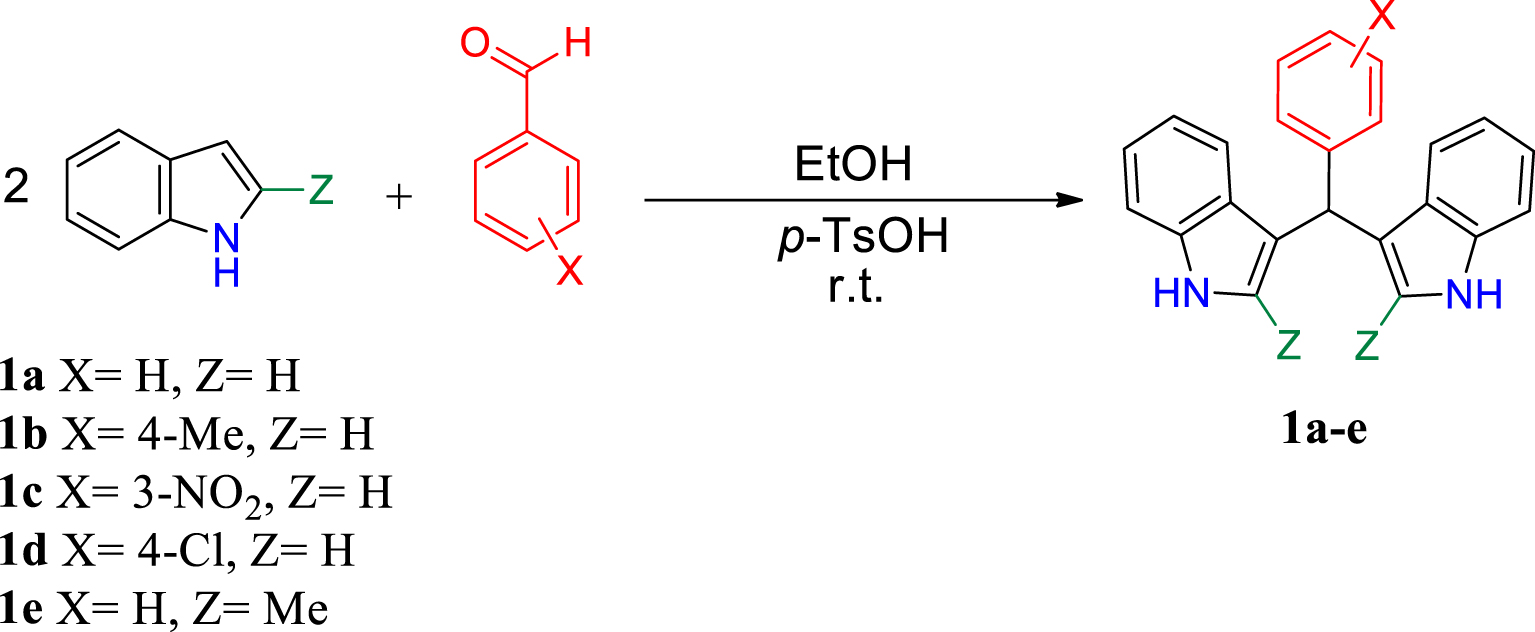
1. Introduction
Indole is probably the most omnipresent compound in nature [1]. That is because its core, which is often found in many biologically active natural products,
Some biologically active 3-substituted indoles.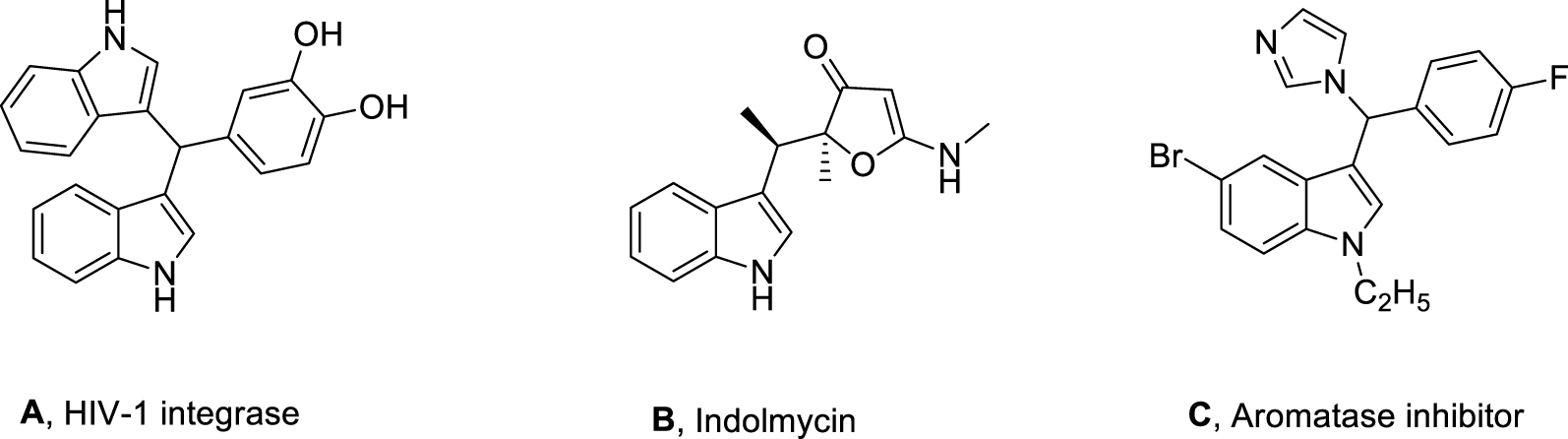
Bis(indolyl)methane natural products.
medicinal, and functional materials [2], can be an essential structural point in pharmaceutical chemistry. Furthermore, indole derivatives, having a wide range of antioxidant activities [2], are the most essential ones and naturally found in fungi [3], plants [4], and marine organisms [5]. Many such derivatives are utilized as antimitotic, antihypertensive, and antineoplastic agents [6]. C-substituted indoles are flexible intermediates for the preparation of a wide range of indole derivatives [7] and are also the critical units of many promising therapeutic agents [8]. For example, as shown in Figure 1, compounds A, B, and C are an HIV-1 integrase inhibitor [9], a potent antibacterial drug Indolmycin [10], and an aromatase inhibitor for breast cancer [11], respectively (Figure 1).
One other critical derivative of indole that has attracted the attention of many chemists and biologists is 3,3-bis(indolyl)methane (BIM), having a lot of biological functions [12].
Some BIM derivatives, for example, are shown in Figure 2, in which Compound D, arundine, isolated from plant roots, shows important anti-cancer properties [13], and Compound E is an unsymmetrical BIM of synthetic origin, which is well known for its anti-cancer activity [9].
On one hand, the common synthetic method concerning the synthesis of symmetrical BIMs, involving an acid-catalyzed condensation of a carbonyl compound with an indole derivative [14, 15], is fairly easy. Both N-alkyl and N-aryl symmetrical BIMs can be prepared by this method [16].
On the other hand, the synthesis of unsymmetrical BIMs is a challenging problem in organic synthesis. In most of the previously reported methods for synthesizing unsymmetrical BIMs, mono N-alkyl BIMs prepared from two different indoles were investigated [17, 18]. In 2019 Zhang et al. reported only one mono N-arylated BIM derivative that was prepared from two different indoles [19]. By virtue of this explanation, the synthesis of mono N-aryl unsymmetrical BIMs appears to be essential in synthetic chemistry.
The current research investigates the C–N cross-coupling reaction of BIMs in the presence of copper-based catalysts for synthesizing the unsymmetrical BIMs. The investigated method is simple, convenient, and inexpensive.
Over the past years, versatile synthetic procedures using transition metals-based catalytic systems such as CuI [20], CuFe2O4 [21], CuO [22], Pd(OAc)2 [23], and Pd/C [24] have been reported for C–N cross-coupling reaction of heterocyclic systems such as indole. In this regard, copper is one of the most interesting and important catalysts, which is used in Ullmann-type reactions of the current study.
2. Experimental
2.1. Materials and methods
All chemicals used were purchased from Merck Chemical Company. The following solvents applied for the reaction were distilled before use: acetonitrile, 1,4-dioxane, ethanol, hexane, and ethyl acetate.
CuFe2O4 used as the catalyst in procedure B was synthesized by a reported solvothermal method with some modifications [25]. The synthesis procedure and the characterization can be found in Supporting Information.
Some recorded data were as follows: IR spectra recorded on a Bruker Tensor-27 FT-IR spectrophotometer using KBr pellets; 1H NMR and 13C NMR spectra recorded on a Bruker 300 AVANCE III NMR magnet (300 MHz for 1H NMR and 75 MHz for 13C NMR) using DMSO-d6 and CDCl3 as solvent; elemental analysis (CHNS) recorded on a Costech-ECS 4010 CHNSO analyzer; and all melting points measured on an Electrothermal-9100 apparatus. The parameters recorded for 1H NMR include chemical shift 𝛿H (ppm), coupling constants J (Hz), integration, and multiplicity (s = singlet, d = doublet, t = triplet, and m = multiplet). Thin-layer chromatography (TLC) plates (silica gel G) were used for the purification of products.
2.2. General procedure for the synthesis of N-aryl bis(indolyl)methanes with method A (2a–k) (3a–k)
A mixture of compound 1a–e (1 mmol), aryl iodide (1 mmol), Cu(OAc)2 (1 mmol), ethylene glycol (1 mmol) and K2CO3 (1 mmol) was stirred in 3 mL of DMF under N2 atmosphere for 24 hours at 120 °C. The promotion of the reaction was monitored by TLC. After completion of the reaction, the solvent was evaporated, and the remaining solid was purified by thin-layer chromatography (TLC) plates (silica gel) in the solvent system n-hexane/ethyl acetate (4:2 v/v).
2.3. General procedure for the synthesis of N-aryl bis(indolyl)methanes with method B (2a–k) (3a–k)
A mixture of compound 1a–e (1 mmol), aryl iodide (1 mmol), CuFe2O4 (120 mg) and K2CO3 (1 mmol) was stirred in 3 mL of DMF under N2 atmosphere for 24 hours at 100 °C (checked by TLC). After completion of the reaction, the solvent was evaporated, and the remaining was purified by thin-layer chromatography (TLC) plates (silica gel) in the solvent system of n-hexane/ethyl acetate (4:2 v/v).
2.3.1. 3-((1H-Indol-3-yl)(phenyl)methyl)-1-phenyl-1H-indole (2a)
Orange solid, mp 94–96 °C. IR (KBr): 2852, 2922, 3045, 3396 cm−1.
1H NMR (300 MHz, DMSO-d6) 𝛿 (ppm): 5.96 (s, 1H, CH), 6.91 (t, J = 7.8 Hz, 1H, arom), 6.98–7.16 (m, 4H, arom), 7.20 (t, J = 6.8 Hz, 2H, arom), 7.28–7.36 (m, 3H, arom), 7.40 (d, J = 8.1 Hz, 2H, arom), 7.47 (t, J = 6.6 Hz, 3H, arom), 7.51–7.53 (m, 4H, arom), 7.56 (d, J = 8.2 Hz, 1H, arom), 10.92 (s, 1H, NH). 13C NMR (75 MHz, DMSO-d6) 𝛿 (ppm): 40.0, 110.8, 120.0, 117.8, 118.8, 119.5, 120.3, 120.5, 121.1, 121.4, 122.9, 124.0, 124.2, 126.5, 126.6, 126.9, 127.0, 128.6, 128.7, 128.8, 130.3, 136.1, 137.0, 139.5, 144.8 .
Anal. Calcd for C29H22N2: C, 87.41; H, 5.56; N, 7.03%. Found: C, 87.49; H, 5.38; N, 7.05%.
2.3.2. 3,3′-(Phenylmethylene) bis(1-phenyl-1H- indole) (3a) [26]
White solid, mp 164–166 °C. IR (KBr): 2848, 2922, 3061 cm−1.
1H NMR (300 MHz, DMSO-d6) 𝛿 (ppm): 6.05 (s, 1H, CH), 7.06 (t, J = 7.5 Hz, 2H, arom), 7.19 (t, J = 7.9 Hz, 3H, arom), 7.24–7.57 (m, 20H, arom). 13C NMR (75 MHz, DMSO-d6) 𝛿 (ppm): 40.0, 110.9, 120.3, 120.4, 123.0, 124.1, 126.6, 127.1, 128.6, 128.7, 128.8, 130.2, 136.0, 139.5, 144.3.
Anal. Calcd for C35H26N2: C, 88.58; H, 5.52; N, 5.90%. Found: C, 88.26; H, 5.45; N, 5.82%.
2.3.3. 3-((1H-Indol-3-yl(phenyl)methyl)-1-(p-tolyl)-1H-indole (2b)
Orange solid, mp 85–87 °C. IR (KBr): 2852, 2920, 3028, 3408 cm−1.
1H NMR (300 MHz, DMSO-d6) 𝛿 (ppm): 2.37 (s, 3H, CH3), 5.94 (s, 1H, CH), 6.90 (t, J = 7.5 Hz, 1H, arom), 6.97–7.33 (m, 10H, arom), 7.39 (t, J = 7.8 Hz, 5H, arom), 7.46 (d, J = 7.6 Hz, 2H, arom), 7.52 (d, J = 8.3 Hz, 1H, arom), 10.90 (s, 1H. NH). 13C NMR (75 MHz, DMSO-d6) 𝛿 (ppm): 21.0, 40.0, 110.8, 112.0, 117.9, 118.8, 119.5, 120.1, 120.4, 120.8, 121.4, 122.8, 124.0, 124.2, 126.4, 127.0, 128.5, 128.6, 128.8, 130.7, 136.0, 136.1, 137.0, 137.1, 144.9.
Anal. Calcd for C30H24N2: C, 87.35; H, 5.86; N, 6.79%. Found: C, 87.28; H, 5.78; N, 6.64%.
2.3.4. 3,3′-(Phenylmethylene) bis(1-(p-tolyl)-1H- indole) (3b)
White solid, mp 63–65 °C. IR (KBr): 2852, 2921, 3029 cm−1.
1H NMR (300 MHz, DMSO-d6) 𝛿 (ppm): 2.33 (s, 6H, 2CH3), 6.03 (s, 1H, CH), 7.03 (t, J = 7.5 Hz, 2H, arom), 7.15 (t, J = 7.6 Hz, 2H, arom), 7.19–7.33 (m, 9H, arom), 7.37 (d, J = 8.2 Hz, 4H, arom), 7.49–7.55 (m, 6H, arom). 13C NMR (75 MHz, DMSO-d6) 𝛿 (ppm): 21.0, 39.7, 110.9, 120.1, 120.2, 120.3, 122.8, 124.0, 126.6, 127.1, 128.4, 128.7, 128.8, 130.6, 136.0, 136.2, 137.0, 144.4.
Anal. Calcd for C37H30N2: C, 88.41; H, 6.02; N, 5.57%. Found: C, 88.01; H, 5.94; N, 5.25%.
2.3.5. 3-((1H-Indol-3-yl)(phenyl)methyl)-1-(4-nitrophenyl)-1H-indole (2c)
Yellow solid, mp 115–118 °C. IR (KBr): 2850, 2920, 3055, 3418 cm−1.
1H NMR (300 MHz, DMSO-d6) 𝛿 (ppm): 5.97 (s, 1H, CH), 6.91 (t, J = 7.5 Hz, 1H, arom), 7.01–7.14 (m, 3H, arom), 7.24 (t, J = 7.3 Hz, 2H, arom), 7.30 (t, J = 7.9 Hz, 3H, arom), 7.40 (d, J = 8.1 Hz, 2H, arom), 7.49 (d, J = 6.4 Hz, 3H, arom), 7.75 (d, J = 8.3 Hz, 1H, arom), 7.82 (d, J = 8.7 Hz, 2H, arom), 8.35 (d, J = 8.7 Hz, 2H, arom), 10.95 (s, 1H, NH). 13C NMR (75 MHz, DMSO-d6) 𝛿 (ppm): 39.8, 111.4, 112.0, 117.4, 118.9, 119.4, 120.8, 121.4, 121.5, 123.4, 123.5, 123.8, 124.3, 125.9, 126.3, 126.6, 127.0, 128.7, 128.8, 129.6, 135.7, 137.0, 144.4, 144.5, 145.0.
Anal. Calcd for C29H21N3O2: C, 78.54; H, 4.77; N, 9.47%. Found: C, 78.28; H, 4.65; N, 9.30%.
2.3.6. 3,3′-(Phenylmethylene) bis(1-(4-nitrophenyl)-1H-indole) (3c)
Yellow solid, mp 130–133 °C. IR (KBr): 2851, 2921, 3080 cm−1.
1H NMR (300 MHz, DMSO-d6) 𝛿 (ppm): 6.07 (s, 1H, CH), 7.14 (t, J = 7.5 Hz, 2H, arom), 7.27 (t, J = 8.0 Hz, 3H, arom), 7.34 (t, J = 7.4 Hz, 2H, arom), 7.50 (s, 2H, arom), 7.59 (dd, J1 = 7.5 Hz, J2 = 5.6 Hz, 4H, arom), 7.75 (d, J = 8.3 Hz, 2H, arom), 7.84 (d, J = 9.0 Hz, 4H, arom), 8.35 (d, J = 9.1 Hz, 4H, arom) 13C NMR (75 MHz, DMSO-d6) 𝛿 (ppm): 39.4, 111.5, 120.6, 121.6, 122.3, 123.6, 123.9, 125.9, 126.7, 126.9, 128.8, 128.9, 129.5, 135.6, 143.5, 144.6, 144.9.
Anal. Calcd for C35H24N4O4: C, 74.46; H, 4.28; N, 9.92%. Found: C, 74.52; H, 4.33; N, 10.08%.
2.3.7. 3-((1H-Indol-3-yl)(p-tolyl)methyl)-1-phenyl-1H-indole (2d)
Orange solid, mp 110–112 °C. IR (KBr): 2852, 2920, 3048, 3413 cm−1.
1H NMR (300 MHz, DMSO-d6) 𝛿 (ppm): 2.27 (s, 3H, CH3), 5.90 (s, 1H, CH), 6.91 (t, J = 7.8 Hz, 1H, arom), 6.96–7.07 (m, 3H, arom), 7.10 (d, J = 7.5 Hz, 3H, arom), 7.17(t, J = 7.7 Hz, 1H, arom), 7.33–7.52 (m, 10H, arom), 7.56 (d, J = 8.2 Hz, 1H, arom), 10.90 (s, 1H, NH). 13C NMR (75 MHz, DMSO-d6) 𝛿 (ppm): 21.1, 39.6, 110.8, 112.0, 118.0, 118.7, 119.5, 120.2, 120.5, 121.3, 121.4, 122.9, 124.0, 124.2, 126.5, 126.9, 127.0, 128.7, 129.2, 130.3, 132.2, 135.3, 136.1, 137.1, 139.6, 141.8.
Anal. Calcd for C30H24N2: C, 87.35; H, 5.86; N, 6.79%. Found: C, 87.58; H, 6.04; N, 7.03%.
2.3.8. 3,3′-(p-Tolylmethylene) bis(1-phenyl-1H- indole) (3d)
White solid, mp 105–107 °C. IR (KBr): 2853, 2920, 3046 cm−1.
1H NMR (300 MHz, DMSO-d6) 𝛿 (ppm): 2.27 (s, 3H, CH3), 5.99 (s, 1H, CH), 7.06 (t, J = 7.4 Hz, 2H, arom), 7.14 (t, J = 6.8 Hz, 2H, arom), 7.20 (d, J = 7.5 Hz, 2H, arom), 7.26 (s, 2H, arom), 7.33–7.38 (m, 2H, arom), 7.42 (d, J = 7.9 Hz, 2H, arom), 7.52–7.57 (m, 12H, arom). 13C NMR (75 MHz, DMSO-d6) 𝛿 (ppm): 21.1, 39.3, 110.9, 120.3, 120.4, 120.6, 122.9, 124.1, 126.6, 127.1, 128.6, 128.7, 129.3, 130.2, 135.5, 136.1, 139.5, 141.3.
Anal. Calcd for C36H28N2: C, 88.49; H, 5.78; N, 5.73%. Found: C, 88.10; H, 5.39; N, 5.65%.
2.3.9. 3-((1H-Indol-3-yl)(p-tolyl)methyl)-1-(p-tolyl)-1H-indole (2e)
Orange solid, mp 102–104 °C. IR (KBr): 2852, 2919, 3041, 3414 cm−1.
1H NMR (300 MHz, DMSO-d6) 𝛿 (ppm): 2.26 (s, 3H, CH3), 2.36 (s, 3H, CH3), 5.89 (s, 1H, CH), 6.90 (t, J = 7.7 Hz, 1H, arom), 6.95–7.11 (m, 6H, arom), 7.16 (t, J = 8.0 Hz, 1H, arom), 7.30–7.44 (m, 9H, arom), 7.51 (d, J = 8.3 Hz, 1H, arom), 10.89 (s, 1H, NH). 13C NMR (75 MHz, DMSO-d6) 𝛿 (ppm): 21.0, 21.1, 39.6, 110.8, 112.0, 118.0, 118.7, 119.5, 120.1, 120.4, 121.0, 121.4, 122.7, 123.9, 124.1, 126.9, 127.0, 128.5, 128.7, 129.2, 130.7, 135.3, 135.9, 136.2, 137.0, 137.1, 141.8.
Anal. Calcd for C31H26N2: C, 87.29; H, 6.14; N, 6.57%. Found: C, 86.97; H, 6.30; N, 6.38%.
2.3.10. 3,3′-(p-Tolylmethylene) bis(1-(p-tolyl)-1H- indole) (3e)
White solid, mp 152–154 °C. IR (KBr): 2852, 2918, 3038 cm−1.
1H NMR (300 MHz, DMSO-d6) 𝛿 (ppm): 2.27 (s, 3H, CH3), 2.37 (s, 3H, CH3), 5.97 (s, 1H, CH), 7.04 (t, J = 7.5 Hz, 2H, arom), 7.11–7.21 (m, 6H, arom), 7.33 (d, J = 8.1 Hz, 4H, arom), 7.39–7.43 (m, 6H, arom), 7.51 (d, J = 8.2 Hz, 3H, arom), 7.70–7.72 (m, 1H, arom). 13C NMR (75 MHz, DMSO-d6) 𝛿 (ppm): 21.0, 22.9, 40.0, 110.8, 120.2, 120.3, 122.8, 124.1, 127.1, 128.4, 128.7, 129.3, 130.6, 132.1, 135.5, 136.0, 136.1, 137.0, 141.4.
Anal. Calcd for C38H32N2: C, 88.34; H, 6.24; N, 5.42%. Found: C, 88.48; H, 6.34; N, 5.63%.
2.3.11. 3-((1H-Indol-3-yl)(p-tolyl)methyl)-1-(4-nitrophenyl)-1H-indole (2f)
Yellow solid, mp 110–113 °C. IR (KBr): 2852, 2920, 3049, 3416 cm−1.
1H NMR (300 MHz, DMSO-d6) 𝛿 (ppm): 2.27 (s, 3H, CH3), 5.91 (s, 1H, CH), 6.88–6.98 (m, 2H, arom), 7.05 (dd, J1 = 7.7 Hz, J2 = 1.4 Hz, 1H, arom), 7.11 (dd, J1 = 7.1 Hz, J2 = 3.5 Hz, 3H, arm), 7.24 (d, J = 7.1 Hz, 1H, arom), 7.28 (s, 1H, arom), 7.34–7.40 (m, 4H, arom), 7.48 (d, J = 7.9 Hz, 1H, arom), 7.74 (d, J = 8.3 Hz, 1H, arom), 7.81 (d, J = 9.1 Hz, 2H, arom), 8.34 (d, J = 9.1 Hz, 2H, arom), 10.92 (s, 1H. NH). 13C NMR (75 MHz, DMSO-d6) 𝛿 (ppm): 21.1, 40.0, 111.4, 112.0, 117.6, 118.8, 119.4, 120.8, 121.4, 123.5, 123.6, 123.8, 124.2, 125.9, 126.3, 127.0, 128.7, 129.3, 129.6, 135.4, 135.7, 137.0, 141.4, 144.5, 145.0.
Anal. Calcd for C30H23N3O2: C, 78.76; H, 5.07; N, 9.18%. Found: C, 78.90; H, 5.15; N, 9.35%.
2.3.12. 3,3′-(p-Tolylmethylene) bis(1-(4- nitrophenyl)-1H-indole) (3f)
Yellow solid, mp 128–130 °C. IR (KBr): 2853, 2922, 3082 cm−1.
1H NMR (300 MHz, DMSO-d6) 𝛿 (ppm): 2.27 (s, 3H, CH3), 6.02 (s, 1H, CH), 7.11-7.16 (m, 4H, arom), 7.27 (t, J = 7.4 Hz, 2H, arom), 7.43–7.47 (m, 4H, arom), 7.58 (d, J = 7.8 Hz, 2H, arom), 7.75 (d, J = 8.3 Hz, 2H, arom), 7.84 (d, J = 9.1 Hz, 4H, arom), 8.35 (d, J = 9.1 Hz, 4H, arom). 13C NMR (75 MHz, DMSO-d6) 𝛿 (ppm): 21.1, 39.0, 111.4, 120.6, 121.6, 122.5, 123.6, 123.9, 125.9, 126.6, 128.7, 129.4, 129.5, 135.6, 135.8, 140.5, 144.6, 144.9.
Anal. Calcd for C36H26N4O4: C, 74.73; H, 4.53; N, 9.68%. Found: C, 74.81; H, 4.59; N, 9.86%.
2.3.13. 3-((1H-Indol-3-yl)(3-nitrophenyl)methyl)-1-phenyl-1H-indole (2g)
Orange solid, mp 85–87 °C. IR (KBr): 2854, 2923, 3055, 3412 cm−1.
1H NMR (300 MHz, DMSO-d6) 𝛿 (ppm): 6.20 (s, 1H, CH), 6.94 (t, J = 7.5 Hz, 1H, arom), 7.04–7.12 (m, 3H, arom), 7.20 (t, J = 7.6 Hz, 1H, arom), 7.25 (s, 1H, arom), 7.35–7.37 (m, 1H, arom), 7.42 (dd, 1 J = 7.9 Hz, 2 J = 5.3 Hz, 2H, arom), 7.49 (d, J = 8.1 Hz, 1H, arom), 7.53–7.64 (m, 6H, arom), 7.94 (d, J = 7.7 Hz, 1H, arom), 8.10 (dd, 1 J = 8.1 Hz, 2 J = 2.3 Hz, 1H, arom), 8.30 (s, 1H, arom), 11.02 (s, 1H, NH). 13C NMR (75 MHz, DMSO-d6) 𝛿 (ppm): 39.3, 111.0, 112.1, 116.8, 119.0, 119.3, 120.0, 120.3, 120.5, 121.6, 121.7, 123.1, 123.2, 124.2, 124.6, 126.7, 126.8, 127.3, 128.4, 130.2, 130.3, 135.6, 136.1, 137.1, 139.4, 147.2, 148.4.
Anal. Calcd for C29H21N3O2: C, 78.54; H, 4.77; N, 9.47%. Found: C, 78.30; H, 4.91; N, 9.15%.
2.3.14. 3,3′-((3-Nitrophenyl)methylene) bis(1- phenyl-1H-indole) (3g)
White solid, mp 89–92 °C. IR (KBr): 2923, 3054 cm−1.
1H NMR (300 MHz, DMSO-d6) 𝛿 (ppm): 6.32 (s, 1H, CH), 7.08 (t, J = 7.4 Hz, 2H, arom), 7.20 (t, J = 7.6 Hz, 2H, arom), 7.32–7.36 (m, 2H, arom), 7.40 (s, 2H, arom), 7.49–7.64 (m, 13H, arom), 8.02 (d, J = 7.7 Hz, 1H, arom), 8.10 (dd, 1 J = 8.1 Hz, 2 J = 2.3 Hz, 1H, arom), 8.42 (s, 1H, arom). 13C NMR (75 MHz, DMSO-d6) 𝛿 (ppm): 39.0, 111.0, 119.4, 120.2, 120.6, 121.9, 123.2, 123.3, 124.2, 126.7, 127.5, 128.3, 130.2, 130.3, 135.6, 136.1, 139.4, 146.7, 148.5.
Anal. Calcd for C35H25N3O2: C, 80.91; H, 4.85; N, 8.09%. Found: C, 80.65; H, 4.71; N, 8.16%.
2.3.15. 3-((1H-Indol-3-yl)(3-nitrophenyl)methyl)-1-(p-tolyl)-1H-indole (2h)
Orange solid, mp 86–88 °C. IR (KBr): 2854, 2922, 3053, 3412 cm−1.
1H NMR (300 MHz, DMSO-d6) 𝛿 (ppm): 2.36 (s, 3H, CH3), 6.19 (s, 1H, CH), 6.93 (t, J = 7.5 Hz, 1H, arom), 7.02–7.34 (m, 7H, arom), 7.41 (d, J = 8.0 Hz, 4H, arom), 7.41–7.54 (m, 2H, arom), 7.60 (t, J = 8.0 Hz, 1H, arom), 7.93 (d, J = 7.7 Hz, 1H, arom), 8.09 (d, J = 8.3 Hz, 1H, arom), 8.30 (s, 1H, arom), 11.02 (s, 1H, NH). 13C NMR (75 MHz, DMSO-d6) 𝛿 (ppm): 21.0, 39.4, 110.9, 112.1, 116.8, 119.0, 119.3, 119.7, 120.3, 121.6, 121.7, 123.0, 123.2, 124.1, 124.6, 126.8, 127.3, 128.2, 130.2, 130.6, 135.6, 136.1, 136.2, 137.0, 137.1, 147.3, 148.4.
Anal. Calcd for C30H23N3O2: C, 78.76; H, 5.07; N, 9.18%. Found: C, 78.45; H, 5.34; N, 8.93%.
2.3.16. 3,3′-((3-Nitrophenyl)methylene) bis(1-(p- tolyl)-1H-indole) (3h)
White solid, mp 73–76 °C. IR (KBr): 2857, 2922, 3035 cm−1.
1H NMR (300 MHz, DMSO-d6) 𝛿 (ppm): 2.35 (s, 6H, 2CH3), 6.29 (s, 1H, CH), 7.06 (t, J = 7.7 Hz, 2H, arom), 7.18 (t, J = 7.7 Hz, 2H, arom), 7.30–7.34 (m, 6H, arom), 7.42 (d, J = 8.4 Hz, 4H, arom), 7.50–7.64 (m, 5H, arom), 8.00 (dt, 1 J = 7.8 Hz, 2 J = 1.3 Hz, 1H, arom), 8.08–8.40 (m, 2H, arom). 13C NMR (75 MHz, DMSO-d6) 𝛿 (ppm): 21.0, 39.1, 111.0, 119.1, 120.1, 120.4, 121.9, 123.0, 123.3, 124.2, 127.5, 128.2, 130.3, 130.6, 135.6, 136.1, 136.2, 136.9, 146.8, 148.4.
Anal. Calcd for C37H29N3O2: C, 81.15; H, 5.34; N, 7.67%. Found: C, 81.13; H, 5.38; N, 7.32%.
2.3.17. 3-((4-Chlorophenyl)(1H-indol-3-yl)methyl)-1-phenyl-1H-indole (2i)
Orange solid, mp 88–91 °C. IR (KBr): 2854, 2922, 3050, 3413 cm−1.
1H NMR (300 MHz, DMSO-d6) 𝛿 (ppm): 5.99 (s, 1H, CH), 6.93 (t, J = 7.5 Hz, 1H, arom), 7.00–7.21 (m, 5H, arom), 7.35 (d, J = 8.1 Hz, 1H, arom), 7.39–7.52 (m, 11H, arom), 7.57 (d, J = 8.4 Hz, 1H, arom), 10.98 (s, 1H, NH). 13C NMR (75 MHz, DMSO-d6) 𝛿 (ppm): 39.3, 110.9, 112.1, 117.4, 118.9, 119.4, 120.3, 120.4, 120.6, 121.5, 123.0, 124.0, 124.3, 126.6, 126.9, 127.0, 128.5, 128.6, 130.2, 130.6, 131.0, 136.1, 137.1, 139.5, 143.9 .
Anal. Calcd for C29H21ClN2: C, 80.45; H, 4.89; N, 6.47%. Found: C, 80.70; H, 5.04; N, 6.86%.
2.3.18. 3,3′-((4-Chlorophenyl)methylene) bis(1- phenyl-1H-indole) (3i) [16]
White solid, mp 88–90 °C. IR (KBr): 2855, 2921, 3038 cm−1.
1H NMR (300 MHz, DMSO-d6) 𝛿 (ppm): 6.08 (s, 1H, CH), 7.06 (t, J = 7.5 Hz, 2H, arom), 7.18 (t, J = 7.4 Hz, 2H, arom), 7.29 (s, 1H, arom), 7.30–7.38 (m, 5H, arom), 7.50–7.57 (m, 14H, arom). 13C NMR (75 MHz, DMSO-d6) 𝛿 (ppm): 39.0, 110.9, 120.0, 120.3, 120.5, 123.1, 124.1, 126.7, 127.2, 128.4, 128.7, 130.2, 130.7, 131.2, 136.1, 139.4, 143.3.
Anal. Calcd for C35H25ClN2: C, 82.58; H, 4.95; N, 5.50%. Found: C, 82.92; H, 5.15; N, 5.87%.
2.3.19. 3-((4-Chlorophenyl)(1H-indol-3-yl)methyl)-1-(p-tolyl)-1H-indole (2j)
Orange solid, mp 82–84 °C. IR (KBr): 2854, 2922, 3041, 3414 cm−1.
1H NMR (300 MHz, DMSO-d6) 𝛿 (ppm): 2.35 (s, 3H, CH3), 5.97 (s, 1H, CH), 6.92 (t, J = 7.4 Hz, 1H, arom), 6.98–7.08 (m, 3H, arom), 7.11 (s, 1H, arom), 7.17 (t, J = 7.7 Hz, 1H, arom), 7.29–7.40 (m, 7H, arom), 7.45 (t, J = 7.6 Hz, 4H, arom), 7.52 (d, J = 8.3 Hz, 1H, arom), 10.97 (s, 1H, NH). 13C NMR (75 MHz, DMSO-d6) 𝛿 (ppm): 21.0, 39.3, 110.9, 112.1, 117.4, 118.9, 119.4, 120.2, 120.3, 121.5, 122.8, 124.0, 124.3, 126.9, 127.0, 128.4, 128.6, 129.1, 130.6, 131.0, 132.0, 136.0, 136.2, 137.0, 137.1, 143.9 .
Anal. Calcd for C30H23ClN2: C, 80.61; H, 5.19; N, 6.27%. Found: C, 80.78; H, 5.26; N, 6.48%.
2.3.20. 3,3′-((4-Chlorophenyl)methylene) bis(1-(p- tolyl)-1H-indole) (3j)
White solid, mp 173–175 °C. IR (KBr): 2852, 2922, 3047 cm−1.
1H NMR (300 MHz, DMSO-d6) 𝛿 (ppm): 2.34 (s, 6H, 2CH3), 6.06 (s, 1H, CH), 7.04 (t, J = 7.4 Hz, 2H, arom), 7.16 (t, J = 7.7 Hz, 2H, arom), 7.23 (s, 2H, arom), 7.31 (t, J = 8.0 Hz, 5H, arom), 7.36–7.40 (m, 5H, arom), 7.52 (dd, J1 = 8.2 Hz, J2 = 5.4 Hz, 6H, arom). 13C NMR (75 MHz, DMSO-d6) 𝛿 (ppm): 21.0, 39.0, 110.9, 119.7, 120.3, 122.9, 124.0, 124.1, 127.2, 128.3, 128.7, 130.6, 130.7, 131.2, 136.0, 136.1, 136.2, 137.0.
Anal. Calcd for C37H29ClN2: C, 82.74; H, 5.44; N, 5.22%. Found: C, 83.07; H, 5.68; N, 5.48%.
2.3.21. 2-Methyl-3-((2-methyl-1H-indol-3-yl)(phenyl)methyl)-1-(p-tolyl)-1H-indole (2k)
Light brown solid, mp 83–85 °C. IR (KBr): 2855, 2920, 3053, 3401 cm−1.
N-arylation of bis(indolyl)methanes with method A and B.
1H NMR (300 MHz, DMSO-d6) 𝛿 (ppm): 2.00 (s, 3H, CH3), 2.12 (s, 3H, CH3), 2.41 (s, 3H, CH3), 6.06 (s, 1H, CH), 6.72–6.98 (m, 7H, arom), 7.24–7.30 (m, 8H, arom), 7.38 (d, J = 7.8 Hz, 2H, arom), 10.82 (s, 1H, NH). 13C NMR (75 MHz, DMSO-d6) 𝛿 (ppm): 11.6, 12.5, 21.2, 39.5, 110.0, 110.9, 112.2, 114.4, 118.5, 118.9, 119.5, 120.1, 121.0, 126.4, 128.2, 128.5, 128.7, 129.2, 130.6, 132.8, 133.9, 135.2, 135.6, 137.5, 137.7, 144.3 .
Anal. Calcd for C32H28N2: C, 87.24; H, 6.41; N, 6.36%. Found: C, 87.31; H, 6.45; N, 6.48%.
2.3.22. 3,3′-(Phenylmethylene) bis(2-methyl-1-(p- tolyl)-1H-indole) (3k)
White solid, mp 260–263 °C. IR (KBr): 2855, 2918, 3028 cm−1.
1H NMR (300 MHz, CDCl3) 𝛿 (ppm): 2.14 (s, 6H, 2CH3), 2.54 (s, 6H, 2CH3), 6.27 (s, 1H, CH), 6.69–7.41 (m, 19H, arom), 7.50 (d, J = 6.6 Hz, 2H, arom). 13C NMR (75 MHz, CDCl3) 𝛿 (ppm): 11.6, 21.3, 40.1, 109.9, 114.0, 119.4, 119.6, 120.6, 126.1, 128.2, 128.3, 128.4, 129.3, 130.0, 134.2, 135.5, 137.5, 137.8, 143.9.
Anal. Calcd for C39H34N2: C, 88.26; H, 6.46; N, 5.28%. Found: C, 88.35; H, 6.51; N, 5.44%.
3. Results and discussion
An efficient method to synthesize the unsymmetrical BIMs using the C–N cross-coupling reaction in the presence of copper catalysts is proposed for the first time here. For this purpose, both homogeneous and heterogeneous copper catalysts were used to increase the yield of unsymmetrical products.
The method investigated here involves two steps; in the first step, a series of BIMs 1a–e were synthesized from the reaction of indole with aromatic aldehydes in the presence of p-toluenesulfonic acid as catalyst (Scheme 1). The procedure can be found in Supporting Information.
In the second step, compounds 1a–e were used in a coupling reaction with several aryl halides, using catalysts of copper salts (method A) and CuFe2O4 (method B) (Scheme 2). The coupling reaction rendered both symmetrical 3a–k and unsymmetrical BIMs 2a–k. The results are reported in Table 3.
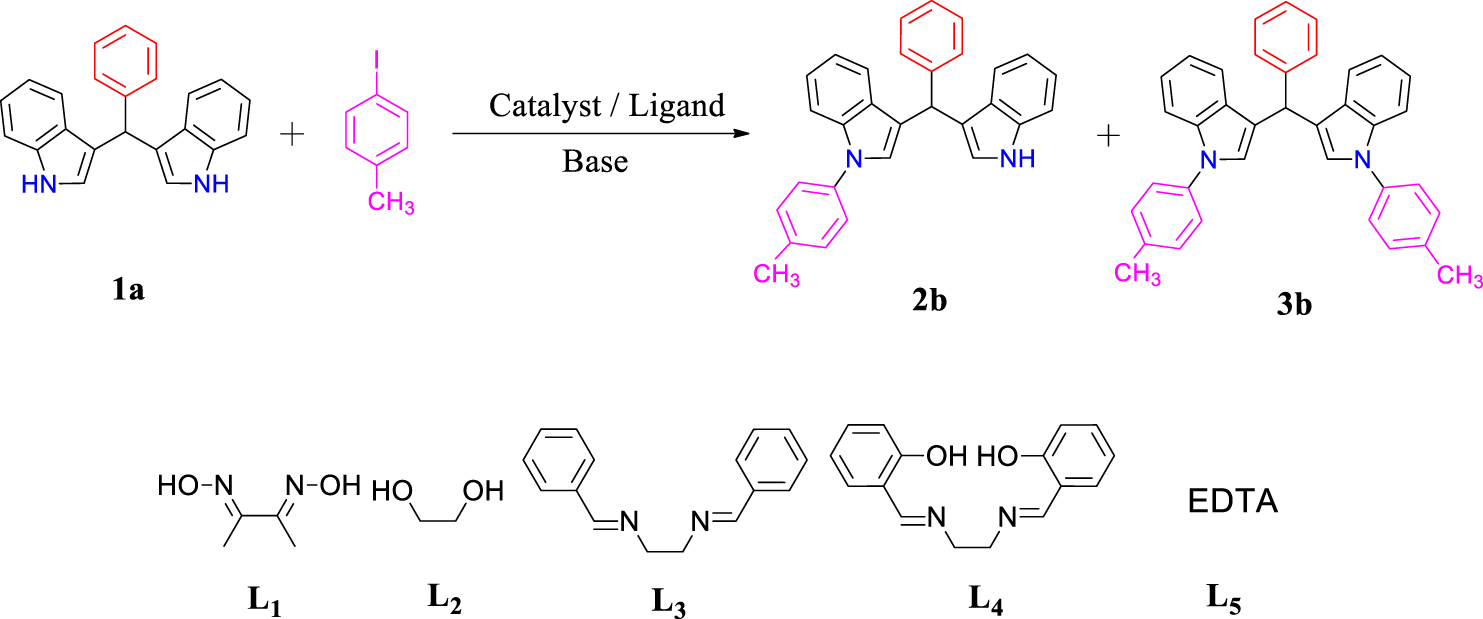
In method A, the optimization of the reaction conditions was performed with a 1:1 molar ratio of aryl iodide and compound 1a. To optimize the reaction conditions, the coupling reaction of compound 1a (1 mmol), 4-iodotoluene (1 mmol), Cu(OAc)2 (1 mmol), and K2CO3 (1 mmol) under N2 at 100 °C condition for 24 hours was selected as the model reaction. Different auxiliary ligands, such as L1, L2, L3, L4, and L5 were used (Table 1). No products were obtained when conducting the mentioned reaction in the presence of ligand L4 and in the no ligand conditions (Table 1, entries 4–6). When running the reaction in the presence of ligands L1, L2, L3, and L5, both products 2b and 3b were formed. The best results were obtained with ligand L2 (ethylene glycol) (Table 1, entry 2).
Other reaction conditions such as temperature, solvent, base, and catalyst were also optimized, and the results are shown in Table 1. Performing the reaction under air atmosphere gave lower yields than under nitrogen atmosphere (Table 1, entry 18). By optimizing the reaction at different temperatures, as can be seen in Table 1, the best yield was obtained at 120 °C (Table 1, entry 20). As the final step in optimizing the reaction conditions, we performed the reaction at 18 and 30 hours, in which the reaction efficiency was significantly reduced compared to 24 hours (Table 1, entries 22, 23).
Optimization of reaction conditions for N-arylation BIMs by method A
 |
| Entry | Catalyst amount (mmol) | Ligand amount (mmol) | Base amount (mmol) | Solvent/Temp. (°C) | Atmosphere | Time (h) | Yield (%)a | |
|---|---|---|---|---|---|---|---|---|
| 2b | 3b | |||||||
| Ligand comparison | ||||||||
| 1 | Cu(OAc)2: (1) | L1: (1) | K2CO3: (1) | DMF/100 °C | N2 | 24 | 15 | 3 |
| 2 | Cu(OAc)2: (1) | L2: (1) | K2CO3: (1) | DMF/100 °C | N2 | 24 | 46 | 10 |
| 3 | Cu(OAc)2: (1) | L3: (1) | K2CO3: (1) | DMF/100 °C | N2 | 24 | 30 | 3 |
| 4 | Cu(OAc)2: (1) | L4: (1) | K2CO3: (1) | DMF/100 °C | N2 | 24 | 0 | 0 |
| 5 | Cu(OAc)2: (1) | L5: (1) | K2CO3: (1) | DMF/100 °C | N2 | 24 | 23 | 3 |
| 6 | Cu(OAc)2: (1) | No ligand | K2CO3: (1) | DMF/100 °C | N2 | 24 | 0 | 0 |
| Catalyst comparison | ||||||||
| 7 | CuI: (1) | L2: (1) | K2CO3: (1) | DMF/ 100 °C | N2 | 24 | 15 | 6 |
| 8 | CuCl2: (1) | L2: (1) | K2CO3: (1) | DMF/ 100 °C | N2 | 24 | 0 | 0 |
| 9 | Cu(NO3)2: (1) | L2: (1) | K2CO3: (1) | DMF/ 100 °C | N2 | 24 | 10 | Trace |
| Base comparison | ||||||||
| 10 | Cu(OAc)2: (1) | L2: (1) | Na2CO3: (1) | DMF/ 100 °C | N2 | 24 | 46 | 13 |
| 11 | Cu(OAc)2: (1) | L2: (1) | Et3N: (1) | DMF/ 100 °C | N2 | 24 | 30 | 6 |
| Solvent comparison | ||||||||
| 12 | Cu(OAc)2: (1) | L2: (1) | K2CO3: (1) | EtOH/ reflux | N2 | 24 | 0 | 0 |
| 13 | Cu(OAc)2: (1) | L2: (1) | K2CO3: (1) | Dioxane/ reflux | N2 | 24 | 0 | 0 |
| 14 | Cu(OAc)2: (1) | L2: (1) | K2CO3: (1) | CH3CN/ reflux | N2 | 24 | 0 | 0 |
| Reaction stoichiometry | ||||||||
| 15 | Cu(OAc)2: (1) | L2: (2) | K2CO3: (1) | DMF/ 100 °C | N2 | 24 | 23 | 3 |
| 16 | Cu(OAc)2: (0.5) | L2: (0.5) | K2CO3: (1) | DMF/ 100 °C | N2 | 24 | 31 | 3 |
| 17 | Cu(OAc)2: (1) | L2: (1) | K2CO3: (2) | DMF/ 100 °C | N2 | 24 | 23 | 3 |
| Reaction atmosphere | ||||||||
| 18 | Cu(OAc)2: (1) | L2: (1) | K2CO3: (1) | DMF/ 100 °C | air | 24 | 31 | 6 |
| Reaction temperature | ||||||||
| 19 | Cu(OAc)2: (1) | L2: (1) | K2CO3: (1) | DMF/ 80 °C | N2 | 24 | 23 | Trace |
| 20 | Cu(OAc)2: (1) | L2: (1) | K2CO3: (1) | DMF/120oC | N2 | 24 | 54 | 19b |
| 21 | Cu(OAc)2: (1) | L2: (1) | K2CO3: (1) | DMF/ 130 °C | N2 | 24 | 30 | 13 |
| Reaction time | ||||||||
| 22 | Cu(OAc)2: (1) | L2: (1) | K2CO3: (1) | DMF/ 120 °C | N2 | 18 | 44 | 18 |
| 23 | Cu(OAc)2: (1) | L2: (1) | K2CO3: (1) | DMF/ 120 °C | N2 | 30 | 37 | 15 |
Reaction conditions: bis(indolyl)methane (1a, 1.0 mmol), 4-iodotoluene (1.0 mmol), catalyst, ligand, base in 3.0 ml of solvent under a N2 and air atmosphere. aIsolated yield. bOptimized conditions.
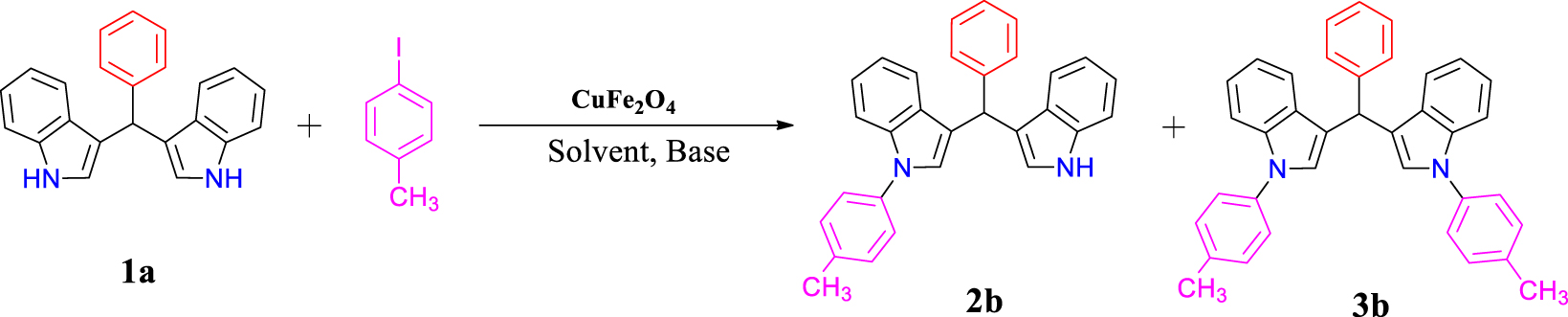
Optimization of reaction conditions for N-arylation BIMs with method B
 |
| Entry | Catalyst (mg) | Base | Solvent | Temp. (°C) | Atmosphere | Time (h) | Yield (%)a | |
|---|---|---|---|---|---|---|---|---|
| 3b | 2b | |||||||
| 1 | 90 | K2CO3 | DMF | 100 | N2 | 24 | 46 | 9 |
| 2 | 120 | K2CO3 | DMF | 100 | N2 | 24 | 60 | 9b |
| 3 | 150 | K2CO3 | DMF | 100 | N2 | 24 | 30 | 12 |
| 4 | 120 | K2CO3 | DMF | 80 | N2 | 24 | 23 | 9 |
| 5 | 120 | K2CO3 | DMF | 120 | N2 | 24 | 31 | 6 |
| 6 | 120 | K2CO3 | DMF | 100 | air | 24 | 30 | 6 |
| 7 | 120 | Na2CO3 | DMF | 100 | N2 | 24 | 23 | 3 |
| 8 | 120 | Et3N | DMF | 100 | N2 | 24 | 32 | 5 |
| 9 | 120 | K2CO3 | EtOH | reflux | N2 | 24 | 0 | 0 |
| 10 | 120 | K2CO3 | Dioxane | reflux | N2 | 24 | 0 | 0 |
| 11 | 120 | K2CO3 | CH3CN | reflux | N2 | 24 | Trace | 0 |
| 12 | 120 | K2CO3 | DMF | 100 | N2 | 18 | 38 | 6 |
| 13 | 120 | K2CO3 | DMF | 100 | N2 | 30 | 54 | 9 |
Reaction conditions: bis(indolyl)methane (1a, 1.0 mmol), 4-iodotoluene (1.0 mmol), CuFe2O4, base in 3.0 mL of solvent under N2 and air atmosphere. aIsolated yield. bOptimized conditions.
In method B, the N-arylation reaction of BIM (1a) with 4-iodotoluene was investigated to optimize the reaction conditions, including CuFe2O4 amount, base, solvent, atmosphere, and temperature. The results are summarized in Table 2. Initially, different amounts of catalyst were checked. As shown in Table 2, the amount of 120 mg of catalyst in DMF solvent and K2CO3 for 24 hours under nitrogen atmosphere is associated with an increase in the unsymmetrical product efficiency (Table 2, entry 2). In the next step, the effect of temperature was investigated. The reaction was performed at 80, 100, and 120 °C, where 100 °C was selected as the best temperature. When performing the reaction in some other solvents such as EtOH, dioxane, and CH3CN, unsatisfactory results were obtained (Table 2, entries 9–11). As can be seen from Table 2, the best result is obtained in DMF. By conducting the reaction in the presence of various bases such as Na2CO3 and Et3N compared to K2CO3, no significant improvement was observed (Table 2, entries 7, 8). The reaction was also performed under air atmosphere, where the yield of product 2b was reduced from 60% to 30% (Table 2, entry 6). Optimizing the time shows that this reaction works best in 24 hours, as listed in Table 2.
After optimization of the reaction conditions with methods A and B, a series of BIMs 1a–e were synthesized using different aryl iodides containing electron-donating and electron-withdrawing groups. The results are presented in Table 3. The spectral data and elemental analysis confirmed the structures of the synthesized compounds.
Synthesis of N-aryl bis(indolyl)methanes with method A and B
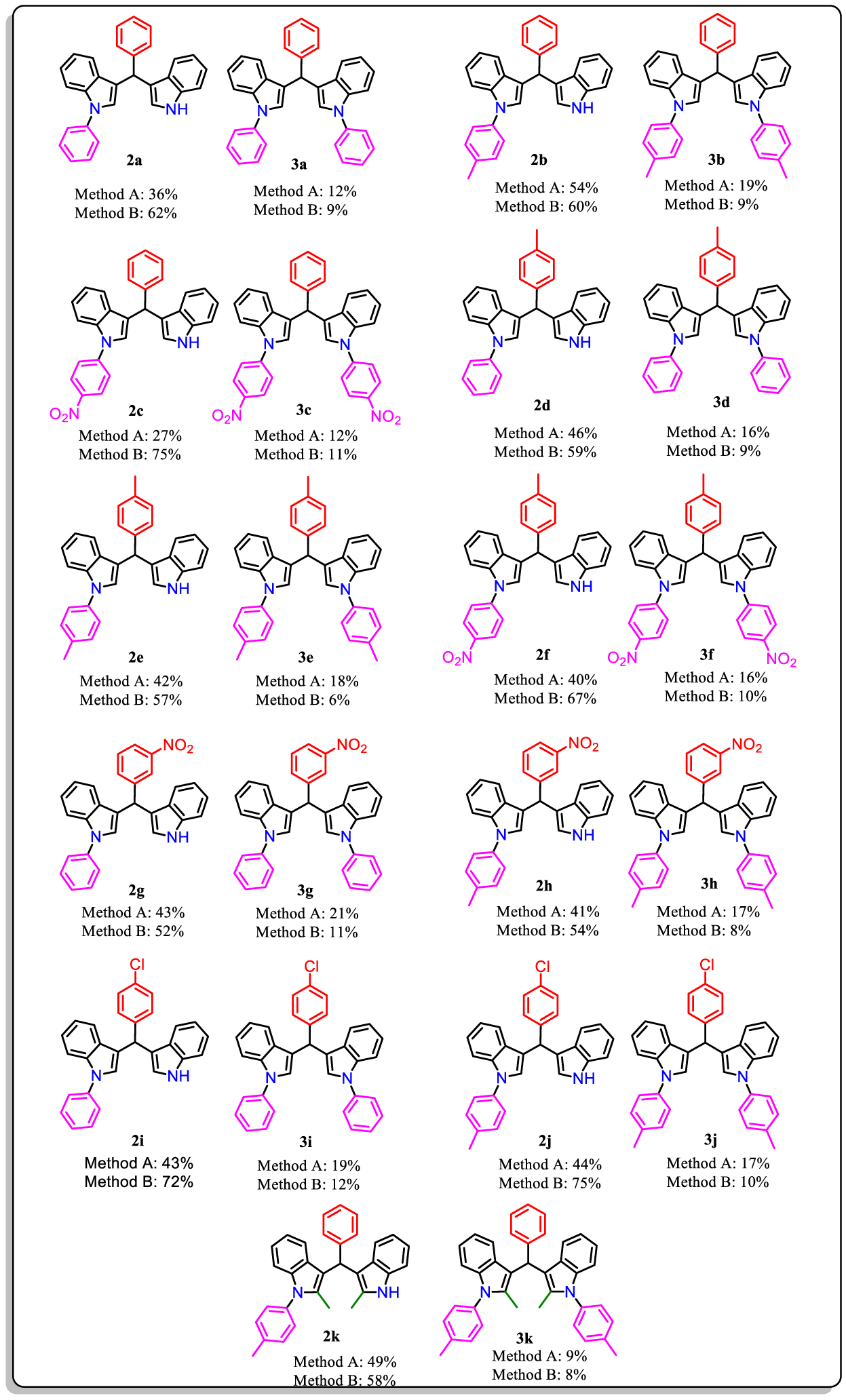 |
Method A: bis (indolyl) methane (1a–e, 1.0 mmol), aryl iodide (1.0 mmol), Cu(OAc)2, ethylene glycol, K2CO3(1.0 mmol) in 3.0 mL of DMF under N2 atmosphere. Method B: bis (indolyl) methane (1a–e, 1.0 mmol), aryl iodide (1.0 mmol), CuFe2O4 (120 mg), K2CO3 (1.0 mmol) in 3.0 mL of DMF under N2 atmosphere. Due to their very low solubility, the products derived from the reaction of compounds 1c, d with 4-NO2-C6H4-I cannot be satisfactorily characterized.
The IR spectrum of compound 2a exhibited a broadband at 3396 cm−1 for the NH group. The 1H NMR spectrum 2a showed one singlet at 𝛿 = 5.96 ppm for the CH group, and the protons of the aromatic ring appeared at 6.88–7.58 ppm. The NH proton was seen as a singlet at 𝛿 = 10.92 ppm. The existence of one NH group shows that the product is an asymmetrical BIM.
The 13C NMR spectrum of this compound has shown 25 signals, being consistent with the proposed structure. CH group was observed at 39.96 ppm. The IR spectrum of the symmetric product (3a) revealed no absorption bands in the range of 3300 cm−1, and 1H NMR spectrum showed protons of the aromatic ring in the field of 7.04–7.57 ppm. Methine proton (–CH) appeared at 6.05 ppm as a singlet. The 13C NMR spectrum of 3a exhibited 15 signals that are consistent with the proposed structure.
The recyclability of the catalyst was checked during the preparation of product 2a. When the reaction was completed in the first run, an external magnet was used for the separation of catalyst from the reaction mixture. The recycled catalyst was washed three times with ethyl acetate, dried at ambient temperature, and used for the next run. The reaction was repeated for up to four consecutive runs, the results of which are shown in Figure 3. Moreover, the XRD pattern (Figure 4) of the CuFe2O4 catalyst after the catalytic test showed no significant variation in the crystal structure, implying the excellent stability of the CuFe2O4 catalyst.
Reusability of CuFe2O4 in the synthesis of unsymmetrical BIM 2a.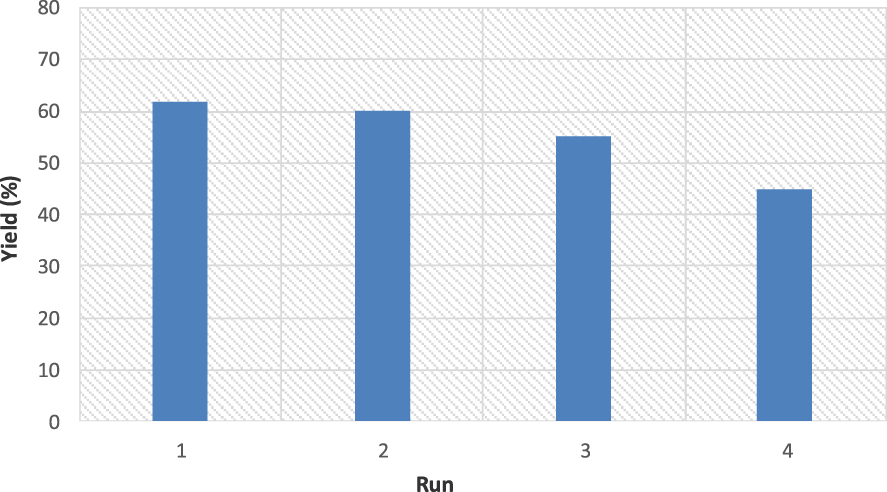
XRD patterns of CuFe2O4 before and after catalytic reaction.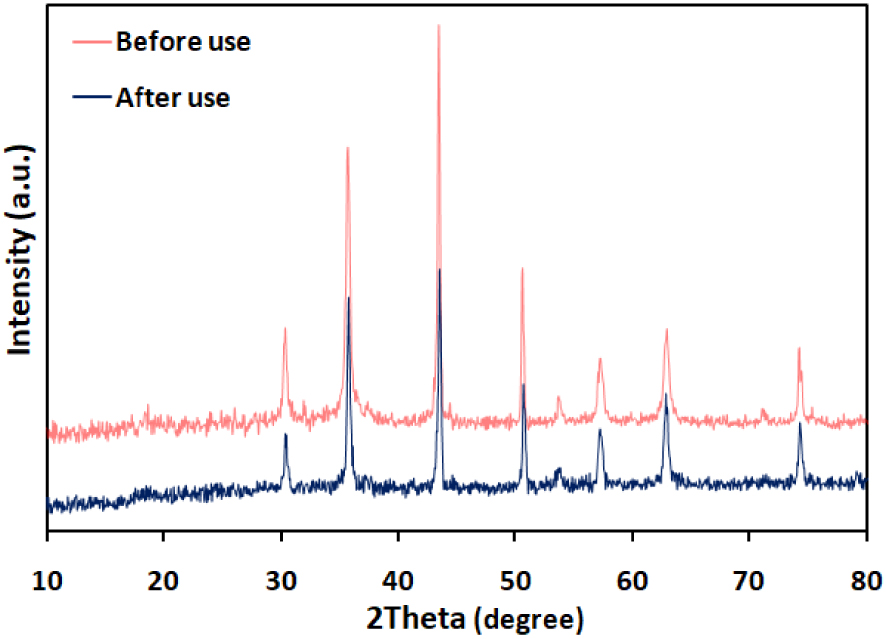
4. Conclusion
In conclusion, we have developed an efficient and mild procedure for the synthesis of unsymmetrical bis(indolyl)methanes via a C–N cross-coupling reaction. The main advantage of this method is its good selectivity for synthesizing mono N-aryl unsymmetrical bis(indolyl)methanes. This procedure complements the existing methods for the synthesis of unsymmetrical bis(indolyl)methanes. Other salient aspects of this method are simple experimental procedure, wide reactant scope, and the use of low-cost Cu-based catalysts.
Acknowledgments
The authors express their appreciation to the Shahid Bahonar University of Kerman Faculty Research Committee for its support of the present investigation.
Supplementary data
Supporting information for this article is available on the journal’s website under https://doi.org/10.5802/crchim.111 or from the author.




 CC-BY 4.0
CC-BY 4.0