1 Introduction
More than a decade ago, we have developed an interesting protocol for the synthesis of Schiff bases in which the condensation process was performed in a less expected solvent for this purpose, that is water [1,2]. By using this aqueous imine synthesis protocol, we planned to perform the synthesis of recalcitrant diimines 1, starting from low-reactivity carbonyl compounds and aliphatic diamines (Scheme 1). Compounds 1 were to be studied for their properties as modulators of multi-drug resistance [3].

Attempted diimine synthesis.
Before attempting the aqueous synthesis of the diimines 1, we initially decided to obtain these compounds through an already known procedure in order to have comparative, standard samples. Screening the scientific literature, we found no examples of such diimines synthesis. Moreover only few examples of such ketones monoimine synthesis have been displayed [4–7]. We then decided to apply a method relying on condensation in refluxing dimethylacetamide [5], but we opted to employ the more usual dimethylformamide as the solvent. To our surprise, this condensation process led to rather unexpected results.
2 Results and discussion
We started with four simultaneous condensation processes by refluxing 1,6-hexamethylenediamine (HMDA) 2 in dimethylformamide (DMF) with diverse aromatic ketones. The progress of the reaction was monitored by TLC (alumina, benzene-ethanol 1:1). The starting ketone spots remained clearly visible, while a new spot corresponding to a reaction product appeared. An experimental detail soon appeared intriguing fact: in all processes the product spots presented the exact same retention factor. Work-ups afforded the same crystalline residues, which were immediately submitted to 1H NMR analysis. All 1H NMR spectra were identical, presenting well-characterized signals in the aliphatic area and a sharp singlet around 8.00 ppm, without any characteristic signals for aromatic protons. At that point we realized our mistake, DMF being known to be “much more than a solvent”, as pointed out by Muzart [8] and namely known as a slow formylation reagent for both aromatic and aliphatic amines [9]. Therefore, the natural conclusion was that a reaction indeed took place and that the reagents in that process were HMDA and DMF, without any participation of the less reactive ketones. The reaction was repeated in the same conditions, using only HMDA and DMF, refluxed for 3 h, and the same reaction product was isolated with excellent yield (95%).
This compound was identified as 4,5,6,7,8,9-hexahydro-1,3-diazonine 3 through 1H and 13C NMR and IR analysis. In particular, the NCH signal at 8.00 ppm was identical with the one appearing in the sole literature reference [10]. Compound 3 was the result of the reaction of some imidazolinium perchlorates with 1,ω-diamines, but was not properly identified.
We supposed that the process was in fact a two-step reaction. In the first step, formylation of one of the two amino moieties occurred and, since formylation of aliphatic amines is quite slow [9], only one function was transformed. In the second step, an intramolecular condensation took place, leading to annulated compound 3 (Scheme 2). Moreover, the condensation step could be affected by the vicinity of an amino group, as it was previously suggested for the formation of imine under the influence of aniline [11].
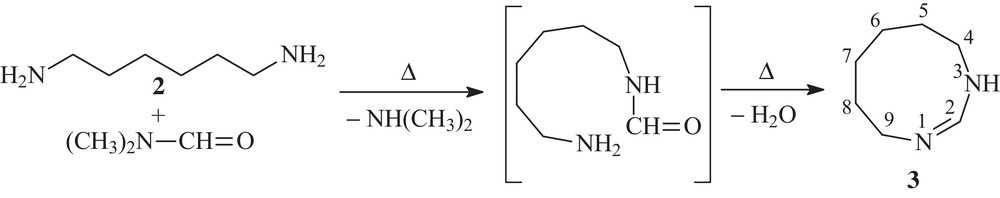
Synthesis of diazonine 3, with probable reaction pathway.
Some cyclic formamidine compounds have been previously obtained through a formylation-condensation strategy, but only as cyclic trimers of aromatic diamines [12]. In that case, triethyl orthoformate was used as the formylation reagent.
In order to emphasize the applicability of this method to the synthesis of non-aromatic nitrogen-containing macrocycles, we refluxed diethylenetriamine 4 and triethylenetetramine 5 with DMF according to the same procedure, followed by the same work-up. In these two cases, after diethylether washing, we were pleased to observe that the condensation reaction delivered the oily macrocycles 6 and 7 in excellent yield. The 1H NMR spectra of the two products 6 and 7 displayed the same proton signal in the vicinity of 8.00 ppm, along with characteristic signals for the ethylene moieties (Scheme 3).

Synthesis of polyazaheterocyclic compounds 6 and 7.
Interestingly, in all 1H NMR spectra, the NH proton appeared highly deshielded, as a broad singlet just over the CH signal, due to a very rapid proton exchange between NHCHN and NCHNH. Introducing two drops of D2O in the NMR probe led to the disappearance of the signal, thus confirming this assumption.
As a limitation, the method seemed applicable only to aliphatic diamines. Indeed starting from o-phenylenediamine the condensation with DMF failed, even after refluxing for 24 h. A possible explanation could be the difference of basicity between the aliphatic and aromatic diamines.
3 General experimental procedure
A solution of the amine (0.1 mol: 1.16 g for hexamethylenediamine, 1.03 g for diethylenetriamine, 1.46 g for triethylenetetramine) in DMF (10 mL) was refluxed for 3 h. Before the end of the process, toluene (5 mL) was added to the reaction mixture which was then submitted to vacuum distillation. Ethyl ether (50 mL) was added to the crude oily residue. The crystalline precipitate of 3 was filtered off. For 6 and 7 the ethereal solution was dried over MgSO4 and concentrated under vacuum to deliver the corresponding oil.
4,5,6,7,8,9-Hexahydro-1H-1,3-diazonine 3 (95%): m.p. = 102-3 °C; 1H NMR (300 MHz, δ ppm, J Hz, DMSO-d6, TMS): 1.24 (4 H, br. s, C6H; C7H), 1.38 (4 H, quint, J = 6.6, C5H; C8H), 3.05 (4 H, dd, J = 6.6, 12.4, C4H; C9H), 7.97 (1 H, s, C2H), 7.99 (1 H, br. s, N3H); 13C NMR (75 MHz, δ ppm, DMSO-d6, TMS): 26.0 (C6; C7), 29.0 (C5; C8), 30.9 (C4; C9), 161.0 (C2); IR (neat, ν, cm−1): 1213 (C–N), 1631 (CN), 2944–3031 (CH), 3279 (NH); Anal (%): calcd. for C7H14N2: C: 66.62; H: 11.18; N: 22.20; found: C: 66.63; H: 11.10; N: 22.27.
1,3,6-Triaza-cyclo-oct-1-ene 6 (94%): oil, 1H NMR (300 MHz, δ ppm, DMSO-d6, TMS): 3.19–3.39 (9 H, m, C4H; C5H; C7H; C8H; N6H), 7.92 (1 H, s, C2H), 8.05 (1 H, br. s, N3H); 13C NMR (75 MHz, δ ppm, DMSO-d6, TMS): 34.7 (C5;C7), 35.5 (C4), 46.1 (C8), 161.5 (C2); IR (neat, ν, cm−1): 1218 (C–N), 1643 (CN), 2958–3020 (CH), 3284 (NH), 3454 (NH).
1,3,6,9-Tetraaza-cyclo-undec-1-ene 7 (92%): oil, 1H NMR (300 MHz, δ ppm, DMSO-d6, TMS): 3.19–3.41 (14 H, m, C4H; C5H; C7H; C8H; C10H; C11H; N6H; N9H), 8.02 (1 H, s, C2H), 8.06 (1 H, br. s, N3H); 13C NMR (75 MHz, δ ppm, DMSO-d6, TMS): 34.7 (C7; C8), 35.4 (C5; C10), 43.6 (C4), 45.7 (C11), 161.6 (C2); IR (neat, ν, cm−1): 1219 (C–N), 1642 (CN), 2876–3055 (CH), 3283 (NH), 3496 (NH).
4 Conclusion
A single-time mentioned, undescribed 9-membered cyclic formamidine has been obtained through a successive formylation-condensation process with dimethylformamide playing a dual role, both as formylation reagent and reaction solvent. The scope of the procedure was broadened by the straightforward and efficient synthesis of two other polyazaheterocycles.


