1 Introduction
Heterocyclic compounds are well-known pharmaceutically active products, and the development of simple and efficient methods of synthesis of compounds incorporating heterocyclic rings has given a new dimension to drug discovery [1]. 1,2,4-triazole and 1,3,4-oxadiazole are important five-membered heterocyclic compounds, which are of great research interest because of their distinct structures with potential applications in synthetic and medicinal chemistry. Heterocyclic compounds containing 1,2,4-triazole and 1,3,4-oxadiazole nuclei have been tested for a wide spectrum of biological activities, including antimicrobial, antitubercular, antiviral, analgesic, anticancer [2–7], and other biological properties, such as genotoxicity and lipid peroxidation [8,9].
Indoles exhibit relatively low toxicity, high biocompatibility, and several pharmacological activities [10,11]. Several 3-substituted indole derivatives have been used as materials for agrochemicals and pharmaceuticals [12–14]. Furthermore, compounds containing 1,3,4-oxadiazole or 1,2,4-triazole rings have been found in many natural products, and have thus become the focus of intense research in recent years on account of their pharmacological activities [15]. Indole unit modification has been widely reported, with a few studies about indole derivatives containing both 1,3,4-oxadiazole and 1,2,4-triazole moieties. According to the pharmacodynamic principle of superposition, 3-substituted indole derivatives containing both 1,3,4-oxadiazole and 1,2,4-triazole groups potentially exhibit effective antibacterial activity.
Green chemistry has been a major inspiration for organic chemists to develop environment-friendly methods for the synthesis of organic compounds with biological value. In recent years, ultrasound irradiation has been utilized to accelerate a number of synthetically useful reactions [16]. Ultrasonic synthesis is a well-established technique in green chemistry that has many advantages compared with the conventional methods, such as enhancement of reactions rates and yield as well as modification of the reaction pathway, greater selectivity, simplicity of operation, and energy-saving protocols. This approach led to the development of a simple purification procedure that also fulfils the concept of green chemistry [17,18].
Our research group has previously reported microwave-assisted synthesis of novel Schiff bases derived from 4-amino-3-[3-(1-benzyl)indole]-5-thiomethyl-1,2,4-triazole [19]. A number of these compounds showed strong antibacterial activity. As a further development of our green agenda [20,21], we report an efficient, high yield, and environment-friendly ultrasound synthesis of novel indole derivatives containing 1,3,4-oxadiazole and 1,2,4-triazole moieties (Scheme 1), as well as an evaluation of the antibacterial activities of these compounds against four pathogenic strains, namely, Escherichia coli (ATCC 35218), Bacillus subtilis (ATCC 6633), Pseudomonas aeruginosa (ATCC 27853), and Staphyloccocus aureus (ATCC 6538).
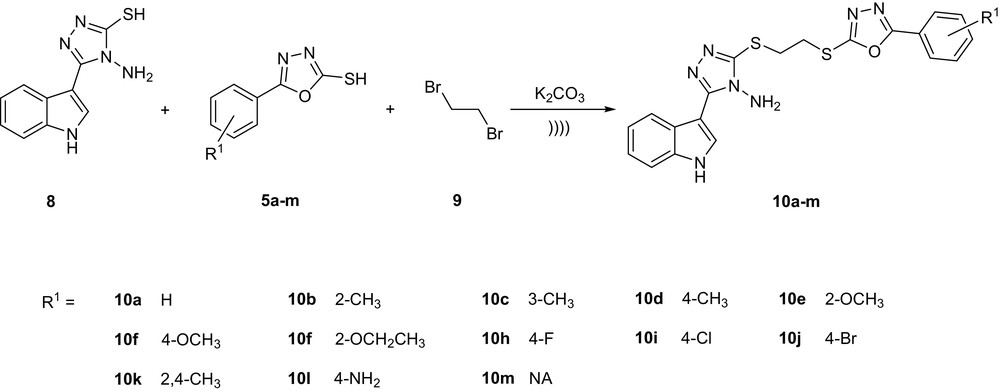
Synthesis protocol of title compounds 10a–m.
2 Results and discussion
We compared the synthesis of 10a–m using ultrasound irradiation, microwave method, and conventional method to study the advantages of the ultrasonic-enhanced reaction. Compared with conventional thermal heating, ultrasonic irradiation decreased the reaction time from 540–900 min to 15–35 min and increased the yields from 40–68% to 82–93% (Table 1). Compared with the microwave method, ultrasonic irradiation increased the yields from 62–86% to 82–93%, and both methods required almost the same reaction time. Thus, ultrasonic irradiation allows a rapid, environment-friendly, and efficient organic synthesis methodology.
Comparison of synthesis of 10a–m using ultrasound irradiation, microwave method, and conventional method.
| Compd. | R1 | Conventional method | Ultrasound method | Microwave method | |||
| t (min) | Yield (%) | t (min) | Yield (%) | t (min) | Yield (%) | ||
| 10a | H | 600 | 56 | 20 | 83 | 15 | 72 |
| 10b | 2-CH3 | 780 | 47 | 25 | 82 | 18 | 69 |
| 10c | 3-CH3 | 720 | 53 | 30 | 87 | 20 | 72 |
| 10d | 4-CH3 | 660 | 62 | 15 | 92 | 12 | 80 |
| 10e | 2-OCH3 | 720 | 54 | 20 | 86 | 15 | 77 |
| 10f | 4-OCH3 | 540 | 68 | 15 | 93 | 10 | 86 |
| 10g | 2-OCH2CH3 | 600 | 57 | 20 | 86 | 16 | 75 |
| 10h | 4-F | 840 | 39 | 30 | 82 | 20 | 68 |
| 10i | 4-Cl | 840 | 47 | 30 | 88 | 20 | 74 |
| 10j | 4-Br | 840 | 42 | 30 | 86 | 20 | 62 |
| 10k | 3,5-CH3 | 720 | 51 | 20 | 85 | 15 | 79 |
| 10l | 4-NH2 | 900 | 40 | 35 | 86 | 22 | 72 |
| 10m | NAa | 780 | 42 | 30 | 82 | 20 | 68 |
a Naphthalene.
Assignment of the selected characteristic infrared (IR) bands of the positions of indole derivatives (10a–m) revealed valuable information about the structure of the compounds. All the compounds exhibited a characteristic strong absorption in the 3208–3452 cm−1 region from the v(N–H) stretching vibration. The IR spectra showed absorption bands characteristic of CN stretching in the 1603–1625 cm−1 region. Intense absorption bands in the 1162–1250, 942–1081, and 699–750 cm−1 regions were attributed to the v(C–S), v(N–N) and v(C–S) stretching vibrations, respectively. Further evidence of indole derivatives was obtained from the proton nuclear magnetic resonance (1H NMR) spectra. The peaks between δ 11.62–11.79 ppm could be assigned to the protons of the indole–NH. Moreover, NH2 protons resonated between δ 5.92–6.19 ppm as a singlet integrating two protons. In addition, the two triplet peaks at 3.58–3.94 ppm and 3.71–4.76 ppm were attributed to the S–CH2 group. The aromatic protons appeared at their usual values. The mass spectra showed the expected high-intensity molecular peaks.
The preliminary results of the antibacterial assays indicated that some of the compounds exhibited moderate to good activity against E. coli and S. aureus. As shown in Table 2, 10b and 10j did not exhibit potent inhibition activity against any of the bacterial strains tested. Notably, 10d was effective in inhibiting Gram-positive bacteria S. aureus, and 10l and 10f were significantly more active against S. aureus than the standard reference drug (amoxicillin). The minimum inhibitory concentration (MIC) values ranged from 2–8 μg/mL (10l and 10f) and 16 μg/mL (10d). Most of compounds showed poor activity against Gram-positive B. subtilis. Table 3 shows that the methoxy derivative 10f and amino-containing 10l were effective at inhibiting Gram-positive S. aureus and E. coli.
Antibacterial activities of 10a-m: Zone of inhibition.
| Compounds | Zone of inhibition (mm)a,b | |||
| B. subtilis | S. aureus | E. coli | P. aeruginosa | |
| 10a | – | 11.3 ± 0.3 | 12.1 ± 0.2 | – |
| 10b | – | – | – | – |
| 10c | – | 11.5 ± 0.4 | 15.6 ± 0.2 | – |
| 10d | – | 20.3 ± 0.3 | 21.8 ± 0.1 | 16.3 ± 0.3 |
| 10e | – | 15.2 ± 0.2 | 15.8 ± 0.3 | – |
| 10f | 18.7 ± 0.3 | 23.8 ± 0.3 | 23.2 ± 0.2 | 18.0 ± 0.3 |
| 10g | 11.8 ± 0.2 | – | – | |
| 10h | 20.3 ± 0.4 | 15.6 ± 0.3 | 15.3 ± 0.4 | – |
| 10i | – | 12.0 ± 0.3 | 12.6 ± 0.2 | – |
| 10j | – | – | – | – |
| 10k | – | – | 15.1 ± 0.2 | 15.9 ± 0.2 |
| 10l | 20.9 ± 0.2 | 25.3 ± 0.3 | 21.1 ± 0.2 | 21.5 ± 0.2 |
| 10m | – | 11.2 ± 0.4 | 15.6 ± 0.2 | – |
| Amoxicillin | 25.9 ± 0.4 | 23.5 ± 0.3 | 22.9 ± 0.4 | 23.5 ± 0.2 |
| Ciprofloxacin | 30.0 ± 0.3 | 29.6 ± 0.5 | 25.3 ± 0.5 | 31.4 ± 0.4 |
| DMSO | – | – | – | – |
a Zone of inhibitions less than 10 mm are not shown in the table.
b Reference and compounds (40 μg/mL).
Antibacterial activities of 10a–m: minimum inhibitory concentration (MIC) and half-maximal inhibitory concentration (IC50).
| Compounds | B. subtilis | S. aureus | E. coli | P. aeruginosa | ||||
| MIC | IC50 | MIC | IC50 | MIC | IC50 | MIC | IC50 | |
| 10a | >128 | >64 | 64 | 44.5 | 64 | 35.5 | >128 | >64 |
| 10b | >128 | >64 | >128 | >64 | >128 | >64 | >128 | >64 |
| 10c | >128 | >64 | 64 | 32.3 | 32 | 17.9 | 128 | >64 |
| 10d | 128 | >64 | 16 | 8.5 | 16 | 8.3 | 64 | 33.8 |
| 10e | >128 | >64 | 32 | 16.9 | 32 | 16.4 | >128 | >64 |
| 10f | 32 | 18.5 | 8 | 4.6 | 8 | 4.5 | 32 | 17.2 |
| 10g | 128 | >64 | 64 | 44.5 | 128 | >64 | 128 | >64 |
| 10h | 16 | 8.5 | 32 | 16.4 | 32 | 16.3 | 128 | >64 |
| 10i | >128 | >64 | 64 | 35.8 | 64 | 32.1 | >128 | >64 |
| 10j | >128 | >64 | >128 | >64 | >128 | >64 | >128 | >64 |
| 10k | >128 | >64 | 128 | >64 | 64 | 16.4 | 64 | 33.6 |
| 10l | 8 | 4.9 | 2 | 1.2 | 8 | 4.7 | 16 | 8.2 |
| 10m | >128 | >64 | 64 | 45.8 | 32 | 16.1 | >128 | >64 |
| Amoxicillin | 2 | 1 | 16 | 6.4 | 16 | 7.3 | 4 | 2.5 |
| Ciprofloxacin | 0.128 | 0.1 | 3.12 | 1.82 | 0.25 | 0.18 | 0.5 | 0.41 |
Determination of MIC and half-maximal inhibitory concentration (IC50) of the active compounds suggested that amino-containing derivative 10l showed maximum activity against most of the strains tested, with MIC of 8 μg/mL for E. coli, 8 μg/mL for B. subtilis, 16 μg/mL for P. aeruginosa and 2 μg/mL for S. aureus. Compound 10l demonstrated notable activity against S. aureus, with MIC values at 2 μg/mL and IC50 at 1.2 μg/mL, showing its potential as a drug against S. aureus. The importance of this study lies in the possibility that the new compounds might be more effective against bacteria. A thorough investigation of the structure–activity relationship, toxicity, and biological effects of the novel compounds may facilitate the design of more potent antibacterial agents for therapeutic use.
3 Conclusion
An ultrasonic-assisted synthesis method was developed for the preparation of novel indole derivatives containing 1,3,4-oxadiazole and 1,2,4-triazole moieties. Compared with the conventional and microwave methods, the present method has many advantages such as shorter reaction times, high yields, and compliance with green chemistry. Compound 10l exhibited promising antibacterial activity against S. aureus similar to that of the standard drugs amoxicillin and ciprofloxacin, indicating that this compound is a promising antibacterial compound for further research. Compounds 10f and 10l exhibited potency against Gram-positive E. coli similar to that of amoxicillin; thus, these compounds are almost comparable to amoxicillin, indicating that they are potentially important pharmacophores for the design of novel antibacterial agents. Studies to establish their in vitro efficacy and safety are recommended for further development.
4 Experimental
4.1 Apparatus, materials and analysis
All chemicals were obtained from commercial suppliers, and the solvents were purified before use. The melting points were obtained on a micro-melting point system with an uncorrected thermometer. The NMR spectra were measured on an Agilent-NMR-VNMRS400 (400 MHz for 1H and 100 MHz for 13C) spectrometer at room temperature using DMSO-d6 as a solvent and TMS as the internal standard. IR spectra were recorded on a 1700 PerkinElmer FTIR spectrometer on KBr disks. Mass spectra were obtained on a Finnigan LCQ-DECA instrument. Elemental analyses (C, H, and N) were performed with an Elementar vario MICRO cube auto analyzer, and the results agreed with the calculated values. All the experiments were monitored by thin-layer chromatography (TLC) on pre-coated silica gel plates. All reactions were performed in a commercial ultrasound and microwave combination apparatus (XH-300UL, ultrasound 25 kHz frequency 0–1,500 W, microwave 0–1,000 W 2,450 MHz, Beijing Xianghu Science and Technology Development Co. LTD, Beijing, China). Hydrazide (3) [22], 2-mercapto-5-substituted-1,3,4-oxadiazoles (5a-m) [23], indole 3-carboxylic acid hydrazide (7) [24], and 4-amino-5-(1H-indol-3-yl)-4H-[1,2,4]triazole-3-thiol (8) [19] were prepared according to previously reported methods (Scheme 2). The melting points of 2-mercapto-5-substituted-1,3,4-oxadiazole 5a–m are shown in Table 4.
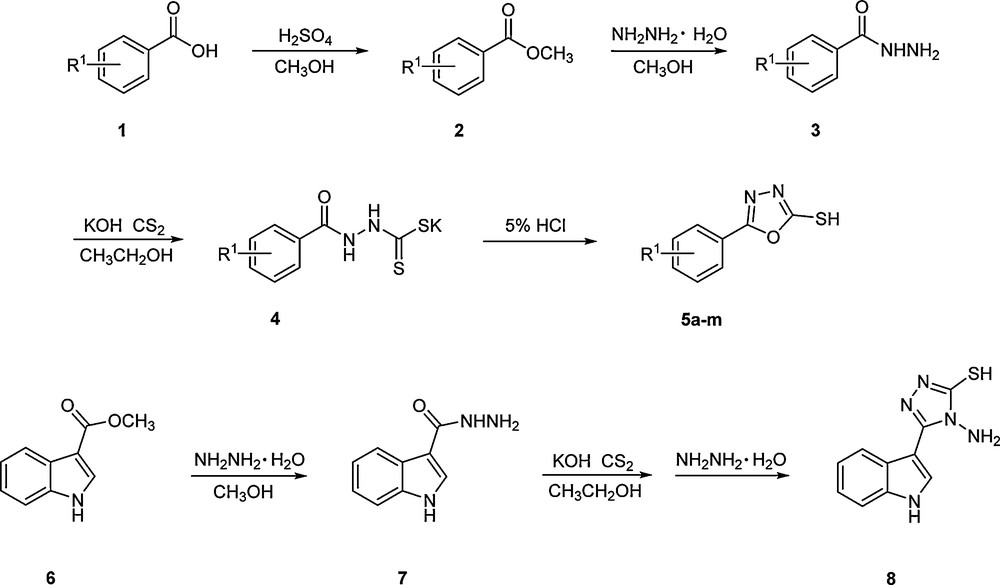
Synthesis protocol of 5a–m and 8.
Melting points of 2-mercapto-5-substituted-1,3,4-oxadiazoles.
| Product | Formula | R1 | Melting point (°C) | Literature melting point (°C) |
| 5a | C8H6N2OS | H | 217–218 | 218 [23] |
| 5b | C9H8N2OS | 2-CH3 | 164–165 | 164–166 [25] |
| 5c | C9H8N2OS | 3-CH3 | 205–206 | 205 [23] |
| 5d | C9H8N2OS | 4-CH3 | 150–151 | 150 [23] |
| 5e | C9H8N2O2S | 2-OCH3 | 212–213 | 213–215 [26] |
| 5f | C9H8N2O2S | 4-OCH3 | 203–204 | 204 [23] |
| 5g | C10H10N2O2S | 2-OCH2CH3 | 195–196 | 195–197 [27] |
| 5h | C8H5FN2OS | 4-F | 157–158 | 156–158 [28] |
| 5i | C8H5ClN2OS | 4-Cl | 174–175 | 175 [23] |
| 5j | C8H5BrN2OS | 4-Br | 205–206 | 205 [29] |
| 5k | C10H10N2OS | 3,5-CH3 | 237–238 | 237–240 [25] |
| 5l | C8H7N3OS | 4-NH2 | 240–241 | 239–240 [29] |
| 5m | C12H8N2OS | NAa | 215–216 | 215–216 [30] |
a Naphthalene.
4.2 Compound 8
4-amino-5-(1H-indol-3-yl)-4H-[1,2,4]triazole-3-thiol: brown solid, yield 80%, mp 258–259 °C (ethanol). 1H NMR (400 MHz, DMSO-d6): δH 5.90 (s, 2H, NH2), 7.22 (t, J = 7.2 and 8.0 Hz, 1H, Indol–H), 7.28 (t, J = 6.8 and 8.0 Hz, 1H, Indol–H), 7.56 (d, J = 8.0 Hz, 1H, Indol–H), 8.16 (d, J = 7.6 Hz, 1H, Indol–H), 8.47 (d, J = 2.8 Hz, 1H, Indol–H), 11.80 (s, 1H, NH), 13.73 (s, 1H, SH). 13C NMR (100 MHz, DMSO-d6): δC 100.97, 112.45, 120.98, 121.21, 122.88, 125.13, 128.33, 136.14, 147.62, 165.62.
4.3 Ultrasound irradiation for the preparation of 10a–m
4-Amino-5-(1H-indol-3-yl)-4H-[1,2,4]triazole-3-thiol (8) (0.1 mmol), 1,2-dibromoethane (9) (0.1 mmol), the 2-mercapto-5-substituted-1,3,4-oxadiazoles (5a–m) (0.1 mmol), K2CO3 (0.2 mmol), and acetonitrile (10 mL) were placed in a 25-mL round-bottom flask. The mixture was sonicated (25 kHz, constant frequency) at 30 °C for 15–35 min. The progress of the reaction was monitored using TLC. When the reaction was complete, the precipitation was filtered, subsequently washed with 5 mL of acetonitrile, and concentrated in vacuo to get the crude solid products. The product was recrystallized from ethanol with 82–93% yields. The physical and spectra data of the compounds 10a–m are as follows.
4.3.1 Compound 10a
3-(1H-indol-3-yl)-5-[[2-[(5-phenyl-1,3,4-oxadiazol-2-yl)thio]ethyl]thio]-4H-1,2,4-triazol-4-amine: yellow solid, yield 83%, mp 113–114 °C (ethanol). IR (KBr, cm−1): v 3423, 3041, 2998, 2953, 2925, 2852, 1609, 1559, 1493, 1468, 1411, 1201, 1198, 1078, 1064, 922, 768, 701. 1H NMR (400 MHz, DMSO-d6): δH 3.67 (t, J = 6.4 and 7.6 Hz, 2H, CH2), 3.81 (t, J = 6.8 and 7.2 Hz, 2H, CH2), 6.19 (s, 2H, NH2), 7.21 (t, J = 7.2 and 7.6 Hz, 1H, Indol–H), 7.27 (t, J = 7.6 and 6.8 Hz, 1H, Indol–H), 7.55 (d, J = 8.0 Hz, 2H, ArH), 7.62–7.68 (m, 3H, Indol–H and ArH), 8.09 (d, J = 7.2 Hz, 1H, ArH), 8.32 (d, J = 9.6 Hz, 1H, Indol–H), 8.24 (s, 1H, Indol–H), 11.72 (s, 1H, NH). 13C NMR (100 MHz, DMSO-d6): δC 31.38, 32.59, 102.10, 112.11, 120.57, 121.76, 122.61, 123.53, 125.79, 126.15, 126.95, 129.81, 132.76, 136.08, 150.88, 152.38, 163.65, 165.71. ESI–MS m/z (%): 436 ([M+1]+, 100). Anal. calcd. for C20H17N7OS2: C, 55.16; H, 3.93; N, 22.51. Found C, 55.27; H, 3.92; N, 22.53.
4.3.2 Compound 10b
3-(1H-indol-3-yl)-5-[[2-[[5-(2-methylphenyl)-1,3,4-oxadiazol-2-yl]thio]ethyl]thio]-4H-1,2,4-triazol-4-amine: white solid, yield 82%, mp 196–197 °C (ethanol). IR (KBr, cm−1): v 3354, 3171, 3112, 3077, 2969, 2927, 1624, 1585, 1474, 1454, 1246, 1206, 1142, 1041, 1006, 944, 730, 699. 1H NMR (400 MHz, DMSO-d6): δH 2.58 (s, 3H, Ar–CH3), 3.58 (t, J = 6.0 and 8.0 Hz, 2H, CH2), 3.71 (t, J = 7.6 and 6.4 Hz, 2H, CH2), 6.09 (s, 2H, NH2), 7.11 (t, J = 7.6 and 7.2 Hz, 1H, Indol–H), 7.17 (t, J = 7.6 and 7.2 Hz, 1H, Indol–H), 7.34–7.41 (m, 2H, ArH), 7.45 (d, J = 7.6 Hz, 2H, ArH), 7.91 (d, J = 8.0 Hz, 1H, Indol–H), 8.21 (d, J = 8.4 Hz, 1H, Indol–H), 8.24 (d, J = 2.0 Hz, 1H, Indol–H), 11.62 (s, 1H, NH). 13C NMR (100 MHz, DMSO-d6): δC 21.90, 31.36, 32.57, 102.08, 112.10, 121.76, 120.58, 122.61, 122.67, 125.78, 126.14, 126.95, 129.24, 131.85, 132.11, 136.07, 137.89, 150.86, 152.37, 163.24, 165.86. ESI–MS m/z (%): 450 ([M+1]+, 100). Anal. calcd. for C21H19N7OS2: C, 56.11; H, 4.26; N, 21.81. Found C, 56.24; H, 4.25; N, 21.78.
4.3.3 Compound 10c
3-(1H-indol-3-yl)-5-[[2-[[5-(3-methylphenyl)-1,3,4-oxadiazol-2-yl]thio]ethyl]thio]-4H-1,2,4-triazol-4-amine: white solid, yield 87%, mp 171–172 °C (ethanol). IR (KBr, cm−1): v 3434, 3250, 3155, 2923, 2852, 1623, 1591, 1561, 1480, 1413, 1239, 1215, 1178, 1066, 1013, 983, 793, 755, 717. 1H NMR (400 MHz, DMSO-d6): δH 2.38 (s, 3H, Ar–CH3), 3.87 (t, J = 5.6 and 6.4 Hz, 2H, CH2), 4.70 (t, J = 6.4 and 6.0 Hz, 2H, CH2), 5.92 (s, 2H, NH2), 7.11 (t, J = 7.2 and 7.6 Hz, 1H, Indol–H), 7.21 (t, J = 7.6 and 7.2 Hz, 1H, Indol-H), 7.42–7.45 (m, 2H, ArH), 7.50 (d, J = 7.6 Hz, 1H, Indol–H), 7.72–7.77 (m, 2H, ArH), 8.12 (d, J = 8.0 Hz, 1H, Indol–H), 8.42 (d, J = 2.4 Hz, 1H, Indol–H), 11.79 (s, 1H, NH). 13C NMR (100 MHz, DMSO-d6): δC 21.22, 30.43, 30.80, 100.36, 112.44, 121.02, 121.28, 122.93, 123.33, 123.96, 125.07, 127.11, 128.96, 129.63, 132.99, 136.13, 139.26, 147.10, 163.34, 165.63, 165.93. ESI–MS m/z (%): 450 ([M+1]+, 100). Anal. calcd. for C21H19N7OS2: C, 56.11; H, 4.26; N, 21.81. Found C, 56.01; H, 4.25; N, 21.88.
4.3.4 Compound 10d
3-(1H-indol-3-yl)-5-[[2-[[5-(4-methylphenyl)-1,3,4-oxadiazol-2-yl]thio]ethyl]thio]-4H-1,2,4-triazol-4-amine: white solid, yield 92%, mp 247–248 °C (ethanol). IR (KBr, cm−1): v 3352, 3282, 3136, 3052, 2922, 1614, 1587, 1500, 1245, 1200, 1182, 1145, 1074, 1008, 936, 743, 723. 1H NMR (400 MHz, DMSO-d6): δH 2.35 (s, 3H, Ar–CH3), 3.60 (t, J = 6.4 and 7.6 Hz, 2H, CH2), 3.74 (t, J = 8.0 and 6.4 Hz, 2H, CH2), 6.11 (s, 2H, NH2), 7.15 (t, J = 6.8 and 7.6 Hz, 1H, Indol–H), 7.21 (t, J = 7.6 and 6.8 Hz, 1H, Indol–H), 7.36 (d, J = 7.6 Hz, 2H, ArH), 7.49 (d, J = 7.6 Hz, 1H, Indol–H), 7.90 (d, J = 8.0 Hz, 2H, ArH), 8.27 (s, 1H, Indol–H), 8.28 (d, J = 2.8 Hz, 1H, Indol–H), 11.65 (s, 1H, NH). 13C NMR (100 MHz, DMSO-d6): δC 21.54, 31.49, 32.55, 102.13, 112.10, 120.55, 120.76, 121.80, 122.61, 125.80, 126.15, 126.89, 130.35, 136.09, 142.48, 150.84, 152.39, 163.624, 165.78. ESI–MS m/z (%): 900 ([M+1]+, 100). Anal. calcd. for C21H19N7OS2: C, 56.11; H, 4.26; N, 21.81. Found C, 56.25; H, 4.27; N, 21.70.
4.3.5 Compound 10e
3-(1H-indol-3-yl)-5-[[2-[[5-(2-methoxyphenyl)-1,3,4-oxadiazol-2-yl]thio]ethyl]thio]-4H-1,2,4-triazol-4-amine: white solid, yield 86%, mp 157–158 °C (ethanol). IR (KBr, cm−1): v 3434, 3353, 3208, 3172, 3107, 3080, 2926, 1624, 1586, 1471, 1268, 1250, 1123, 1044, 1017, 924, 745, 700. 1H NMR (400 MHz, DMSO-d6): δH 3.62 (t, J =7.2 and 6.8 Hz, 2H, CH2), 3.73 (t, J = 7.6 and 6.4 Hz, 2H, CH2), 3.89 (s, 3H, Ar–OCH3), 6.13 (s, 2H, NH2), 7.10–7.23 (m, 3H, Indol–H and ArH), 7.25 (d, J = 8.8 Hz, 1H, ArH), 7.49 (d, J = 8.0 Hz, 1H, Indol–H), 7.57–7.62 (m, 1H, ArH), 7.86 (d, J = 7.2 Hz, 1H, ArH), 8.26 (d, J = 7.6 Hz, 1H, Indol–H), 8.28 (s, 1H, Indol–H), 11.66 (s, 1H, NH). 13C NMR (100 MHz, DMSO-d6): δC 31.30, 32.55, 56.43, 102.08, 112.10, 112.35, 113.06, 120.59, 121.20, 121.76, 122.61, 125.78, 126.16, 130.56, 133.99, 136.08, 150.84, 152.37, 157.70, 163.37, 164.45. ESI–MS m/z (%): 466 ([M+1]+, 100). Anal. calcd. for C21H19N7O2S2: C, 54.18; H, 4.11; N, 21.06. Found C, 54.02; H, 4.12; N, 21.13.
4.3.6 Compound 10f
3-(1H-indol-3-yl)-5-[[2-[[5-(4-methoxyphenyl)-1,3,4-oxadiazol-2-yl]thio]ethyl]thio]-4H-1,2,4-triazol-4-amine: white solid, yield 93%, mp 213–214 °C (ethanol). IR (KBr, cm−1): v 3358, 3145, 3092, 2928, 1619, 1588, 1482, 1243, 1246, 1162, 1038, 933, 793, 702. 1H NMR (400 MHz, DMSO-d6): δH 3.60 (t, J = 8.0 and 6.4 Hz, 2H, CH2), 3.74 (t, J = 6.8 and 8.0 Hz, 2H, CH2), 3.78 (s, 3H, Ar–OCH3), 6.12 (s, 2H, NH2), 7.08 (d, J = 8.4 Hz, 2H, ArH), 7.15 (t, J = 7.6 and 7.2 Hz, 1H, Indol–H), 7.21 (t, J = 7.2 and 8.0 Hz, 1H, Indol–H), 7.49 (d, J = 8.0 Hz, 1H, Indol–H), 7.94 (d, J = 8.8 Hz, 2H, ArH), 8.28 (s, 1H, Indol–H), 8.29 (d, J = 3.6 Hz, 1H, Indol–H), 11.65 (s, 1H, NH). 13C NMR (100 MHz, DMSO-d6): δC 31.53, 32.54, 55.83, 102.13, 112.11, 115.22, 115.87, 120.57, 121.78, 122.61, 125.79, 126.17, 128.81, 136.10, 150.88, 152.39, 162.40, 162.79, 165.62. ESI–MS m/z (%): 466 ([M+1]+, 100). Anal. calcd. for C21H19N7O2S2: C, 54.18; H, 4.11; N, 21.06. Found C, 54.02; H, 4.11; N, 21.14.
4.3.7 Compound 10 g
3-(1H-indol-3-yl)-5-[[2-[[5-(2-ethoxyphenyl)-1,3,4-oxadiazol-2-yl]thio]ethyl]thio]-4H-1,2,4-triazol-4-amine: white solid, yield 86%, mp 244–245 °C (ethanol). IR (KBr, cm−1): 3332, 3141, 3105, 2976, 2926, 2880, 1603, 1585, 1495, 1452, 1245, 1190, 1039, 940, 745, 704. 1H NMR (400 MHz, DMSO-d6): δH 1.35 (t, J = 6.8 Hz, 3H, Ar–OCH2CH3), 3.60–3.63 (m, 2H, CH2), 3.70–3.74 (m, 2H, CH2), 4.17 (q, J = 6.8 Hz, 2H, Ar–OCH2CH3), 6.11 (s, 2H, NH2), 7.08–7.18 (m, 2H, Indol–H), 7.22 (t, J = 8.4 and 7.2 Hz, 2H, ArH), 7.48 (d, J = 8.0 Hz, 1H, Indol–H), 7.54–7.59 (m, 1H, ArH), 7.86 (dd, J = 2.0 and 1.6 Hz, 1H, ArH), 8.24 (d, J = 8.0 Hz, 1H, Indol–H), 8.26 (d, J = 2.4 Hz, 1H, Indol–H), 11.64 (s, 1H, NH). 13C NMR (100 MHz, DMSO-d6): δC 14.94, 31.37, 32.53, 64.63, 102.09, 112.10, 112.62, 114.02, 120.58, 121.14, 121.76, 122.61, 125.79, 126.15, 130.54, 133.93, 136.09, 150.80, 152.38, 157.01, 163.29, 164.65. ESI–MS m/z (%): 480 ([M+1]+, 100). Anal. calcd. for C22H21N7O2S2: C, 55.10; H, 4.41; N, 20.44. Found C, 55.25; H, 4.40; N, 20.39.
4.3.8 Compound 10 h
3-(1H-indol-3-yl)-5-[[2-[[5-(4-fluorophenyl)-1,3,4-oxadiazol-2-yl]thio]ethyl]thio]-4H-1,2,4-triazol-4-amine: yellow solid, yield 82%, mp 244–245 °C (ethanol). IR (KBr, cm−1): v 3301, 3193, 3089, 2967, 2914, 1615, 1540, 1500, 1487, 1264, 1189, 1081, 1030, 964, 789, 705. 1H NMR (400 MHz, DMSO-d6): δH 3.62 (t, J = 6.4 and 8.0 Hz, 2H, CH2), 3.75 (t, J = 7.6 and 6.8 Hz, 2H, CH2), 6.12 (s, 2H, NH2), 7.15 (t, J = 7.2 and 7.6 Hz, 1H, Indol–H), 7.21 (t, J = 7.6 and 6.8 Hz, 1H, Indol–H), 7.49 (d, J = 8.0 Hz, 1H, Indol–H), 7.56–7.64 (m, 2H, ArH), 8.03 (d, J = 6.8 Hz, 2H, ArH), 8.26 (d, J = 10.8 Hz, 1H, Indol–H), 8.27 (s, 1H, Indol–H), 11.65 (s, 1H, NH). 13C NMR (100 MHz, DMSO-d6): δC 31.39, 32.59, 102.10, 112.10, 120.58, 121.76, 122.61, 123.52, 125.79, 126.15, 126.95, 129.81, 132.37, 136.08, 150.88, 152.38, 163.65, 165.71. ESI–MS m/z (%): 454 ([M+1]+, 100). Anal. calcd. for C20H16FN7OS2: C, 52.97; H, 3.56; N, 21.62. Found C, 52.85; H, 3.57; N, 21.67.
4.3.9 Compound 10i
3-(1H-indol-3-yl)-5-[[2-[[5-(4-chlorophenyl)-1,3,4-oxadiazol-2-yl]thio]ethyl]thio]-4H-1,2,4-triazol-4-amine: yellow solid, yield 88%, mp 233–234 °C (ethanol). IR (KBr, cm−1): v 3363, 3176, 3071, 3054, 2928, 1605, 1469, 1407, 1247, 1198, 1090, 1071, 1009, 842, 747, 722. 1H NMR (400 MHz, DMSO-d6): δH 3.61 (t, J = 6.0 and 8.0 Hz, 2H, CH2), 3.75 (t, J = 7.6 and 6.8 Hz, 2H, CH2), 6.11 (s, 2H, NH2), 7.15 (t, J = 7.2 and 7.6 Hz, 1H, Indol–H), 7.21 (t, J = 7.6 and 6.8 Hz, 1H, Indol–H), 7.49 (d, J = 7.6 Hz, 1H, Indol–H), 7.63 (d, J = 8.4 Hz, 2H, ArH), 8.05 (d, J = 8.4 Hz, 2H, ArH), 8.25 (d, J = 8.4 Hz, 1H, Indol–H), 8.26 (s, 1H, Indol–H), 11.65 (s, 1H, NH). 13C NMR (100 MHz, DMSO-d6): δC 31.41, 32.57, 102.08, 112.12, 120.56, 121.73, 122.42, 122.62, 125.77, 126.14, 128.79, 129.96, 136.09, 137.04, 150.91, 152.39, 163.90, 164.94. ESI–MS m/z (%): 471 ([M+1]+, 100). Anal. calcd. for C20H16ClN7OS2: C, 51.11; H, 3.43; N, 20.86. Found C, 51.22; H, 3.43; N, 20.81.
4.3.10 Compound 10j
3-(1H-indol-3-yl)-5-[[2-[[5-(4-bromophenyl)-1,3,4-oxadiazol-2-yl]thio]ethyl]thio]-4H-1,2,4-triazol-4-amine: yellow solid, yield 86%, mp 211–212 °C (ethanol). IR (KBr, cm−1): v 3352, 3125, 3011, 2976, 1607, 1453, 1406, 1252, 1173, 1096, 1012, 962, 847, 743, 708. 1H NMR (400 MHz, DMSO-d6): δH 3.60 (t, J = 6.4 and 8.0 Hz, 2H, CH2), 3.74 (t, J = 8.0 and 6.4 Hz, 2H, CH2), 6.12 (s, 2H, NH2), 7.15 (t, J = 7.2 and 7.6 Hz, 1H, Indol–H), 7.21 (t, J = 7.2 and 6.8 Hz, 1H, Indol–H), 7.49 (d, J = 7.6 Hz, 1H, Indol–H), 7.78 (d, J = 8.4 Hz, 2H, ArH), 7.99 (d, J = 8.4 Hz, 2H, ArH), 8.26 (d, J = 10.4 Hz, 1H, Indol–H), 8.27 (s, 1H, Indol–H), 11.66 (s, 1H, NH). 13C NMR (400 MHz, DMSO-d6): δC 31.39, 32.55, 102.08, 112.14, 120.58, 121.74, 122.64, 122.74, 125.77, 125.94, 126.14, 128.91, 132.89, 136.09, 150.94, 152.40, 163.92, 165.05. ESI–MS m/z (%): 516 ([M+2]+, 100). Anal. calcd. for C20H16BrN7OS2: C, 46.70; H, 3.14; N, 19.06. Found C, 46.79; H, 3.14; N, 19.03.
4.3.11 Compound 10k
3-(1H-indol-3-yl)-5-[[2-[[5-(3,5-dimethylphenyl)-1,3,4-oxadiazol-2-yl]thio]ethyl]thio]-4H-1,2,4-triazol-4-amine: yellow solid, yield 85%, mp 189–190 °C (ethanol). IR (KBr, cm−1): v 3427, 3355, 3113, 2973, 2922, 1625, 1587, 1471, 1246, 1179, 1026 1007, 940, 744, 724. 1H NMR (400 MHz, DMSO-d6): δH 2.31 (s, 6H, Ar–CH3), 3.59 (t, J = 6.4 and 6.8 Hz, 2H, CH2), 3.71 (t, J = 7.6 and 6.0 Hz, 2H, CH2), 6.09 (s, 2H, NH2), 7.10 (t, J = 7.2 and 7.6 Hz, 1H, Indol–H), 7.17 (t, J = 7.6 and 8.4 Hz, 1H, Indol–H), 7.19 (s, 1H, ArH), 7.45 (d, J = 8.0 Hz, 1H, Indol–H), 7.59 (s, 2H, ArH), 8.21 (d, J = 7.6 Hz, 1H, Indol–H), 8.24 (s, 1H, Indol–H), 11.62 (s, 1H, NH). 13C NMR (100 MHz, DMSO-d6): δC 21.14, 31.36, 32.57, 102.10, 112.08, 120.54, 121.76, 122.60, 123.32, 124.45, 125.79, 126.14, 133.81, 136.08, 139.20, 150.88, 152.36, 163.44, 165.89. ESI–MS m/z (%): 464 ([M+1]+, 100). Anal. calcd. for C22H21N7OS2: C, 57.00; H, 4.57; N, 21.15. Found C, 57.12; H, 4.58; N, 21.20.
4.3.12 Compound 10l
3-(1H-indol-3-yl)-5-[[2-[[5-(4-aminophenyl)-1,3,4-oxadiazol-2-yl]thio]ethyl]thio]-4H-1,2,4-triazol-4-amine: brown solid, yield 86%, mp 251–252 °C. IR (KBr, cm−1): v 3452, 3346, 3215, 3051, 2922, 2854, 1609, 1585, 1505, 1479, 1454, 1247, 1177, 1107, 1070, 942, 740, 700. 1H NMR (400 MHz, DMSO-d6): δH 3.59 (t, J = 6.4 and 7.2 Hz, 2H, CH2), 3.69 (t, J = 8.0 and 6.0 Hz, 2H, CH2), 5.94 (s, 2H, Ar–NH2), 6.12 (s, 2H, NH2), 6.67 (d, J = 8.4 Hz, 2H, ArH), 7.16 (t, J = 7.2 and 8.0 Hz, 1H, Indol–H), 7.21 (t, J = 8.0 and 6.8 Hz, 1H, Indol–H), 7.49 (d, J = 7.6 Hz, 1H, Indol–H), 7.66 (d, J = 8.4 Hz, 2H, ArH), 8.26 (d, J = 8.8 Hz, 1H, Indol–H), 8.27 (s, 1H, Indol–H), 11.65 (s, 1H, NH). 13C NMR (100 MHz, DMSO-d6): δC 31.41, 32.59, 102.10, 109.81, 112.09, 113.98, 120.62, 121.78, 122.60, 125.80, 126.16, 128.46, 136.08, 150.87, 152.37, 152.79, 161.33, 165.55. ESI–MS m/z (%): 451 ([M+1]+, 100). Anal. calcd. for C20H18N8OS2: C, 53.32; H, 4.03; N, 24.87. Found C, 53.41; H, 4.02; N, 24.91.
4.3.13 Compound 10m
3-(1H-indol-3-yl)-5-[[2-[[5-(naphthalen-1-yl)-1,3,4-oxadiazol-2-yl]thio]ethyl]thio]-4H-1,2,4-triazol-4-amine: yellow solid, yield 82%, mp 175–176 °C (ethanol). IR (KBr, cm−1): v 3418, 3278, 3174, 3056, 2923, 2852, 1624, 1590, 1538, 1513, 1331, 1291, 1181, 1122, 1067, 963, 773, 750. 1H NMR (400 MHz, DMSO-d6): δH 3.94 (t, J = 6.0 and 6.4 Hz, 2H, CH2), 4.76 (t, J = 6.4 and 6.0 Hz, 2H, CH2), 5.93 (s, 2H, Ar–NH2), 7.09 (t, J = 7.2 and 7.6 Hz, 1H, Indol–H), 7.20 (t, J = 7.2 and 7.6 Hz, 1H, Indol–H), 7.49 (d, J = 8.0 Hz, 1H, Indol–H), 7.60–7.74 (m, 3H, ArH), 8.08 (d, J = 7.6 Hz, 1H, ArH), 8.14–8.20 (m, 3H, Indol–H and ArH), 8.41 (d, J = 2.8 Hz, 1H, Indol–H), 9.03 (d, J = 8.0 Hz, 1H, ArH), 11.77 (s, 1H, NH). 13C NMR (100 MHz, DMSO-d6): δC 30.87, 47.89, 100.36, 112.43, 119.75, 121.01, 121.28, 122.92, 125.08, 125.69, 125.82, 127.23, 128.61, 128.93, 128.96, 129.28, 129.42, 132.99, 133.80, 136.13, 147.13, 163.33, 165.41, 165.99. ESI–MS m/z (%): 486 ([M+1]+, 100). Anal. calcd. for C24H19N7OS2: C, 59.36; H, 3.94; N, 20.19. Found C, 59.42; H, 3.93; N, 20.22.
4.4 Conventional procedure for the preparation of compounds (10a–m)
4-Amino-5-(1H-indol-3-yl)-4H-[1,2,4]triazole-3-thiol (8) (0.1 mmol), 1,2-dibromoethane (9) (0.1 mmol), the 2-mercapto-5-substituted-1,3,4-oxadiazoles (5a–m) (0.1 mmol), K2CO3 (0.2 mmol), and acetonitrile (10 mL) were placed in a 25-mL round-bottom flask. After the mixture was completely dissolved, it was stirred for 540–900 min at 82 °C. The progress of the reaction was monitored using TLC. When the reaction was complete, the precipitate was filtered, subsequently washed with 5 mL of acetonitrile, and concentrated in vacuo to get the crude solid products. The products were purified through column chromatography using ethyl acetate: petroleum ether (1:3) as an eluent with 40–68% yield.
4.5 Microwave procedure for the preparation of compounds (10a–m)
4-Amino-5-(1H-indol-3-yl)-4H-[1,2,4]triazole-3-thiol (8) (0.1 mmol), 1,2-dibromoethane (9) (0.1 mmol), the 2-mercapto-5-substituted-1,3,4-oxadiazoles (5a-m) (0.1 mmol), K2CO3 (0.2 mmol), and acetonitrile (10 mL) were placed in a vessel, which was then shaken to homogenize the mixture. The mixture was then irradiated in a microwave oven for 10–22 min at 350 W. The progress of the reaction was monitored using TLC. When the reaction was complete, the precipitate was filtered, subsequently washed with 5 mL of acetonitrile, and concentrated in vacuo to get the crude solid products. The products were purified through column chromatography using ethyl acetate: petroleum ether (1:3) as an eluent with 62–86% yield.
4.6 Bioactivity
The in vitro antimicrobial activity of the structurally promising 10a–m against Gram-positive (B. subtilis and S. aureus) and Gram-negative (E. coli and P. aeruginosa) bacteria were investigated using disc diffusion and microdilution assays along with reference drugs amoxicillin and ciprofloxacin for comparison. Dimethylformamide was used as a negative control.
4.6.1 Disc diffusion assay
The compounds were tested for their antibacterial activities through disc diffusion using a nutrient agar medium [31,32]. Serial dilutions of the test compounds were prepared in DMSO to final concentrations of 40, 20, 10, 5 and 0 μg/mL. Sterile paper discs (diameter = 6 mm) were then placed on the nutrient agar medium, and 20 μL of the compounds were added to each disc in five replications. Then, the paper discs impregnated with the compounds were placed on the surface of the media inoculated with the microorganism. The plates were incubated at 37 °C for 16–18 h (Table 2). The IC50 was computed from the diameter values of growth inhibition zones.
4.6.2 Microdilution assay
The MICs of the compounds were determined by microdilution [33] using standard inocula of 2 × 106 CFU/mL. Serial dilutions of the test compounds were prepared to final concentrations of 256, 128, 64, 32, 16, 8, 4, 2, 1, 0.5, 0.25 and 0 μg/mL in DMSO. A bacterial fluid (1 mL of 0.5 McFarland standard) was added to each tube. The MIC was visually determined after incubation for 18 h, at 37 °C (Table 3).
Acknowledgments
This research was financially supported by the Fundamental Research Funds for the Central Universities, Southwest University for Nationalities (No. 2015NZYQN23), the Science and Technology Department of Sichuan Province (Nos. 2012SZ0160, 2012JY0028).


