as an electrolyte was studied at room temperature. As it is indicated in Table 1, the current density 12 mA/cm2 (I = 60 mA, electrode surface 5 cm2) in n-PrOH was found to be the optimum one for the electrochemically induced chain process and afforded the highest yield (98%) of 4a. The current density increase up to 16 mA/cm2 (I = 80 mA) results in a slight decrease of the reaction yield, which may be connected with the activation of undesired direct electrochemical processes possible under these conditions and leading to the oligomerization of the starting material.
Electrocatalytic transformation of phthalhydrazide (1), 3-nitrobenzaldehyde (2a) and malononitrile (3) into 3-amino-1-(3-nitrophenyl)-5,10-dioxo-5,10-dihydro-1H-pyrazolo[1,2-b]phthalazine-2-carbonitrile (4a)a.
| Entry | Alcohol | I (mA) | Current density (mAcm−2) | Time (min) | Electricity passed (F mol−1) | Yield (%) |
| 1 | EtOH | 30 | 6 | 8 | 0.15 | 79 |
| 2 | n-PrOH | 30 | 6 | 8 | 0.15 | 85 |
| 3 | n-PrOH | 50 | 10 | 5 | 0.15 | 90 |
| 4 | n-PrOH | 60 | 12 | 4 | 0.15 | 98 |
| 5 | n-PrOH | 80 | 16 | 3 | 0.15 | 93 |
a Phthalhydrazide (1 mmol), 3-nitrobenzaldehyde (1 mmol), malononitrile (1 mmol), NaBr (0.1 mmol), alcohol (10 mL), iron cathode (5 cm2), platinum anode (5 cm2), room temperature.
Under the optimal conditions (current density 12 mA/cm2), the electrolysis of phthalhydrazide 1, aromatic aldehydes 2a–k, and malononitrile 3 in an undivided cell gives rise to the corresponding 1H-pyrazolo[1,2-b]phthalazine-5,10-diones 4a–k in short reaction times (4–8 min) and high yields (Table 2). The electronic nature of the substituent on the aromatic ring showed no particular effect on the conversion.
Electrocatalytic transformation of phthalhydrazide (1), aromatic aldehydes (2a–k), and malononitrile (3) into 1H-pyrazolo[1,2-b]phthalazine-5,10-dione (4a–k)a.
| Product | Aldehyde | Current density (mAcm−2) | Time (min) | Electricity passed (F mol−1) | Yield (%) | Mp (°C) | Mp (°C) (lit. [11d]) |
| 4a | 12 | 4 | 0.15 | 98 | 269–270 | 269–271 | |
| 4b | 12 | 6 | 0.22 | 98 | 267–269 | 265–267 | |
| 4c | 12 | 5 | 0.19 | 85 | 228–229 | 228–229 | |
| 4d | 12 | 5 | 0.19 | 98 | 275–277 | 275–276 | |
| 4e | 12 | 5 | 0.19 | 95 | 259–261 | 259–260 | |
| 4f | 12 | 7 | 0.26 | 91 | 249–250 | 248–251 | |
| 4g | 12 | 6 | 0.22 | 87 | 259–261 | 257–259 | |
| 4h | 12 | 8 | 0.30 | 98 | 272–274 | 270–272 | |
| 4i | 12 | 6 | 0.22 | 85 | 267–268 | 263–264 | |
| 4j | 12 | 7 | 0.26 | 86 | 266–268 | – | |
| 4k | 12 | 5 | 0.19 | 98 | 276–278 | – |
a Phthalhydrazide (1 mmol), aromatic aldehydes (1 mmol), malononitrile (1 mmol), NaBr (0.1 mmol), alcohol (10 mL), iron cathode (5 cm2), platinum anode (5 cm2), room temperature.
Taking the above results into consideration, the following mechanism for this electrocatalytic chain transformation is proposed. As the initiation step of the catalytic cycle, the deprotonation of an alcohol at the cathode leads to the formation of propioxide anion. The subsequent reaction in solution between propioxide anion and malononitrile gives rise to malononitrile anion (Scheme 2).

Formation of propioxide anion at the cathode.
Then, Knoevenagel condensation of malononitrile anion with aromatic aldehydes 2a–k takes place in the solution with elimination of hydroxide anion and formation of arylidene malononitrile 5. The subsequent propioxide anion promoted Michael addition of phetalhydrazide 1 to electron-deficient Knoevenagel adduct 5, followed by intramolecular cyclization to the corresponding 1H-pyrazolo[1,2-b] phthalazine-5,10-diones 4a-k (Scheme 3).

Proposed mechanism for the preparation of 1H-pyrazolo[1,2-b]phthalazine-5,10-diones 4a–k.
3 Conclusions
In conclusion, the use of an EGB in comparison with conventional chemistry [11] has advantages such as (i) in situ generation of base, (ii) one-pot reaction in excellent yields under milder conditions, (iii) avoidance of polluting or hazardous chemicals or the addition of base or probase; moreover, its work-up procedure is easy.
4 Experimental
4.1 General
All reagents were purchased from Merck and Fluka and used without further purification. The melting points were obtained in open capillary tubes and were measured on an Electrothermal IA 9100 apparatus. IR spectra were recorded on KBr pellets with a Shimadzu FT-IR 8600 spectrophotometer. 1H and 13C NMR spectra were determined with a Bruker DRX-400 Avance instrument at 400 and 100 MHz. Elemental analysis were carried out using a Thermo Finnigan Flash EA 1112 series instrument.
4.2 General procedure for electrochemical synthesis of pyrazolo[1,2-b]phthalazines
A solution of phthalhydrazide (1.0 mmol), various aryl aldehydes (1.0 mmol), and malononitrile (1.0 mmol) and sodium bromide (0.1 mmol) in n-propanol (10 mL) was electrolyzed in an undivided cell equipped with a magnetic stirrer, a platinum anode and an iron cathode at room temperature under a constant current density of 12 mA/cm2 (I = 60 mA, electrodes square 5 cm2).
The progress of the reaction was monitored by thin layer chromatography. After completion of the reaction (4–8 minutes), the obtained precipitate was filtered, and the filter cake was washed with ethanol to yield pure products (4a–k).
4.2.1 3-Amino-1-(4-nitrophenyl)-5,10-dioxo-5,10-dihydro-1H-pyrazolo[1,2-b]phthalazine-2-carbonitrile (4c)
Yellow powder; yield: 85%; mp = 228–229 °C; 1H NMR (400 MHz, DMSO-d6): δH 8.27–8.29 (m, 1H), 8.22 (d, J = 8.6 Hz, 2H), 8.20 (brs, 2H, NH2), 8.08–8.10 (m, 1H), 7.97–7.99 (m, 2H), 7.81 (d, J = 8.6 Hz, 2H), 6.30 (s, 1H) ppm; 13C NMR (100 MHz, DMSO-d6): 157.2, 154.2, 151.4, 151.3, 147.8, 146.3, 135.1, 134.3, 129.4, 128.9, 128.5, 127.8, 127.2, 124.2, 116.3, 62.8 ppm; IR (KBr): υ = 3433, 3321, 3076, 2160, 1658, 1558, 1515 cm−1.
4.2.2 3-Amino-1-(2-methoxyphenyl)-5,10-dioxo-5,10-dihydro-1H-pyrazolo[1,2-b]phthalazine-2-carbonitrile (4e)
Yellow powder; yield: 95%; mp = 259–261 °C; 1H NMR (400 MHz, DMSO-d6): δH 8.30–8.27 (m, 1H), 8.11–8.09 (m, 1H), 8.02 (brs, 2H, NH2), 8.01–7.97 (m, 2H), 7.32–7.27 (m, 2H), 7.04 (d, J = 7.6 Hz, 1H), 6.91 (dt, J = 7.6, 0.8 Hz, 1H), 6.35 (s, 1H), 3.74 (s, 3H, OCH3) ppm; 13C NMR (100 MHz, DMSO-d6): 157.1, 156.9, 153.7, 151.5, 135.2, 134.2, 129.8, 129.1, 129.0, 127.9, 127.8, 127.2, 126.3, 121.2, 116.5, 112.1, 61.1, 59.4, 56.3 ppm; IR (KBr): υ = 3380, 3250, 3184, 2199, 1657, 1564, 1379 cm−1.
4.2.3 3-Amino-1-(3-methoxyphenyl)-5,10-dioxo-5,10-dihydro-1H-pyrazolo[1,2-b] phthalazine-2-carbonitrile (4f)
Yellow powder; yield: 91%; mp = 249–250 °C; 1H NMR (400 MHz, DMSO-d6): δH 8.25–8.27 (m, 1H), 8.08–8.10 (m, 3H, NH2, H), 7.95–7.99 (m, 2H), 7.28 (t, J = 7.6 Hz, 1H), 6.99–7.01 (m, 2H), 6.89 (dd, J = 8.0, 1.6 Hz, 1H), 6.10 (s, 1H), 3.74 (s, 3H, OCH3) ppm; 13C NMR (100 MHz, DMSO-d6): 159.8, 157.1, 154.1, 151.1 138.1, 134.2, 130.1, 129.5, 129.3, 129.1, 127.3, 127.1, 124.2, 119.2, 113.8, 113.1, 64.0, 55.6 ppm; IR (KBr): υ = 3361, 3259, 3056, 2190, 1654, 1566 cm−1.
4.2.4 3-Amino-1-(2-chlorophenyl)-5,10-dioxo-5,10-dihydro-1H-pyrazolo[1,2-b]phthalazine-2-carbonitrile (4g)
Yellow powder; yield: 87%; mp = 259–261 °C; 1H NMR (400 MHz, DMSO-d6): δH 8.29–8.27 (m, 1H), 8.14 (brs, 2H, NH2), 8.11–8.07 (m, 1H), 8.01–7.96 (m, 2H), 7.60 (dd, J = 7.0, 2.0 Hz, 1H), 7.48–7.46 (m, 1H), 7.38–7.31 (m, 2H), 6.46 (s, 1H) ppm; 13C NMR (100 MHz, DMSO-d6): 157.1, 154.0, 151.6, 135.8, 135.2, 134.3, 131.7, 130.4, 130.2, 129.5, 129.2, 128.8, 128.3, 127.8, 127.2, 116.2, 61.1, 60.2 ppm; IR (KBr): υ = 3367, 3232, 3171, 2206, 1655, 1568, 1379 cm−1.
4.2.5 3-Amino-1-(4-chlorophenyl)-5,10-dioxo-5,10-dihydro-1H-pyrazolo[1,2-b]phthalazine-2-carbonitrile (4h)
Yellow powder; yield: 98%; mp = 272–274 °C; 1H NMR (400 MHz, DMSO-d6): δH 8.27-8.24 (m, 1H), 8.13 (brs, 2H, NH2), 8.10–8.06 (m, 1H), 7.99–7.94 (m, 2H), 7.52 (d, J = 8.8 Hz, 2H), 7.43 (d, J = 8.8 Hz, 2H), 6.15 (s, 1H) ppm; 13C NMR (100 MHz, DMSO-d6): 157.1, 154.1, 151.2, 151.1, 137.1, 135.1, 134.2, 133.3, 129.3, 129.4, 129.0, 128.1, 127.7, 127.1, 116.4, 62.7 ppm; IR (KBr): υ = 3371, 3257, 3114, 2196, 1656, 1566 cm−1.
4.2.6 3-Amino-1-(4-Bromophenyl)-5,10-dioxo-5,10-dihydro-1H-pyrazolo[1,2-b]phthalazine-2-carbonitrile (4i)
Yellow powder; yield: 85%; mp = 267–268 °C; 1H NMR (400 MHz, DMSO-d6): δH 8.27-8.25 (m, 1H), 8.13 (brs, 2H, NH2), 8.10-8.07 (m, 1H), 7.98–7.96 (m, 2H), 7.56 (d, J = 8.8 Hz, 2H), 7.46 (d, J = 8.8 Hz, 2H), 6.13 (s, 1H) ppm; 13C NMR (100 MHz, DMSO-d6): 157.1, 154.1, 151.2, 138.4, 135.1, 134.2, 131.9, 129.6, 129.3, 129.0, 127.7, 127.1, 121.9, 116.4, 62.8, 61.3 ppm; IR (KBr): υ = 3371, 3257, 3193, 2194, 1655, 1560 cm−1.
4.2.7 3-Amino-1-(pyridine carbaldehyde)-5,10-dioxo-5,10-dihydro-1H-pyrazolo[1,2-b]phthalazine-2-carbonitrile (4j)
Yellow powder; yield: 86%; mp = 266–268 °C; 1H NMR (400 MHz, DMSO-d6): δH 8.73 (d, J= 1.6 Hz, 1H), 8.54 (dd, J = 4.8, 1.2 Hz, 1H), 8.27–8.25 (m, 1H), 8.17 (brs, 2H, NH2), 8.10–8.08 (m, 1H), 7.99–7.96 (m, 2H), 7.94–7.92 (m, 1H), 7.40 (dd, J = 7.8, 4.4 Hz, 1H), 6.22 (s, 1H) ppm; IR (KBr): υ = 3364, 3259, 3193, 2189, 1652, 1569, 1383 cm−1; Anal. calcd. for C17H11N5O2: C, 64.35; H, 3.49; N, 22.07. Found: C, 64.66; H, 3.27; N, 21.55.
4.2.8 3-Amino-1-(1-naphtalen-1-yl)-5,10-dioxo-5,10-dihydro-1H-pyrazolo[1,2-b] phthalazine-2-carbonitrile (4k)
Yellow powder; yield: 98%; mp = 276–278 °C; 1H NMR (400 MHz, DMSO-d6): δH 8.30–8.28 (m, 1H), 8.16 (brs, 2H, NH2), 7.89–8.08 (m, 7H), 7.53–7.60 (m, 3H), 6.30 (s, 1H) ppm; 13C NMR (100 MHz, DMSO-d6): 157.2, 154.2, 151.1, 151.0 136.3, 135.1, 134.2, 133.3, 133.1, 129.3, 129.1, 128.9, 128.3, 128.1, 127.8, 127.1, 126.9, 126.8, 126.5, 124.8, 116.6, 63.7 ppm; IR (KBr): υ = 3365, 3255, 3193, 2190, 1652, 1560 cm−1; Anal. calcd. for C22H14N4O2: C, 72.12; H, 3.85; N, 15.29. Found: C, 72.71; H, 3.50; N, 15.52.
Acknowledgements
Financial support for this work by the research council of Islamic Azad University, Rasht Branch is gratefully acknowledged.


