1 Introduction
Compounds possessing the 1,5-benzodiazepine core are well recognized for their broad spectrum of pronounced biological and pharmacological activities [1,2]. Thus, interest in their chemistry continues unabated. Moreover, new insights have recently been found into the development of more efficient and versatile methods for the synthesis of these 1,5-benzodiazepinic scaffolds [3,4]. In a previous work, our research team has explored the condensation between the [1]benzopyrano[4,3-c][1,5]benzodiazpin-7(8H)one 1 and a series of N,N-binucleophiles to give the aforementioned heterocoumarins as a result of a multiple-step sequence involving successive ring-opening and recyclization processes [5,6].
As a continuation of our ongoing efforts directed towards the synthesis of novel polyheterocycles containing the 1,5-benzodiazepine moiety, especially promising bioactive compounds [7–10], we were prompted to study the behavior of 1 towards 2-aminopyridine derivatives as 1,3-dinitrogenated nucleophile and we report herein our results, which are of particular interest.
2 Results and discussion
Keeping in mind our previous report on the synthesis of new heterocoumarins [5,6], we firstly carried out the reaction between 1 and 2-aminopyridine under drastic acidic conditions, aiming thereby to achieve the 3(N-pyridinylamino)methylene-1,5-benzodiazepinone 6 via a nucleophilic attack of the amino group at the C-2 of the benzopyran ring. The key intermediate 6, if obtained, would probably undergo a series of rearrangements to afford interesting benzodiazepine derivatives.
Nevertheless, under such vigorous conditions, all attempts led to the formation of complex crudes and directed us to opt for milder conditions using n-butanol instead of acetic acid. Thus, when a mixture of benzopyrano-benzodiazepine 1 and 2-aminopyridine 2a or 2-amino-4-methylpyridine 2b in n-butanol was heated under reflux for 3 h, the attempted 3(N-pyridinylamino)methylene-1,5-benzodiazepinone 6 was not obtained. On the basis of their spectral data, the isolated products were identified as a self-assembled molecular system 5a,b linked via an intermolecular hydrogen bonding between the 6-(O-butyl)benzochromeno-1,5-benzodiazepinone 4a and the 2-aminopyridine 2 (Scheme 1).

Synthesis of the self-assembled systems 5b in one-pot.
According to this finding, we made a careful review of the literature that revealed a continual growing interest in benzodiazepinic systems for their specificity to recognize and bind through intermolecular hydrogen bonding and π–π stacking to a modulatory site on GABA receptors [11]. In addition, Pavlovsky et al. previously described the arrangement of 3-arylidene-1,2-dihydro-3H-1,4-benzodiazepin-2-one in dimers due to the N(1)-H···O(2) hydrogen bonds [12]. On another hand, the 2-aminopyridine also revealed a great tendency to develop intermolecular hydrogen bonding [13,14]. Such self-assembled systems continuously attract the researcher's interest due to their potential to be applied in the field of chemistry, biology and physics [15–17].
Here, one can return to the first spectroscopic evidence that emerged from the mass spectrum (ES + mode) of the self-assembled system 5b (4a + 2b) chosen as an illustration example which exhibited a molecular ion peak [MH+] at m/z = 445.3. In fact, this value represents the sum of the molecular weights of diazepine 1, 2-amino-4-methylpyridine 2b, and n-butanol. On the other hand, the 1H NMR spectrum of 5b showed a large singlet (4.51 ppm, 1H), which was attributed to the free amino group of the pyridinic ring. This confirmed that the 2-amino-4-methylpyridine 2b did not participate in the reaction. However, it is worth noting that all the protons of the 2-aminopyridine were easily located in the 1H NMR spectrum. Moreover, the absence of the deshielded phenolic hydrogen singlet usually observed around 15 ppm [18] excluded from the beginning a nucleophilic attack of the amino group at the C-2 of the benzopyran moiety and the consequent ring-opening. Hence, the set of both singlets (5.93 ppm, 1H and 3.29 ppm, 1H) were then assigned to H-6 and H-6a of the dihydrobenzopyrane moiety as a result of an O-attack of the butanol [19] at the benzopyran carbon followed by a concomitant proton shift and a subsequent reduction of C6 = C6a. The C6-linked n-butoxy chain was readily assigned on the basis of chemical shifts and multiplicities. Here, the non-coupling between H-6 and H-6a was accidental and undoubtedly due to the non-planarity of the fused heptatomic–hexatomic ring systems.
This finding led us to consider the HMBC 2D-map that did not reveal any spot pointing out a long-range C–H correlation between protons H-6 and carbons of the pyridine moiety, whereas it has been found that protons H-2a′ (3.56 ppm) and H-2b′ (3.76 ppm) correlates with C-6 (96.2 ppm). Consequently, it can be stated that derivative 2b is not directly attached to the benzodihydropyrane moiety, but more probably linked through an intermolecular hydrogen bonding.
The event of a H(amide)5a·····N(pyridine)2b hydrogen bonding was deeply supported by an nOesy study that showed a correlation between the amidic hydrogen H-8 (8.97 ppm) and the free amino group (4.51 ppm). This observation highlights the proximity between the diazepine 4a and the 2-aminopyridine ring 2.
At this stage of the work, the 6-(O-butyl) benzochromeno-1,5-benzodiazepinone 4a was isolated to further study its behavior towards 2-aminopyridines 2a,b. Indeed, the reaction between the key intermediate 1 and n-butanol has been carried out, the corresponding benzodiazepinone 4a was obtained in good yield. As a second step, the condensation between 4a and 2a,b under reflux in tetrahydrofuran, led to the expected self-assembled structures 5a,b (Scheme 2).

Synthesis of the self-assembled systems 5a–f, path a: a one-pot synthesis, path b: two-step synthesis.
In order to get more proofs of the establishment of the intermolecular hydrogen bonding, we have carried out a 1H NMR titration experiment [20,21]. While the concentration of compound 4a was kept constant, we have progressively increased the concentration of 2b. Consequently, apparent down field shift of the amidic proton signal of 4a was observed as a result of a strong NH5a····N2b intermolecular hydrogen bonding between the diazepine 4a and the 2-amino-4-methylpyridine 2b (Fig. 1).

1H NMR titration of 4a (c = 2.10−2 M) with 2b in CDCl3 at room temperature (the molar ratio of 4a/2b is shown in the figure).
Infrared studies gave an additional evidence for the hydrogen bonding between 4a and 2b. In fact, the hydrogen bonding interaction has lowered the frequencies of the amino NH bands (νN–H = 3411 cm−1). Moreover, the asymmetric and symmetric stretching vibrations of the amino group were weakened and shifted to 3411 cm−1 and 3222 cm−1 as can be seen when compared with the frequencies of the corresponding mode for the non-interacting 2-aminopyridine [22]. It should be pointed out that the N–H and NH2 vibrations bands are almost undetectable in the Raman spectrum. Such pattern in the changes of frequencies and intensities in the IR and Raman spectra unambiguously points out the formation of intermolecular H-bonds [23].
As a summary from our experimental results, it is reasonable now to assume that the outcome of the reaction between benzopyrano-1,5-benzodiazepinone 1 and 2-aminopyridines 2a,b in n-butanol is different from what we had previously observed [5] and follows the pathway anticipated and described in Scheme 1.
With this encouraging result in hand, giving the corresponding self-assembled structures in high yield, we decided to extend this study to other alcoholic solvents, such as methanol and ethanol as O-nucleophilic agents, and see whether similar H-bonding would link 2-aminopyridine to structure 4 (Scheme 2).
The 1D and 2D-NMR studies enabled the establishment of a whole set of linkages and complete assignment of the self-assembled molecular structures 5c–f (see Experimental section).
A single cristal suitable for an X-ray diffraction study was obtained for 5e by recrystallization in EtOH. The ORTEP diagram of the crystal structure of the self-assembly 5e with the total atomic numbering scheme is shown in Fig. 2.
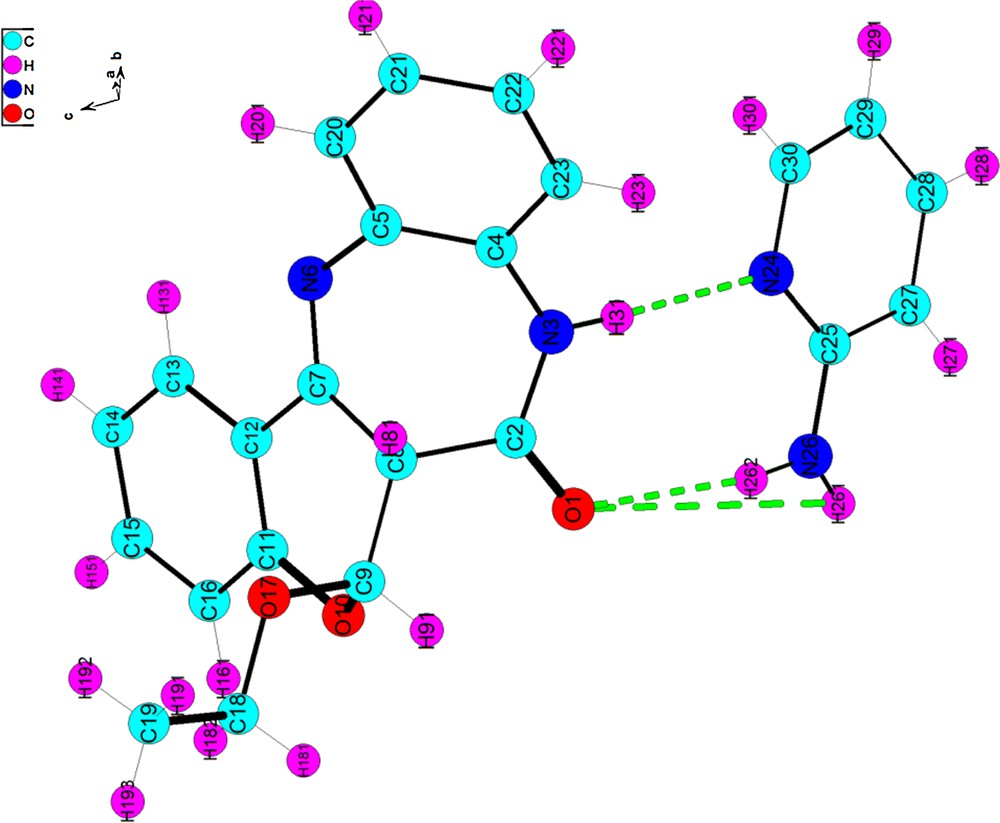
(Color online.) The structure of the self-assembly 5e showing the total atomic numbering scheme. Intermolecular hydrogen bonds are shown by dashed lines. For convenience, the numbering of atoms given in the figure does not correspond to the IUPAC nomenclature system.
The molecular structure of the self-assembly 5e reveals two intermolecular hydrogen bonds. The amidic hydrogen of the O-ethylbenzodiazepine, which is bonded to the N(24) of the 2-aminopyridine, represents the first center of hydrogen bonding. This bond is similar in its parameters to the hydrogen bond characteristic of benzodiazepines unsubstituted at N(3) [24–26]. The second interaction is observed between both hydrogens of the amino group H(261) and H(262) and oxygen O(1) of the carbonyle. Hydrogen bonding dimensions for 5e are listed in Table 1.
Hydrogen bonding distances (Å) and angles [°] in the structure of self-assembled 5e.
| D–H···A | D–H | H···A | D···A | D–H···A |
| N3–H31···N24 | 0.86 | 2.06 | 2.920(3) | 177 |
| N26–H261···O1i | 0.87 | 2.23 | 3.024(3) | 152(1) |
| N26–H262···O1 | 0.87 | 2.21 | 3.048(3) | 162 |
| C27–H271···O10i | 0.93 | 2.46 | 3.275(3) | 146(1) |
It can be observed that the O-ethylbenzochromenobenzodiazepinone 5e is not planar with the folding of the seven-membered ring, which adopts a boat conformation along the N…N hinge [27]. The loss of the planarity in the pyran moeity as a consequence of the O-attack of ethanol is presumably the cause of its molecular shape. It is worth mentioning that the addition of ethanol is not enantioselective as it results in the formation of a racemic mixture. Another interesting feature of the compound packing is the π–π interaction. Each self-assembled structure is stacked face to face with the other ones along a crystallographic 0a axis (Fig. 3).
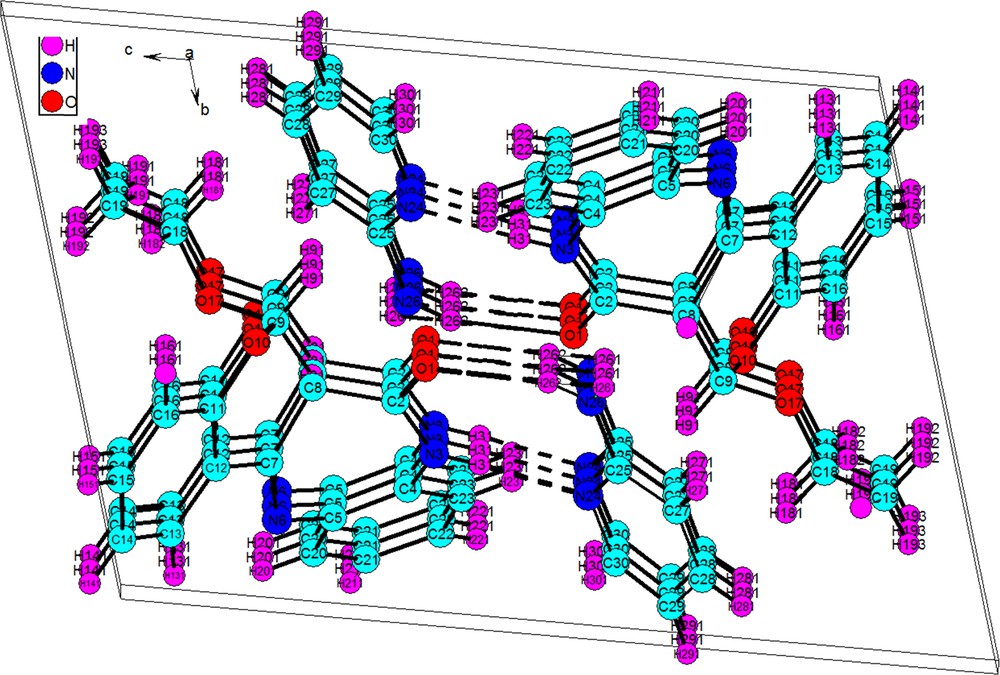
(Color online.) View along the Oa axis on the layered supramolecular structure in crystal 5e.
3 Conclusion
In this work, we have successfully developed an efficient and straightforward preparation of novel O-alkylatedbenzochromeno-1,5-benzodiazepinones starting from readily available materials. We have demonstrated that these new diazepines revealed a great ability to establish strong NH····N and CO·····N intermolecular hydrogen bonding with 2-aminopyridines derivatives. A wide range of 1,3-nucleophiles, such as 2-aminobenzothiazole derivatives and 2-aminobenzimidazole could be applied to this process, thereby giving a reliable method for the synthesis of new self-assembled structures with enhanced biological and pharmacological activities.
4 Experimental
All solvents were purified and dried prior to use. 1H and 13C NMR spectra were recorded with an AC-300 Bruker spectrometer with tetramethylsilane as an internal reference. Chemical shifts are reported in parts per million. Two-dimensional NMR experiments were performed with an Avance-500 Bruker spectrometer. High-resolution mass spectra of compounds 4a–c and the self-assembled 5a–f were performed within a Hewlett-Packard 5890/5970 GC–mass spectrometer. The [M]+ mass for self-assembled 5a–f were never observed due to their instability in HRMS mode. Low-resolution mass spectra of compound 5a–b were obtained with a Micromass LCT spectrometer (ESI technique, positive mode).
The FT–IR spectra were measured from KBr pellets using a PerkinElmer 1750 spectrophotometer. To complete the results of the FT–IR spectroscopy, only in case of the self-assembled system 5b, Raman spectra of samples in sealed glass cell (2 mm in diameter) were obtained at room temperature with a Horibo Jobin Yvon HR800 microcomputer system instrument using a conventional scanning Raman instrument equipped with a Spex 1403 double monochromator (with a pair of 600 grooves/mm gratings) and a Hamamatsu 928.
All the reactions were followed by TLC using aluminum sheets of Merck silica gel 60 F254, 0.2 mm. The starting material 1 was prepared according to the literature [8]. Melting points of benzodiazepines 4a–c were determined on a Buchi 510 capillary melting point apparatus. However, melting points of the self-assembled systems 5a–f could not be determined because they decompose on heating.
4.1 General procedure for the synthesis of the self-assembly (5a–f)
To a stirred suspension of compound 1 (262 mg, 1 mmol) in refluxed n-alcohol (80 mL) was added the 2-aminopyridine 2a (94 mg, 1 mmol) or the 4-methyl-2-aminopyridine 2b (108 mg, 1 mmol), respectively. The solution was allowed to react for 3 to 5 h and its progress was monitored by a TLC control (cyclohexane–ethylacetate 80:20). After the completion of the reaction, the solvents were removed in vacuo and to the resulting yellow crude oil was added the petroleum ether; the so-formed white precipitate was collected and crystallized from a mixture of THF and petroleum ether to give the corresponding products 5a–f, respectively.
4.1.1 Intermolecular hydrogen bonded self-assembly 5a (4a + 2a)
White cottony, yield 73%. 1H NMR (300 MHz, CDCl3) δH 9.46 (s, 1H, N–H), 8.26 (dd, 1H, H-1, J = 7,5 Hz), 8.02 (d, 1H, H-6′′, J = 4.2 Hz), 7.49 (d, 1H, H-12, J = 7.5 Hz), 7.42 (t, 1H, H-3), 7.40 (t, 1H, H-4′′), 7.28–7.20 (m, 2H, H-10,11), 7.10–7.01 (m, 3H, H-2,4,9), 6.59 (t, 1H, H-5′′), 6.45 (d, 1H, H-3′′, J = 8.1 Hz), 5.93 (s, 1H, H-6), 4.68 (s, 2H, NH2), 3.78 (m, 1H, H-2b′), 3.58 (m, 1H, H-2a′), 3.29 (s, 1H, H-6a), 1.43 (m, 2H, H-3′), 1.15 (m, 2H, H-4′), 0.77 (t, 3H, H-5′); 13C NMR (75.47 MHz, CDCl3) δC 166.9 (C-7), 158.6 (C-2′′), 154.2 (C-4a), 151.1 (C-13a), 147.8 (C-6′′), 139.7 (C-12a), 137.8 (C-4′′), 133.5 (C-3), 129.0 (C-12), 128.4 (C-8a), 126.5 (C-1), 126.3 (C-10), 125.2 (C-11), 122.2 (C-2), 121.8 (C-9), 120.2 (C-13b), 118.6 (C-4), 113.8 (C-5′′), 108.8 (C-3′′), 96.3 (C-6), 68.4 (C-2′), 49.2 (C-6a), 31.2 (C-3′), 19.0 (C-4′), 13.6 (C-5′); MS (ES+) m/z 431.2 [M5a + H]+, m/z 337.2 [M4a + H]+, m/z 359.2 [M4a + Na]+ and m/z 695.3 [2 M4a + Na]+; HRMS (m/z): calcd [M5a(4a+2a)]+ for C25H26N4O3: 430.2005 was not observed, found: 359.1386 [M4a + Na]+ and 95.0568 [M2a + H]+; νmax(KBr): 3415, 3336, 3221, 1678, 1617, 1459, 1305, 1095, 758 cm−1.
4.1.2 Intermolecular hydrogen bonded self-assembly 5b (4a + 2b)
White cottony, yield 75%. 1H NMR (300 MHz, CDCl3) δH 8.97 (s, 1H, N-H), 8.28 (dd, 1H, H-1, J = 8.1 Hz), 7.91 (d, 1H, H-6′′, J = 5.4 Hz), 7.52 (d, 1H, H-12, J = 7.8 Hz), 7.40 (t, 1H, H-3), 7.28–7.18 (m, 2H, H-11, 10), 7.09–7.01 (m, 3H, H-2,4, H9), 6.46 (d, 1H, H-5′′, J = 5.1 Hz), 6.31 (s, 1H, H-3′′), 5.93 (s, 1H, H-6), 4.51 (s, 2H, NH2), 3.76 (m, 1H, H-2b′), 3.56 (m, 1H, H-2a′), 3.29 (s, 1H, H-6a), 2.22 (s, 3H, CH3–4a′′), 1.41 (m, 2H, CH2–3′), 1.13 (m, 2H, CH2–4′), 0.77 (t, 1H, CH3–5′), 13C NMR (75.47 MHz, CDCl3) δC 166.8 (C-7), 158.6 (C-2′′), 154.1 (C-4a), 151.1 (C-13a), 148.9 (C-4′′), 147.4 (C-6′′), 139.6 (C-12a), 133.5 (C-3), 128.9 (C-8a), 128.3 (C-12), 126.5 (C-1), 126.2 (C-10), 125.2 (C-11), 122.2 (C-2), 121.7 (C-9), 120.1 (C-13b), 118.6 (C-4), 115.4 (C-5′′), 109.0 (C-3′′), 96.3 (C-6), 68.4 (C-2′), 49.1 (C-6a), 31.2 (C-3′), 21.0 (C-4a′′), 19.0 (C-4′), 13.6 (C-5′); MS (ES+) m/z 445.3 [M5b + H]+, m/z 337.2 [M4a + H]+, m/z 359.2 [M4a + Na]+ and m/z 695.3 [2 M4a + Na]+, HRMS (m/z): calcd [M5b(4a+2b)]+ for C26H28N4O3: 444.2161 was not observed, found: 359.1387 [M4a + Na]+, 695.2849 [2 M4a + Na]+ and 131.1608 [M2b + Na]+; νmax(KBr): 3417, 3337, 3222, 1679, 1618, 1460, 1306, 1098, 760 cm−1.
4.1.3 Intermolecular hydrogen bonded self-assembly 5c (4b + 2a)
White cottony, yield 62%. 1H NMR (300 MHz, CDCl3) δH 8.27 (dd, 1H, H-1, J = 7.8 Hz), 8.22 (s, 1H, N-H), 8.06 (dd, 1H, H-6′′, J = 5.1 Hz), 7.52 (d, 1H, H-12, J = 7.8 Hz), 7.43 (m, 1H, H-4′′), 7.42 (t, 1H, H-3), 7.25–7.22 (m, 2H, H-10, 11), 7.07–7.02 (m, 3H, H-2,4,9), 6.62 (m, 1H, H-5′′), 6.49 (dd, 1H, H-3′′, J = 8.1), 5.85 (s, 1H, H-6), 4.49 (s, 2H, NH2), 3.48 (s, 1H, OCH3–2′), 3.34 (s, 1H, H-6a); 13C NMR (75.47 MHz, CDCl3) δC 166.5 (C-7), 158.6 (C-2′′), 154.0 (C-4a), 151.0 (C-13a), 147.9 (C-6′′), 139.6 (C-12a), 137.7 (C-4′′), 133.4 (C-3), 128.4 (C-8a), 128.3 (C-12), 126.5 (C-1), 126.4 (C-10), 125.5 (C-11), 122.2 (C-2), 120.1 (C-13b), 118.6 (C-4), 113.9 (C-5′′), 108.9 (C-3′′), 97.2 (C-6), 55.9 (C-2′), 49.1 (C-6a); HRMS (m/z): calcd [M5c(4b+2a)]+ for C22H20N4O3: 388.1535 was not observed, found: 317.0914 [M4b + Na]+ and 95.0567 [M2a + H]+; νmax(KBr): 3410, 3335, 3220, 1677, 1618, 1460, 1302, 1075, 754 cm−1.
4.1.4 Intermolecular hydrogen bonded self-assembly 5d (4b + 2b)
White cottony, yield 58%. 1H NMR (300 MHz, CDCl3) δH 8.25 (d, 1H, H-1, J = 7.8 Hz), 8.38 (s, 1H, N-H), 7.84 (d, 1H, H-6′′, J = 4.2 Hz), 7.48 (d, 1H, H-12, J = 7.8 Hz), 7.42 (t, 1H, H-3), 7.26–7.22 (m, 2H, H-1110), 7.08–7.02 (m, 3H, H-2, 9, 4), 6.48 (d, 1H, H-5′′, J = 5.7 Hz), 6.36 (s, 1H, H-3′′), 5.83 (s, 1H, H-6), 5.02 (s, 2H, NH2), 3.45 (s, 3H, OCH3). 3.32 (s, 1H, H-6a), 2.25 (s, 3H, CH3). 13C NMR (75.47 MHz, CDCl3) δC 166.5 (C-7), 157.3 (C-2′′), 153.8 (C-4a), 151.0 (C-13a), 150.08 (C-4′′), 143.6 (C-6′′), 139.5 (C-12a), 133.6 (C-3), 128.6 (C-8a), 128.2 (C-12), 126.5 (C-1), 126.3 (C-10), 125.4 (C-11), 122.3 (C-2), 121.7 (C-9), 120.0 (C-13b), 118.6 (C-4), 115.2 (C-5′′), 110.07 (C-3′′), 97.3 (C-6), 55.9 (C-2′), 48.9 (C-6a), 21.29 (C-4a′′). HRMS (m/z): calcd [M5d(4b+2b)]+ for C23H22N4O3: 402.1692 was not observed, found: 317.0915 [M4b + Na]+ and 109.0732 [M2b + H]+; νmax(KBr): 3411, 3338, 3223, 1679, 1618, 1461, 1306, 1085, 774 cm−1.
4.1.5 Intermolecular hydrogen bonded self-assembly 5e (4c + 2a)
White cottony, yield 41%. 1H NMR (300 MHz, CDCl3) δH 8.28 (d, 1H, H-1, J = 7.8 Hz), 8.23 (s, 1H, N-H), 8.05 (d, 1H, H-6′′, J = 4.2 Hz), 7.49 (d, 1H, H-12, J = 7.8 Hz), 7.43 (m, 1H, H-4′′), 7.38 (t, 1H, H-3), 7.30–7.21 (m, 3H, H-2,10, 11), 7.04–7.01 (m, 2H, H-4,9), 6.61 (m, 1H, H-5′′), 6.47 (d, 1H, H-3′′, J = 8.4 Hz), 5.95 (s, 1H, H-6), 4.47 (s, 2H, NH2), 3.85 (m, 1H, H-2b′), 3.67 (m, 1H, H-2a′), 3.31 (s, 1H, H-6a), 1.09 (t, 3H, CH3–3′). 13C NMR (75.47 MHz, CDCl3) δC 166.6 (C-7), 158.6 (C-2′′), 154.2 (C-4a), 151.2 (C-13a), 148.0 (C-6′′), 139.7 (C-12a), 137.7 (C-4′′), 133.6 (C-3), 128.6 (C-8a), 128.4 (C-12), 126.5 (C-1), 126.3 (C-10), 125.4 (C-11), 122.2 (C-2), 121.6 (C-9), 120.1 (C-13b), 118.6 (C-4), 114.0 (C-5′′), 108.6 (C-3′′), 96.1 (C-6), 64.2 (C-2′), 49.1 (C-6a), 14.8 (C-3′); HRMS (m/z): calcd [M5e(4c+2a)]+ for C23H22N4O3: 402.1692 was not observed, found: 309.1198 [M4c + H]+ and 95.0569 [M2a + H]+; νmax(KBr): 3410, 3337, 3223, 1679, 1615, 1460, 1305, 1082, 765 cm−1.
4.1.6 Intermolecular hydrogen bonded self-assembly 5f (4c + 2b)
White cottony, yield 55%. 1H NMR (300 MHz, CDCl3) δH 8.40 (s, 1H, N-H), 8.28 (d, 1H, H-1, J = 7.8 Hz), 7.90 (d, 1H, H-6′′, J = 5.4 Hz), 7.50 (dd, 1H, H-12, J = 7.8 Hz), 7.39 (t, 1H, H-3), 7.30–7.24 (m, 3H, H-11, 10, 2), 7.08–7.05 (m, 2H, H-4,9), 6.47 (d, 1H, H-5′′, J = 5.4 Hz), 6.32 (s, 1H, H-3′′), 5.96 (s, 1H, H-6), 4.44 (s, 2H, NH2), 3.83 (m, 1H, H-2b′), 3.64 (m, 1H, H-2a′), 3.32 (s, 1H, H-6a), 2.22 (s, 3H, CH3), 1.09 (t, 3H, H-3′). 13C NMR (75.47 MHz, CDCl3) δC 166.7 (C-7), 158.4 (C-2′′), 154.2 (C-4a), 151.1 (C-13a), 150.8 (C-4′′), 147.5 (C-6′′), 139.6 (C-12a), 133.6 (C-3), 128.7 (C-8a), 128.3 (C-12), 126.5 (C-1), 126.3 (C-10), 125.3 (C-11), 122.2 (C-2), 121.6 (C-9), 120.1 (C-13b), 118.6 (C-4), 115.5 (C-5′′), 109.0 (C-3′′), 96.0 (C-6), 64.2 (C-2′), 49.1 (C-6a), 21.0 (C-4a′′), 14.8 (C-3′); HRMS (m/z): calcd [M5f(4c+2b)]+ for C24H24N4O3: 416.1848 was not observed, found: 309.1198 [M4c + H]+ and 109.0729 [M2b + H]+; νmax(KBr): 3411, 3338, 3223, 1679, 1618, 1461, 1306, 1085, 774 cm−1.
4.2 General procedure for the synthesis of the 6(O-alkylated)benzochromeno-1,5-benzodiazepinones (4a–c)
Compounds 4a–c were synthesized from 262 mg of 1 in 80 mL of n-butanol, methanol and ethanol, respectively. The mixture was refluxed under stirring for five hours. The reaction was stopped due to the formation of some decomposition products as evidenced by TLC analysis. Concentration in vacuo of the obtained solution gave a pale yellow precipitate. Successive recrystallization using diethyl ether, THF and petroleum ether allowed the isolation of compounds 4a in good yield, and respectively 4b and 4c in lower yield.
4.2.1 6-(O-butyl) benzochromeno [4,3-c][1,5] benzodiazepin-7(10H)-one (4a)
White cottony, yield 74%. Mp = 147.5 °C. 1H NMR (300 MHz, CDCl3) δH 8.25 (dd, 1H, H-1, J = 7.8 Hz), 7.93 (s, 1H, N-H), 7.50 (dd, 1H, H-12, J = 7.8 Hz), 7.38 (t, 1H, H-3), 7.28 (t, 1H, H-10), 7.21 (m, 1H, H-9), 7.10–7.00 (m, 3H, H-2, 4, 11), 5.93 (s, 1H, H-6), 3.76 (m, 1H, H-2b′), 3.56 (m, 1H, H-2a′), 3.31 (s, 1H, H-6a), 1.41 (m, 2H, CH2–3′), 1.16 (m, 2H, CH2–4′), 0.77 (t, 3H, CH3–5′). 13C NMR (75.47 MHz, CDCl3) δC 166.6 (C-7), 154.2 (C-4a), 151.2 (C-13a), 139.6 (C-12a), 133.5 (C-3), 128.6 (C-8a), 128.4 (C-12), 126.5 (C-1), 126.3 (C-9), 125.4 (C-10), 122.2 (C-4), 121.6 (C-11), 120.1 (C-13b), 118.6 (C-2), 96.3 (C-6), 68.4 (C-2′), 49.2 (C-6a), 31.2 (C-3′), 19.0 (C-4′), 13.6 (C-5′). ESI (HRMS) m/z calcd for C20H20N2O3 336.1474 found 337.1566 [M4a + H]+ and 359.1387 [M4a + Na]+; νmax(KBr): 3466, 1678 cm−1.
4.2.2 6-(O-methyl) benzochromeno [4,3-c][1,5] benzodiazepin-7(10H)-one (4b)
White cottony, yield 45%. Mp = 167.5 °C. 1H NMR (300 MHz, CDCl3) δH 8.26 (dd, 1H, H-1, J = 7.8 Hz), 7.83 (s, 1H, N-H), 7.49 (d, 1H, H-12, J = 7.8 Hz), 7.39 (t, 1H, H-3), 7.27 (t, 1H, H-10), 7.18 (m, 1H, H-9), 7.11–6.99 (m, 3H, H-2, 4, 11), 5.83 (s, 1H, H-6), 3.45 (s, 3H, OCH3), 3.32 (s, 1H, H-6a), 13C NMR (75.47 MHz, CDCl3) δC 166.4 (C-7), 153.9 (C-4a), 150.9 (C-13a), 139.6 (C-12a), 133.7 (C-3), 128.5 (C-8a), 128.4 (C-12), 126.6 (C-1), 126.3 (C-9), 125.5 (C-10), 122.4 (C-2), 121.6 (C-11), 120.1 (C-13b), 118.6 (C-4), 97.4 (C-6), 55.9 (C-2′). 49.0 (C-6a); ESI (HRMS) m/z calcd for C17H14N2O3 294.1004 found 295.1078 [M4b + H]+ and 317.0914 [M4b + Na]+; νmax(KBr): 3469, 1674.
4.2.3 6-(O-ethyl) benzochromeno [4,3-c][1,5] benzodiazepin-7(10H)-one (4c)
White cottony, yield 49%. Mp = 154.5 °C. 1H NMR (300 MHz, CDCl3) δ (ppm) 8.26 (d, 1H, H1, J = 7.8 Hz), 7.79 (s, 1H, N-H), 7.49 (d, 1H, H-12, J = 7.8 Hz), 7.39 (t, 1H, H-3), 7.27 (t, 1H, H-10), 7.21 (m, 1H, H-9), 7.05–7.09 (m, 3H, H-2, 4, 11), 5.93 (s, 1H, H-6), 3.82 (m, 1H, H-2b′), 3.63 (m, 1H, H-2a′), 3.32 (s, 1H, H-6a), 1.10 (t, 3H, H-3′). 13C NMR (75.47 MHz, CDCl3) δ(ppm) 166.6 (C-7), 153.9 (C-4a), 150.9 (C-13a), 139.7 (C-12a), 133.7 (C-3), 128.7 (C-8a), 128.4 (C-12), 126.4 (C-1), 126.3 (C-9), 125.4 (C-10), 122.2 (C-2), 121.6 (C-11), 120.1 (C-13b), 118.6 (C-4), 96.1 (C-6), 64.2 (C-2′), 49.1 (C-6a), 14.8 (C-3′); ESI (HRMS) m/z: calcd for C18H16N2O3 308.1161 found 309.1198 [M4c + H]+ and 331.0978 [M4c + Na]+; νmax(KBr): 3467, 1678.
4.3 Reaction of the 6-(O-alkyl)benzochromeno-1,5-benzodiazepinones (4a–c) with 2-aminopyridine (2a,b)
A mixture of compounds 4a–c respectively with 1 equiv of 2-aminopyridine 2a and 1 equiv of 2-amino-4-méthylpyridine 2b respectively in 50 mL of dry THF was heated at 40 °C for 3 h. The resulting mixture was allowed to stand at room temperature and then kept under stirring for 12 h. Then the mixture was evaporated to dryness under reduced pressure to give a pale yellow oil residue, which solidified after adding petroleum ether. Recrystallization from THF/petroleum ether (1:3) afforded the corresponding self-assembled 5a–f.
4.4 Crystallography
Single-crystal diffraction data were collected on an Xcalibur, Atlas, Gemini ultra diffractometer, Cu Kα radiation (λ = 1.5418 Å) for compound 5e. The structure was solved by direct methods using the SIR97 program and then refined with the aid of the WINGX program.
Cristal data for compound 5e (C18H16N2O3·C5H6N2); M = 402.45, T = 150 K; triclinic , a = 6.849 (5) Å, b = 10.387 Å, c = 15.298 Å, α = 106.251 (5)°, β = 91.102 (5)°, γ = 97.942 (5)°, v = 1032.9 (10) A3, Z = 2, d = 1.294 Mg m−3, μ = 0.71 mm−1, A final refinement on F2 with unique intensities and parameters converged at ωR(F2) = 0.085.
5 Supplementary material
Crystallographic data for structural analysis have been deposited with the Cambridge Crystallographic Data Centre, No. 948433, for self-assembly 5e. Copies of this information may be obtained free of charge from the CCDC, 12 Union Road, Cambridge CB2, IEZ, UK (e-mail: deposit@ccdc.cam.ac.uk or http://www.ccdc.ac.uk).
Acknowledgements
We gratefully acknowledge Dr. Marie-Thérèse Martin for 2D-NMR experiments. The authors acknowledge Dr. Sébastien Vidal and Dr. Erwann Jeanneau (University Claude-Bernard Lyon-1) for X-ray crystallographic analysis.


