1 Introduction
Selective partial oxidation of alkanes is an important topic with potential in terms of economic and ecological perspectives of sustainable chemistry [1]. However, efficient catalytic oxidation of alkanes [2] still remains as a challenging topic. The development of more active catalysts, under mild conditions, at room temperature and using low-toxicity media and oxidizing agents is needed. Hydrogen peroxide is one of the best options in this regard since H2O is the sole by-product. Thus, peroxidative (with H2O2) alkane oxidations were the selected reactions for the present study.
Cyclohexane was the chosen substrate in view of the significance of the oxidized products (cyclohexanol and cyclohexanone) for the manufacturing of adipic acid, Nylon-6,6’ and polyamide-6 [1(a)–(i)]. Moreover, the industrial process of cyclohexane oxidation needs to be improved, since it requires considerably harsh conditions (150 °C) and leads to low yields (ca. 4%) to assure a good selectivity (ca. 85%) [1(j)].
The use of multicopper complexes as catalysts for cycloalkane oxidation reactions [2(a),2(g),3,4] is rather promising, since copper is cheap and widely found in nature, being present in the active sites of many oxidation enzymes. An example is particulate methane monooxygenase (pMMO), that bears an active site with a multicopper cluster with N,O-environment, and appears to be a unique copper enzyme capable of catalyzing the hydroxylation of alkanes and alkene epoxidation [3(p), 3(q), 3(r)]. The use of a multicopper complex to mimic the action of this enzyme was initiated by our group [3(f)]. However, in spite of other subsequent works [2(a)Ch.3, 3(g)], the application of such a type of complexes for the oxidation of alkanes under mild conditions still remains an underdeveloped area of research.
A topic of high importance in catalysis is the role of the used solvents. The increasing awareness of the detrimental health and environmental effects of some organic solvents led to the search for greener technologies [2(m)]. Ionic liquids (ILs) have received a great deal of attention for a possible replacement of volatile organic solvents (VOCs) in catalytic oxidations, mainly due to their non-measurable vapor pressure and good solubility for other salts, as well as the possibility of recycling the catalyst and enhancing the yield and selectivity of the product [5(a)], using a simple protocol. Reaction types successfully performed in ILs include Diels–Alder [5(b), 5(d), 6], aldol [5(b), 5(d), 7(a)], and several oxidations [5(a), 5(b), 5(d)]. In addition, ILs may act as catalysts, co-catalysts or supports in the catalytic process [5(b), 5(d)]. However, their use in the fields of alkane functionalization remains virtually unexplored [5(b), 5(d), 5(f)].
Ionic conductivity, chemical and thermal stability, polarity and electrochemical potential window [4(m)] are some properties to take in account in the selection of the most suitable IL as a solvent for a certain reaction. For example, the conductivity of an IL can allow a faster mass transfer, but its high viscosity can act as an obstacle in the same process, and a careful choice of ILs is always required. 1-Butyl-3-methylimidazolium hexafluorophosphate, [bmim][PF6] (Fig. 1), was the chosen IL due to its appealing properties, such as good thermal stability, high polarity, hydrophobicity, and ability to dissolve many compounds, as well as the fact this IL is typically inert and stable, namely when used as a solvent in chemical reactions. In particular, it is known to be resistant towards oxidation [8(a), 8(b)] and degradation in the presence of hydrogen peroxide [8(c)]. In addition, a preliminary electrochemical study, by cyclic voltammetry, of [bmim][PF6] was performed in this study in order to confirm its redox stability and suitable application in oxidative catalysis.
1-Butyl-3-methylimidazolium hexafluorophosphate ([bmim][PF6]).
Hence, a main aim of this study is to extend the catalytic oxidation of cyclohexane to an IL medium, in particular [bmim][PF6], in replacement of a conventional organic solvent, and check if the catalytic performance could be improved by using such a type of medium.
Taking in account our recent successful application of the tetranuclear complex, containing a cuboid cage, bis(μ4-(ae)-cyclohexane-1,4-dicarboxylato-O,O′,O′′,O′′′)-tetracopper(II) [(CuL)2(μ4-O,O′,O′′,O′′′′-CDC)]2·2H2O (1) (Fig. 2) as a catalyst for the peroxidative (with H2O2) oxidation of cyclohexane in acetonitrile [3(a)], we have selected this catalyst for the current study in IL. Its catalytic performance is compared with those observed in three molecular solvents, and the effects of additives are also investigated.

(Color online.) Bis(μ4-(ae)-cyclohexane-1,4-dicarboxylato-O,O′,O′′,O′′′)-tetracopper(II) [(CuL)2(μ4-O,O′,O′′,O′′′′-CDC)]2·2H2O (1) [HL = 2-(2-pyridylmethyleneamino)benzenesulfonic acid, CDC = cyclohexane-1,4-dicarboxylate] [3(a)].
2 Experimental
2.1 Materials and physical methods
All the reagents and solvents were purchased from commercial sources and used as received. Water used in all the syntheses and catalytic studies was double distilled. The bis(μ4-(ae)-cyclohexane-1,4-dicarboxylato-O,O′,O′′,O′′′)-tetracopper(II) complex 1 has been synthesized according to the reported procedure and characterized accordingly [3(a)].
1-Butyl-3-methylimidazolium hexafluorophosphate, [bmim][PF6], was prepared by anion exchange of 1-butyl-3-methylimidazolium bromide upon reaction with Na[PF6], using a standard literature method [3(o)] and used after drying for 24 h at 70 °C under high vacuum whilst stirring. The 1H and 13C NMR spectra were recorded at ambient temperature on a Bruker Avance II + 300 (UltraShield™ Magnet) spectrometer operating at 300.130 and 75.468 MHz for proton and carbon-13, respectively. The chemical shifts are reported in ppm using tetramethylsilane as an internal reference.
The electrochemical experiments were performed on an EG&G PAR 273A potentiostat/galvanostat connected to a personal computer through a GPIB interface. Cyclic voltammetry (CV) studies were undertaken in 0.2 M [nBu4N][BF4]/NCMe or [bmim][PF6], at a platinum disc working electrode (d = 0.5 mm) and at room temperature. A Pt wire was employed as the counter-electrode. The solutions were saturated with N2 by bubbling this gas for ca. 1 min before each run. The redox potentials were measured versus the ferrocene/ferricinium (Fc/Fc+) redox pair.
2.2 General procedure for the peroxidative oxidation of cyclohexane
The cycloalkane oxidations were carried out under air, in a biphasic based IL system, contained in a round bottom flask, with vigorous stirring, and using [bmim][PF6] as a solvent (up to a total volume of 5.0 mL). Typically, the copper catalyst was added to the solvent as a solid or in the form of a stock solution in the IL. Cyclohexane (2.3 mmol) was then introduced, and the reaction started when hydrogen peroxide (50% in H2O, 0.68 mL, 11 mmol) was added in one portion. The final concentrations of the reactants in the reaction mixture were as follows: catalyst precursor (2·10−4–2·10−2 mol·L−1), substrate (0.46 mol·L−1), H2O2 (2.2 mol·L−1) and pyridine (0.005 mol·L−1). The reaction was stopped and 5 mL of diethylether were added for extraction of the organic products.
The catalyst's recyclability was investigated using the IL medium, for up to three consecutive cycles. Each cycle was initiated after the preceding one upon addition of new typical portions of all other reagents. After completion of each run, the products were analyzed and the IL with the catalyst was recovered by drying in vacuo overnight at 70 °C.
All products formed were identified by GC and their retention times confirmed using those of commercially available products. Nitromethane (0.05 mL) was used as a GC internal standard. Chromatographic measurements were performed in a Fisons Instruments GC 8000 series gas chromatograph with a BP20/SGE (30 m × 0.22 mm × 0.25 mm) capillary column (FID detector) and using helium as a carrier gas, whereas the analyses of the chromatographic peaks were done by the corresponding Jasco-Borwin v.1.50 software. A PerkinElmer Clarus 600 gas chromatograph, equipped with two capillary columns (SGE BPX5; 30 m 0.32 mm 25 mm), one having an EI–MS (electron impact) detector and the other one with a FID detector, were used for analyzing the reaction mixtures. Helium was used as the carrier gas. The reaction mixtures were analyzed twice by GC: with and without adding an excess of solid triphenylphosphine. The addition of this phosphine to the final organic phase reduces cyclohexyl hydroperoxide, if present, to the corresponding alcohol, and hydrogen peroxide to water. Comparison of the results of both analyses allows estimate the amount of cyclohexyl hydroperoxide, following a method developed by Shul’pin [9]. Blank experiments in ionic liquid were performed and confirmed that no alkane oxidation products (or only traces, below 1%) were obtained in the absence of the metal catalyst. The catalytic activity of the ligand was also tested and no products were detected.
3 Results and discussion
The bis(μ4-(ae)-cyclohexane-1,4-dicarboxylato-O,O′,O′′,O′′′)-tetracopper(II) complex [(CuL)2(μ4-O,O′,O′′,O′′′′-CDC)]2·2H2O (1) [HL = 2-(2-pyridylmethyleneamino)benzenesulfonic acid, CDC = cyclohexane-1,4-dicarboxylate] (Fig. 2) was tested as a catalyst for the peroxidative (with H2O2) oxidation of cyclohexane (CyH), using 1-butyl-3-methylimidazolium hexafluorophosphate ([bmim][PF6]) as a solvent, at 50 °C (Scheme 1).
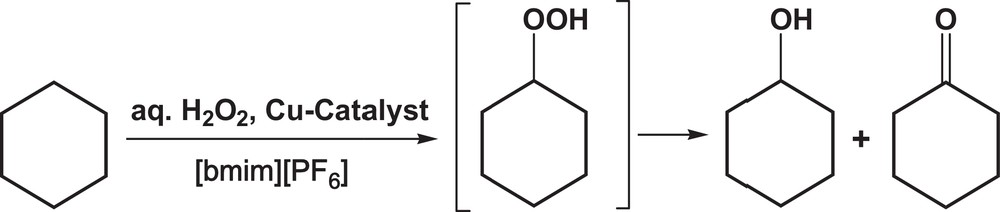
Peroxidative oxidation of cyclohexane catalyzed by the tetranuclear Cu(II) complex 1, in [bmim][PF6].
In view of the oxidative nature of the catalysis, the redox stability of [bmim][PF6] was first investigated by cyclic voltammetry (CV) at a platinum-working electrode at room temperature. It did not exhibit any redox activity in the wide potential range from–2.0 to 2.0 V vs. Fc/Fc+ (internal reference) as depicted in Fig. 3. However, this wide electrochemical window, which is favorable to the application of the IL, is significantly reduced in the presence of water (Fig. 3) on account not only of the redox properties of water but also, e.g., of the susceptibility of [PF6]– to hydrolysis [4(b),4(m)]. Hence, the presence of water cannot be neglected. In any case, the higher stability of [PF6]– relatively to other common used anions in ILs [10], together with the ability of [bmim][PF6] to easily dissolve 1, makes this IL a good choice for use as a solvent.
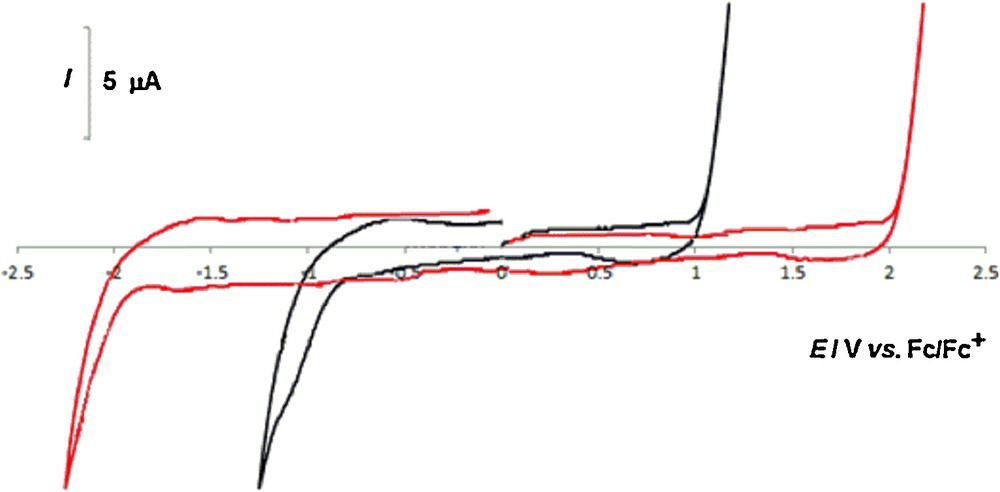
(Color online.) Cyclic voltammogram, initiated by the anodic sweep, of [bmim][PF6], at a Pt disc working electrode (d = 0.5 mm), run at a scan rate of 200 mV·s−1. () vacuum-dried [bmim][PF6]; () wet [bmim][PF6].
Cyclohexane is oxidized to cyclohexanol (CyOH) and cyclohexanone (CyO), via cyclohexyl hydroperoxide (CyOOH, primary product), according to Scheme 1, and the results are shown in Table 1. Shul’pin‘s method [9] was used, where the addition of PPh3 to the samples prior to the GC analysis is applied to prove indirectly the presence of CyOOH. PPh3 reduces CyOOH to CyOH, resulting in an increase in the alcohol amount and a decrease in that of CyO. This behavior has been observed in several peroxidative oxidation systems [2(h),2(r),3(g),11,12].
Oxidation of cyclohexane to cyclohexanol and cyclohexanone catalyzed by the tetranuclear Cu(II) complex 1, in [bmim][PF6]a.
| Entry | Catalyst amount, (mol·L−1) | Additive (Py) (mol·L−1) | Time (min) | Yield %b | Total TONc | Total TOF (h−1) | [Keto]/[Alc]d | Conversione % | ||
| Cyclo-hexanone | Cyclo-hexanol | Total | ||||||||
| 1 | 2·10−4 | 0.005 | 15 | 3.2 | 1.1 | 4.3 | 99 | 396 | 2.9 | 4.8 |
| 2 | 60 | 9.8 | 2.4 | 12.2 | 281 | 281 | 4.0 | 12.4 | ||
| 3 | 120 | 19.0 | 4.0 | 23.0 | 529 | 265 | 4.8 | 23.1 | ||
| 4 | 240 | 15.2 | 5.1 | 20.3 | 467 | 117 | 3.0 | 24.0 | ||
| 5 | 2·10−3 | 0.005 | 15 | 5.0 | 1.8 | 6.8 | 16 | 63 | 2.8 | 7.0 |
| 6 | 60 | 10.2 | 3.9 | 14.0 | 32 | 32 | 2.6 | 15.3 | ||
| 7 | 120 | 25.3 | 8.9 | 34.2 | 79 | 39 | 2.9 | 34.9 | ||
| 8 | 240 | 22.0 | 9.0 | 31.0 | 71 | 18 | 2.4 | 35.0 | ||
| 9 | 2·10−2 | 0.005 | 15 | 7.3 | 1.5 | 8.8 | 2.0 | 8.1 | 4.7 | 9.0 |
| 10 | 60 | 14.8 | 3.2 | 18.0 | 4.1 | 4.1 | 4.6 | 19.0 | ||
| 11 | 120 | 28.9 | 7.1 | 36.0 | 8.3 | 4.1 | 4.1 | 36.1 | ||
| 12 | 240 | 23.6 | 9.7 | 33.3 | 7.7 | 1.9 | 2.4 | 36.3 | ||
| 13f | 2·10−2 | 0.005 | 120 | 7.7 | 4.3 | 12.0 | 2.8 | 1.4 | 1.8 | 12.2 |
| 14g | 7.9 | 5.3 | 13.2 | 3.0 | 1.5 | 1.5 | 13.2 | |||
| 15h | 8.6 | 7.3 | 15.9 | 3.7 | 1.8 | 1.2 | 16.3 | |||
| 16 | 2·10−2 | _ | 120 | 24.8 | 6.6 | 31.4 | 7.2 | 3.6 | 3.8 | 31.4 |
| 17i | 0.005 | 15.7 | 7.7 | 23.4 | 5.4 | 2.7 | 2.0 | 23.5 | ||
| 18 | – | – | 120 | 0.7 | 0.1 | 0.8 | – | – | 7.0 | 0.9 |
| 19j | 2·10−2 | 0.005 | 120 | 2.7 | 1.2 | 3.9 | 0.9 | 0.5 | 2.3 | 4.0 |
a Reaction conditions, unless stated otherwise: [cyclohexane]0 = 0.46 mol·L−1, [total H2O2]0 = 2.2 mol·L−1 (50% aqueous), [bmim][PF6] up to 5 mL total volume, 50 °C.
b Based on GC analysis, after treatment with PPh3; moles of cyclohexanol + cyclohexanone per 100 moles of cyclohexane.
c Total turnover number (moles of cyclohexanol + cyclohexanone per mole of catalyst).
d Ratio between the concentrations of cyclohexanone (Keto) and cyclohexanol (Alc).
e Conversion (%) = [1 – (number of unreacted moles of reagent after reaction/initial number of moles of reagent)] × 100.
f Using acetone as a solvent.
g Using methanol as a solvent.
h Using acetonitrile as a solvent.
i Using TFA as an additive.
j Using CuCl2 as a catalyst.
The effects of a basic and an acid additive were also tested and a yield-enhancing effect of pyridine was found (Table 1, entries 11 and 16), whereas no promoting effect was observed upon addition of trifluoroacetic acid (TFA) (Table 1, entries 16 and 17). Hence, the reactions were typically studied in the presence of pyridine. This base conceivably promotes the proton-transfer steps involved in the formation of the hydroxyl radical from H2O2 [13(a)].
A blank control experiment carried out in [bmim][PF6] but in the absence of 1 resulted in a negligible product yield (lower than 1%, Table 1, entry 18).
The effect of catalyst loading in the overall yield was investigated, and the highest yield (36%) was obtained at the highest concentration of catalyst used (2·10−2, Table 1, entry 11 and Fig. 4). The highest yield is obtained after a 2-h reaction time, beyond which a minor decrease is observed on account of a slight overoxidation, as shown namely by the appearance of 1,4-cyclohexanedione, detected by GC–MS. This constitutes a significant improvement on the reaction time relatively to the optimized system in acetonitrile [3a], which, to obtain the same total yield of products, requires a double reaction time (4 h). The faster kinetics in the present case may be related to the IL influence on the energy of transitional states along the reaction [13].
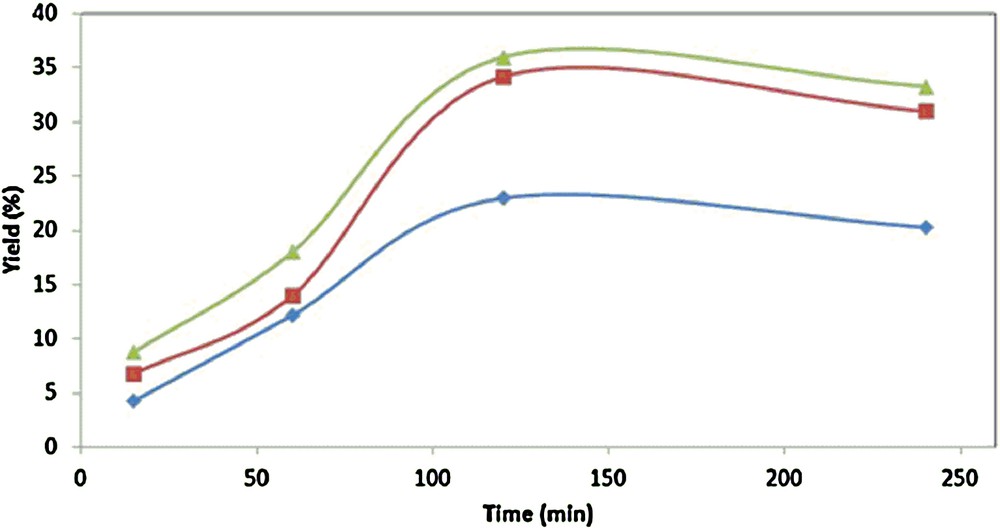
(Color online.) Overall product yield vs. reaction time in the oxidation of cyclohexane catalyzed by 1 in [bmim][PF6] at different catalyst concentrations: = 2·10−2 mol·L−1of 1, = 2·10−3 mol·L−1 of 1 and = 2·10−4 mol·L−1of 1.
Another advantage, besides the shorter reaction times, in the use of [bmim][PF6] relatively to acetonitrile and other tested molecular solvents (acetone and methanol, see below) concerns the increased selectivity towards cyclohexanone, which eventually may have potential commercial significance [1]. The obtained cyclohexanone/cyclohexanol molar ratio, for [bmim][PF6], varies between 2.4 and 4.8 (Table 1 and Fig. 5), whereas it is lower than 2 for all the tested molecular solvents (Table 1, entries 13–15).
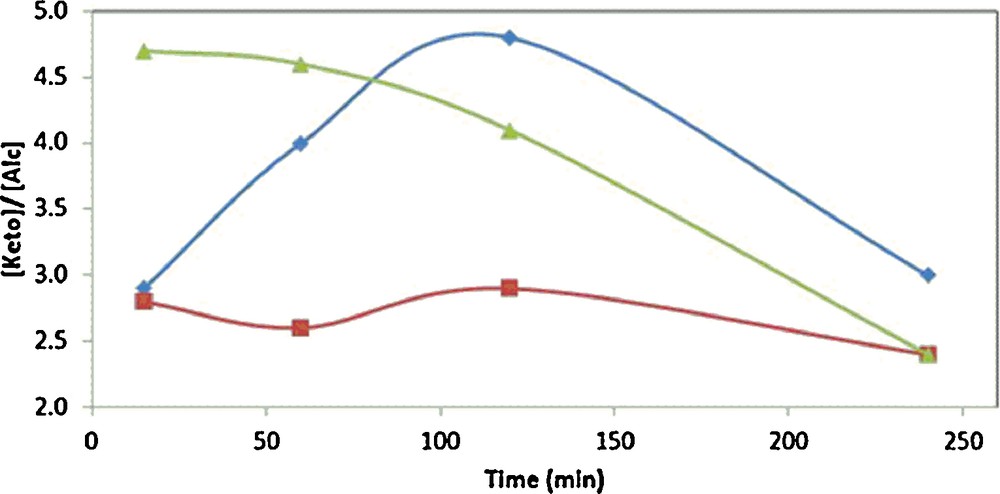
(Color online.) Variation of the molar ratio of cyclohexanone/cyclohexanol products along with the reaction time in cyclohexane oxidation catalyzed by 1 in [bmim][PF6]. = 2·10−2 mol·L−1 of 1, = 2·10−3 mol·L−1 of 1 and = 2·10−4 mol·L−1 of 1.
A comparison of the obtained product yields and turnover numbers (TONs) for the IL and the tested organic solvents (Table 1, entries 11 and 13–15) under identical experimental conditions shows the following order, which agrees with their increasing polarity: acetone < methanol < acetonitrile < [bmim][PF6]. Although further studies and examples are necessary to confirm such a tendency and to understand the promoting effect of this IL, it could be related with its non-coordinating ability, thus making the catalyst active site more accessible to the substrate. Such a trend is consistent with those reported in a few other cases [13(a), 14(g)]. The charge and the steric bulkiness of this IL, which can be described as a polymeric supramolecular species with weak interactions [14], can possibly create an electrostatic and steric colloid-type stabilization of the copper(II) catalyst. The complete solubility of 1 in the ionic medium all along the reaction is also a favorable feature.
We have also performed a control experiment with the IL, the catalyst and H2O2, but without cyclohexane, and no product formation was observed.
Thus, the investigated IL appears to act as a solvent only and, to our knowledge, the possible competition of an IL with an alkane has never been mentioned [8(b), 8(c), 14(h)].
A similar enhancement of yield, TON and selectivity was found by some of us for the oxidation of cyclohexane catalyzed by multinuclear copper (II) AHBD complexes in the related [bmim][BF4] medium [5(a)].
In order to get an insight into the mechanism of the catalytic reaction, the oxidation of cyclohexane was performed in the presence of a radical trap (CBrCl3 or Ph2NH as a trap for C- or O-centered radicals, respectively) also used in homogeneous conditions [3(a)]. The drastic decrease in the yield (by ca. 95–87%) suggests that the reaction proceeds mainly via a radical mechanism. By analogy with the proposed mechanisms for 1 in acetonitrile [3(a)], other copper(II) catalysts [2(r),3(a)–(i), 4(c)–(f), 5(a)] and other Mn+1/n (e.g., V, Re, Fe or Au) systems [4(h),4(i),11(a),11(b),15], we can propose (see Scheme 2) the metal-catalyzed decomposition of hydrogen peroxide leading to the oxygen-centered radicals HOO• and HO• upon oxidation by Cu(II) or reduction by Cu(I), respectively (reactions 1 and 2). Water is formed and is believed to catalyze H+-shift steps towards the formation of HO• [13(a), 16(a), 16(c)]. The cycloalkyl radical Cy• is then formed upon H-abstraction from cycloalkane CyH by HO• (reaction 3). Reaction of Cy• with dioxygen leads to CyOO• (reaction 4), and CyOOH can then be formed upon H-abstraction from H2O2 by CyOO• (reaction 5) or upon reduction of the latter to CyOO− by Cu(I) followed by protonation. Metal-assisted decomposition of CyOOH to CyO• and CyOO• (reactions 6 and 7) would then lead ultimately to the cyclohexanol (CyOH) and cyclohexanone (Cy-H = O) products (reactions 8 and 9) [2(h), 3(a), 3(g), 11(a), 15(e), 16(c)–(f)].
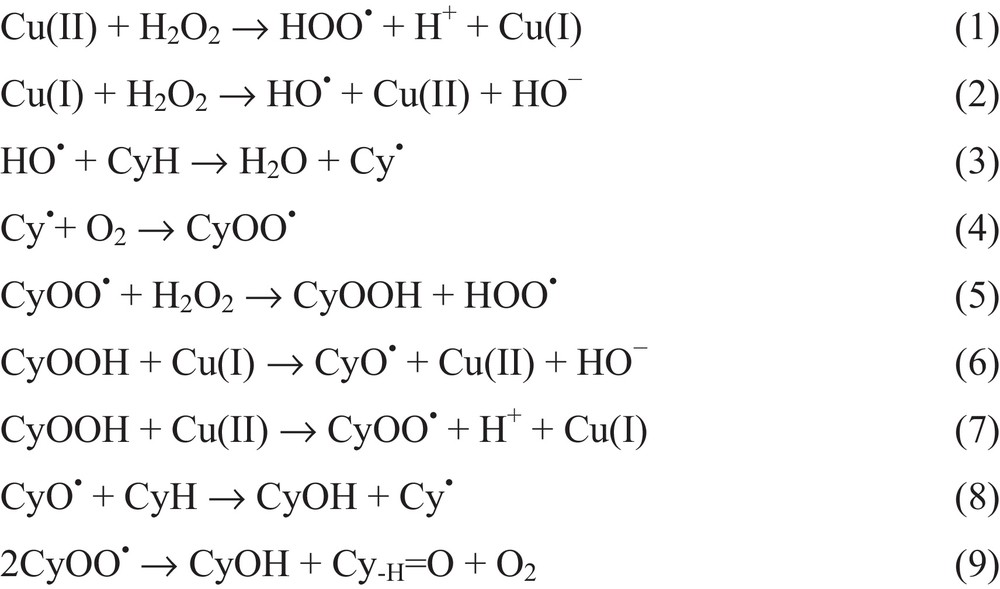
Possible pathways for catalytic oxidation of cycloalkane (CyH) to cycloalkanol (CyOH) and cycloalkanone (Cy-H = O).
Another factor with significance in the type of mechanism is provided by the size of the copper cage in complex 1. In a free-radical mechanism, the cyclohexane substrate does not need to interact directly with the metal center for the reaction to occur. In fact, X-ray diffraction analysis of 1 [3(a)] shows that the cage presents an available empty space of 16.1 Å3, which is too small to accept a cyclohexane molecule (estimated volume of 108 Å3). However, it is consistent with the free-radical mechanism proposed above.
The catalyst recyclability was investigated using the IL medium, for up to three consecutive cycles. Each new cycle was initiated upon addition of new portions of all reagents, except copper catalyst 1. After completion of each run, the products were analyzed as previously and the IL with the catalyst was recovered by drying in vacuo overnight at 70 °C. The activity and selectivity of catalyst 1 is maintained through the three consecutive cycles (Fig. 6, Table 2).
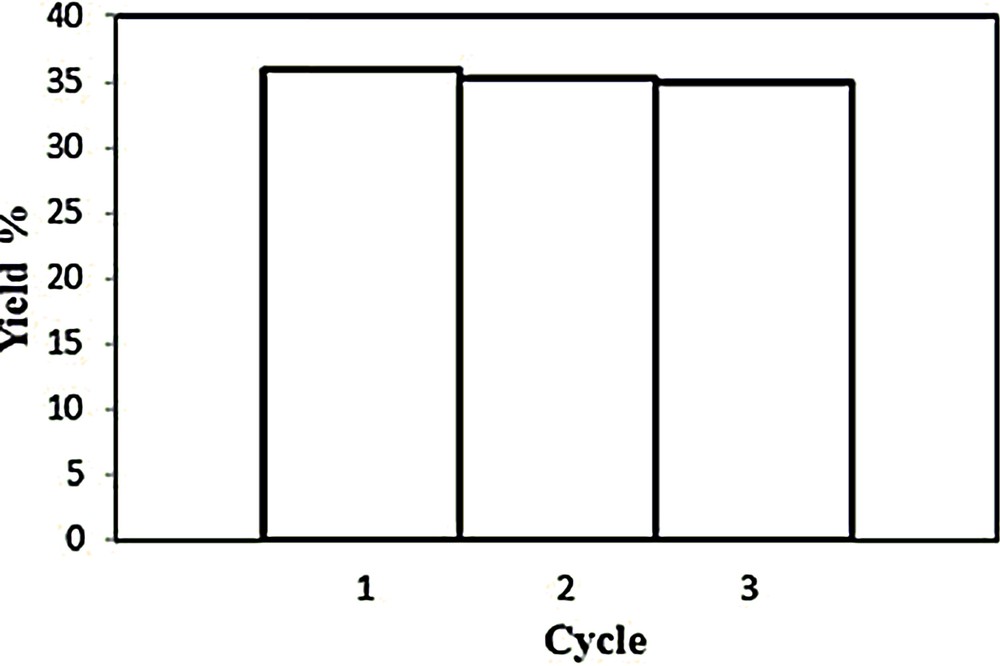
Effect of catalyst 1 recycling on the overall product yield, in the peroxidative oxidation of cyclohexane, in IL medium. Reaction conditions: [cyclohexane]0 = 0.46 mol·L−1, [total H2O2]0 = 2.2 mol·L−1 (50% aqueous), [1] = 2·10−3 mol·L−1. [bmim][PF6] up to 5 mL total volume, 50 °C. Reaction time: 120 min. Each bar represents a run, and the respective yield values are taken from Table 2.
Recycling in the peroxidative oxidation of cyclohexane to cyclohexanol and cyclohexanone catalyzed by the Cu(II) complex (1) in [bmim][PF6]a.
| Entry | Catalyst amount, mol·L−1 | Yield % | Total TONc | [Keto]/[Alc]d | Level of activity (%) | ||
| Cyclo-hexanone | Cyclo-hexanol | Totalb | |||||
| 1 | 2·10−2 | 28.9 | 7.1 | 36 | 8 | 4.1 | 100 |
| 2 | 28.9 | 6.4 | 35.3 | 8 | 4.5 | 98.1 | |
| 3 | 28.7 | 6.3 | 35.0 | 8 | 4.6 | 97.2 |
a Reaction conditions, unless stated otherwise: [cyclohexane]0 = 0.46 mol·L−1, [total H2O2]0 = 2.2 mol·L−1 (50% aqueous), [bmim][PF6] up to 5 mL total volume, 50 °C, 120 min.
b Based on GC analysis, after treatment with PPh3; moles of cyclohexanol + cyclohexanone per 100 moles of cyclohexane.
c Total turnover number (moles of cyclohexanol + cyclohexanone per mole of catalyst).
d Ratio between the concentrations of cyclohexanone (Keto) and cyclohexanol (Alc).
It is worth to mention that the catalyst recyclability and reuse represents another important improvement relatively to the homogeneous peroxidative oxidation of cyclohexane catalyzed by the same complex for which its separation and recycling was not possible [3(a)].
4 Conclusions
We have applied a biphasic IL system to the catalytic cyclohexane oxidation in the presence of a multinuclear copper catalyst. The produced oxidized products were extracted to the organic phase, minimizing the overoxidation by H2O2.
The 1/H2O2/[bmim][PF6] system, in comparison with the conventional 1/H2O2/NCMe homogeneous one having the most common organic solvent [3(a)], revealed significant improvements on the catalytic performance of 1, in terms of products yield, TON, reaction time, selectivity towards cyclohexanone, easy recycling and reuse (not possible in homogeneous conditions). They are of significance towards the establishment of a green catalytic process.
The interactions between ILs and the catalyst, substrate, oxidant and even reaction intermediates can make ILs act as multi-functional solvents for the catalytic oxidation reactions with H2O2.
However, although ILs can be quite stable towards both oxidation and reduction, which is a good advantage over common organic solvents in the field of redox catalysis, care should be taken when water is present as shown by cyclic voltammetry, which indicates a decrease in the electrochemical window of the IL, at both the anodic and cathodic limits, upon increasing the water content.
More studies should be performed to understand how the properties of the IL (e.g., charge, bulkiness, ionic mobility, viscosity and density) can affect the catalytic route. The generality of the above advantages in the field of alkane oxidation should also be tested by extending the investigation to other alkanes and a variety of catalysts.
Acknowledgments
The financial support from the Fundação para a Ciência e a Tecnologia (FCT), Portugal, for fellowships (SFRH/BPD/90883/2012 to A.P.C.R and SFRH/BPD/78264/2011 to S.H.) and the UID/QUI/00100/2013 project is gratefully acknowledged.


