1 Introduction
Lipases (EC 3.1.1.3) are ubiquitous enzymes that catalyze the hydrolysis of triacylclycerols to glycerols and free fatty acids at the lipid–water interface [1]. They are an important group of biotechnologically relevant enzymes widely used in food, dairy, detergent and pharmaceutical industries [2]. It is well known that lipases are the most widely used enzymes in organic synthesis and more than 20% of biotransformations are performed with lipases [3]. Besides low stability under application conditions and the impossibility of re-use, native lipases tend to form aggregates that decrease their catalytic performances. To improve the technical performance of industrial processes and their economy, lipases, as well as other enzymes, are usually immobilized on appropriate solid supports. Solid supports can effectively distribute enzyme molecules, prevent their aggregation, and stabilize the active forms. Immobilized enzymes can also be easily separated from the reaction medium and recycled for repeated use [4]. Moreover, compared to free enzymes, their immobilized counterparts have advantages in biocatalytic processes because the attachment of the enzyme onto a solid support usually leads to increases in their operational, thermal and pH stability.
Although the first data of the enzyme immobilization on solid supports date back to the 1950s [5], and since then a variety of organic and inorganic materials have been persuaded extensively, there is no universal one that meets all the requirements of an ideal carrier. The development of new immobilization methods and carriers still remains the main subject of research in enzyme engineering [6]. Recent interest in nanotechnology has provided a wealth of diverse nano scaffolds that have attracted great attention due to their potential application in biotechnology, immunosensing and biomedical areas [7], which could potentially support enzyme immobilization. Recently, nanomaterials have attracted enormous attention in this field, and enzymes have been immobilized on nano-sized spheres, fibers and tubes [8]. The effective enzyme loading on nanoparticles could be achieved up to 10 wt. % due to the large surface area per unit mass of nanoparticles [9]. Compared to enzymes immobilized on micro-scale supports, a nanobiocatalyst could achieve a much higher enzyme loading capacity and significantly enhanced mass transfer efficiency [7]. Among various carbonaceous nanomaterials, nanotubes are particularly suitable because their intrinsic length enables easy recovery from the reaction mixture via simple filtration, and that is a property that other nanoparticles do not possess [10]. To date, a large number of different enzymes, including lipases, have been attached on single- and multi-walled carbon nanotubes (SWCNTs and MWCNTs, respectively) [11]. First enzymes were physically adsorbed [12], and later the possibility of chemical modification of the surface of nanotubes opened up new opportunities for covalent bonding to their surface. Huang and coworkers were the first to publish the attachment of bovine serum albumin (BSA) on carboxylated CNTs in the presence of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) as a coupling reagent [13]. To increase the stability of the formed intermediate and the coupling efficiency, Hazani et al. modified the procedure by adding N-hydroxysuccinimide (NHS) to the reaction mixture [14], and later, to avoid intramolecular conjugation of protein in the presence of coupling reagents, Jiang and coworkers performed a two-step activation [15]. Since then, researchers have used these methods, based on amide linkage formation, to immobilize various biomolecules, such as DNA [14], proteins [16], and antibodies [17]. Although lipases represent one of the most extensively studied enzyme classes, only a few papers report covalent immobilization on carbon nanotubes [18,19].
In a previous report, we studied lipase adsorption onto unmodified MWCNTs [20], while herein, we report the immobilization of Candida rugosa lipase on oxidized multi-walled carbon nanotubes (o-MWCNTs). To investigate the effect of functionalization of MWCNTs on the surface hydrophobicity and the extent of non-specific interactions between lipase and o-MWCNTs, in addition to immobilization under covalent promoting conditions, a set of experiments without activating agents was also performed. The influence of the incubation time and initial enzyme concentration on enzyme coupling and activity yield was examined, and the immobilized preparations were characterized by Fourier transformation infrared spectroscopy (FT-IR) and thermogravimetric analysis (TGA).
2 Experimental
2.1 Materials
MWCNTs were purchased from Sigma–Aldrich, and used as received without purification. The purity of the MWCNTs was more than 95%. Lipase from C. rugosa (EC 3.1.1.3), Type VII, activity 1410 U mg−1 of solid was purchased from Sigma Chemical Co. (St. Louis, USA). Triton X-100, p-nitrophenylpalmitate (p-NPP), Bradford reagent and NHS were provided by Sigma–Aldrich and EDC was purchased from Acros Organics. PTFE membrane filters with a pore size of 0.05 μm were purchased from Carl Roth GmbH. All other chemicals used in this work were of analytical grade, and used without further purification.
2.2 Oxidation of MWCNTs
MWCNTs were oxidized according to the procedure described by Vuković et al. [21]. Raw MWCNTs (100 mg) were treated with a mixed solution of concentrated H2SO4 and HNO3 (3:1 v/v). The mixture was then sonicated at 40 °C for 3 h. After cooling to room temperature, the mixture of o-MWCNTs was added dropwise to 300 cm3 of cold deionized water and vacuum filtered through a PTFE membrane filter. After filtration, the sample was dried in a vacuum oven at 80 °C for 8 h.
2.3 Adsorption of lipase on o-MWCNTs
o-MWCNTs (3 mg) were added to 3 cm3 of lipase solution (50 mM phosphate buffer, pH 7.0) and sonicated for 1 min to redisperse the nanotubes. Immobilization was realized at a constant temperature (20 °C) under stirring (150 rpm). When the immobilization was finished (0.25, 0.5, 1 and 2 h), the immobilized enzyme was separated and washed three times with 3 cm3 of the same buffer.
2.4 Immobilization of lipase on o-MWCNTs through carbodiimide chemistry
o-MWCNTs (3 mg) were added to 1.5 cm3 of phosphate buffer (50 mM, pH 6.2) and 1.5 cm3 of 400 mM NHS in the same buffer. The mixture was sonicated for 30 min and then 20 mM of EDC was added and the mixture was stirred at 400 rpm for 30 min. After completion of the activation, the nanotubes were filtered and washed with the phosphate buffer (50 mM pH 6.2) to remove the excess of NHS and EDC and transferred to 3 cm3 of lipase solution (50 mM phosphate buffer, pH 7.0). The immobilized enzyme was separated after a certain period (0.25, 0.5, 1 and 2 h) and washed with the same buffer to remove the unbound enzyme.
2.5 Determination of the lipase activity
The hydrolytic activity of lipase was measured using p-nitrophenylpalmitate as the substrate. The substrate solution was prepared by dissolving 0.01586 g p-NPP, 0.017 g sodium dodecyl sulfate (SDS) and 0.1 g Triton X-100 in 100 cm3 of distilled water, mixed vigorously and heated at 80 °C for 15 min. The assay mixture consisted of 1.25 cm3 of substrate, 1.25 cm3 of 0.1 M Tris–HCl buffer pH 8.2, and 0.5 cm3 of test solution. The absorbance was read at 410 nm every 30 s for 3 min. One unit of activity was expressed as 1 μmol of p-nitrophenol released per minute under the assay conditions.
2.6 Determination of the protein loading
The lipase concentration was determined according to the method of Bradford [22], using BSA as the standard. The amount of bound enzyme was determined indirectly from the difference between the amount of enzyme introduced into the coupling reaction mixture and the amount of enzyme in the filtrate after immobilization.
2.7 FT-IR spectroscopy
Fourier-transform infrared (FT-IR) spectra of o-MWCNTs and immobilized preparations were recorded using a BOMEM (Hartmann & Braun) spectrophotometer. One mass % of sample was ground thoroughly with potassium bromide and the resulting powder was pressed into a transparent pellet in a hydraulic press. The FT-IR spectra were collected in the transmission mode between 400 and 4000 cm−1 at a resolution of 4 cm−1.
2.8 Thermogravimetric analysis
Thermogravimetric analysis (TGA) was realized on an SDT Q600 instrument (TA Instruments) up to 700 °C in a nitrogen atmosphere (flow rate: 100 cm3 min−1; heating rate: 20 °C min−1) using less than 10 mg samples.
3 Results and discussion
3.1 Effect of initial enzyme concentration and immobilization time on enzyme loading
Due to the lack of strong bonding interactions between the adsorbed enzyme molecules and the support surface, there are possibilities of enzyme desorption and loss of activity and stability. To prevent potential enzyme leakage, the possibility of forming true chemical bonds between the lipase amino groups and the carboxylic groups of o-MWCNTs was examined. However, amide bond formation is a slow and not spontaneous process at room temperature and the direct condensation can only be achieved if the reaction mixture is heated to more than 200 °C [23], which destroys the enzyme structure, resulting in loss of activity. Therefore, various compounds are used as coupling reagents to initiate the reaction [24]. Among them carbodiimides are the most frequently used and in this work, other than EDC, NHS was added to the reaction mixture to stabilize the formed intermediate and increase the coupling efficiency [25]. Considering that carbon nanotubes possess a great affinity toward proteins, it is also expected that a certain amount of protein is adsorbed even after oxidation and introduction of carboxylic groups. Therefore, concurrently, the same experimental set was performed but without the activating agents. A schematic representation of the immobilization procedure is depicted in Fig. 1. The influence of the incubation time on the lipase loading for different initial enzyme concentrations (for both experimental sets) is shown in Fig. 2.
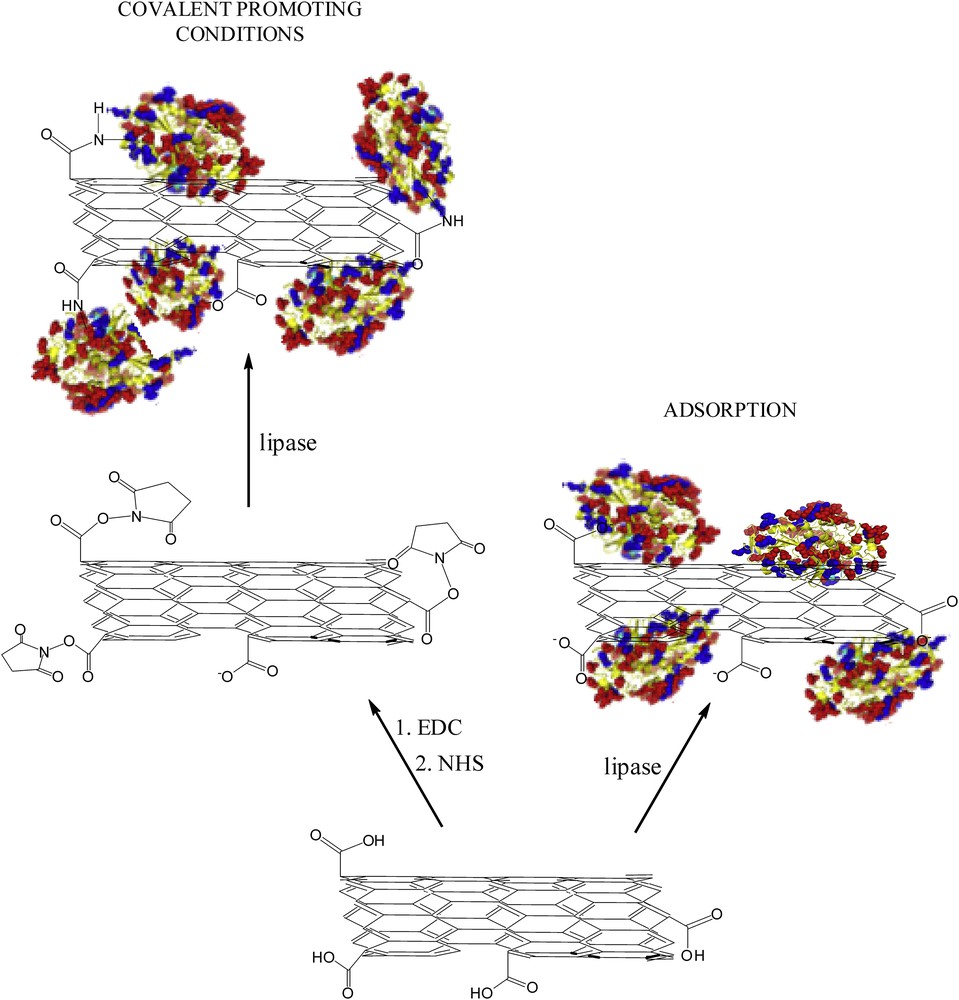
Schematic representation of the immobilization procedure.
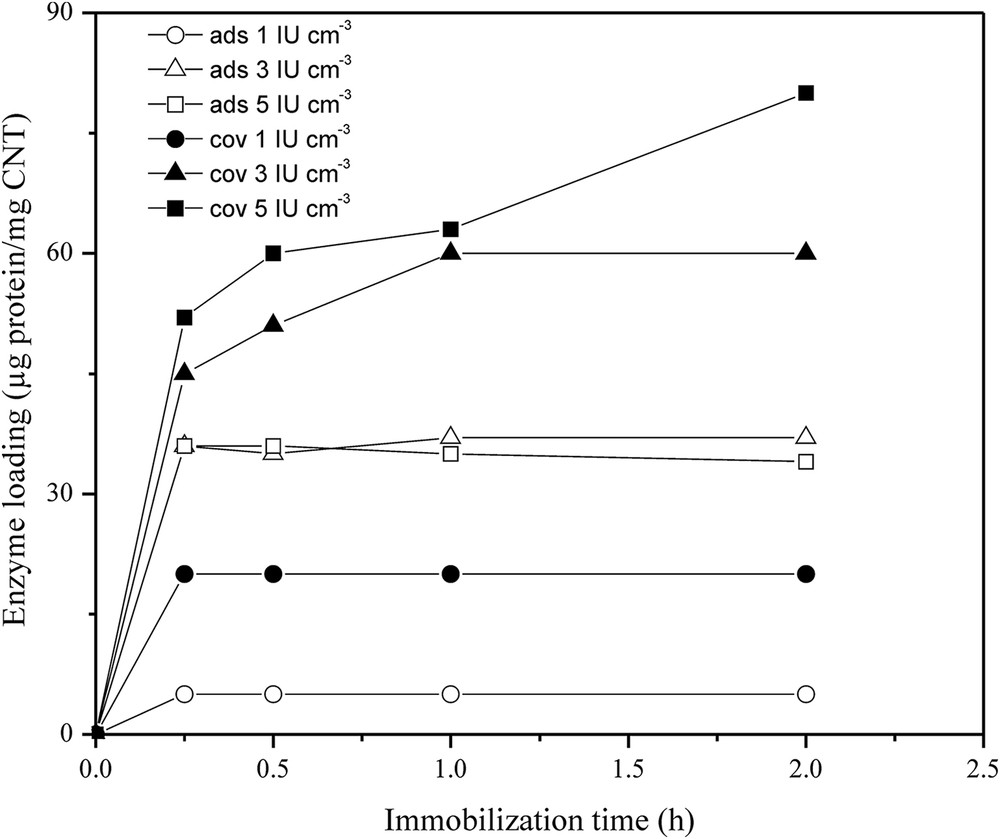
The influence of the initial enzyme concentration and immobilization time on the enzyme loading.
Increasing the initial enzyme concentration leads to increased enzyme loading on the activated o-MWCNTs, while with unmodified o-MWCNTs, the loading reached a maximum after 15 min of incubation (37 μg mg−1 CNT), at an initial enzyme concentration of 3 IU cm−3, which represents an enzyme coupling yield of 61.7%. Comparing this maximal adsorbed value to the previously reported results for the adsorption of lipase on non-oxidized MWCNTs [20], it could be seen that the capacity of o-MWCNTs for lipase adsorption was three times lower. This is because the oxidative treatment reduced the hydrophobicity of nanotubes, the main property for lipase adsorption [26]. In addition, the contribution of local negative charges on the surface of the support due to the presence of carboxyl groups introduced by oxidation cannot be neglected. It can be seen (Fig. 3) that the occurrence of amino acid residues with a negative charge (Asp and Glu) on the surface of lipase from C. rugosa is very frequent and well-distributed. Therefore, it is plausible that the lower capacity of o-MWCNTs in comparison to that of MWCNTs is due to repulsive forces between the enzyme and the charged areas of the support. This hypothesis is also in agreement with the observed increase of loading capacity after activation with EDC and NHS, since carboxyls are consumed in this activation process and the negative charges are eliminated.
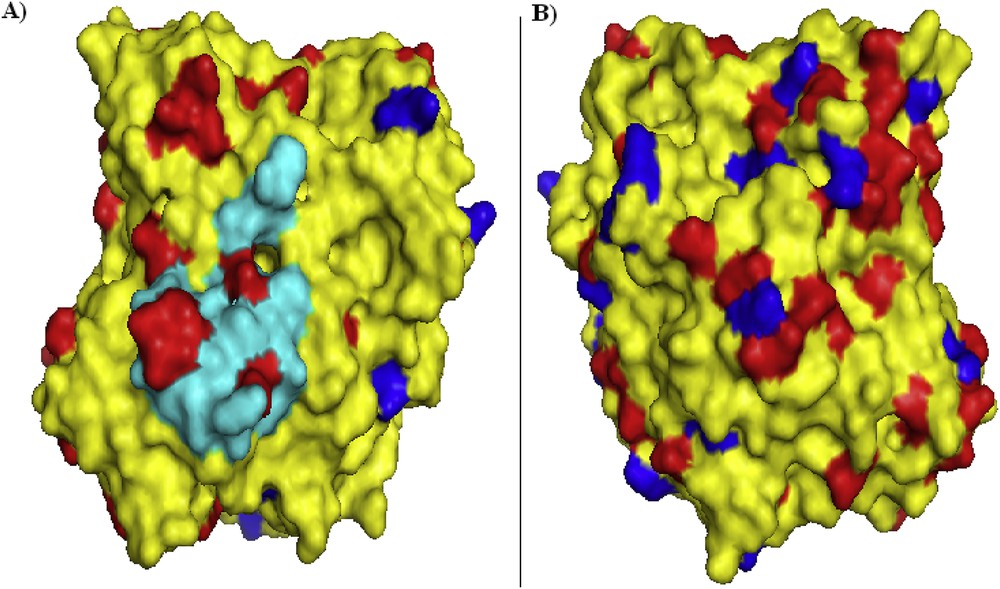
Distribution of the charged residues on the surface of the lipase from C. rugosa. Positively charged residues are colored in dark blue, negatively charged residues in red, and part of the lid that covers the active site in light blue. (A) Front view of the catalytic site. (B) 1800 rotation of the front view in the x-y plane. The 3D structure was obtained using Pymol vs. 0.99 and data obtained from Protein Data Bank (PDB). PDB code for lipase from C. rugosa is 1CRL.
Under covalent promoting conditions, the time required to achieve the maximum amount of attached lipase increased with increasing initial enzyme concentration. For the lowest and the lipase concentration of 3 IU cm−3, total amount of the offered protein was attached after 15 min and 1 h, respectively, while for the lipase concentration of 5 IU cm−3, the amount of attached protein increased in the overall examined incubation time. For the highest enzyme loading of 80 μg per mg of CNTs, an enzyme coupling yield of 80% was achieved after 2 h of incubation. Such behavior is expected since the formation of covalent chemical bonds is time consuming as opposed to the fast adsorption process.
These results clearly show that when o-MWCNTs were directly used without activating agents, a significant amount of enzyme loading was observed indicating that non-specific bonding existed to some extent but was less pronounced with increasing concentration. Considering that there was no increase in the mass of the adsorbed protein when the above initial enzyme concentration of 3 IU cm−3 was applied, it could be assumed that 37 μg of protein per mg of CNTs represents the maximum amount of the enzyme that can be adsorbed on the o-MWCNTs under the given experimental conditions. Although the introduction of carboxylic groups makes the surface of carbon nanotubes more hydrophilic, some hydrophobic fragments remain, allowing lipase adsorption. In addition, the functional –COOH groups of the o-MWCNTs would offer potential electrostatic, hydrogen bonds and hydrophilic interactions with the charged residues of protein molecules [27]. Therefore, it is not surprising that enzymes adsorb onto o-MWCNTs. Through the years, contradictory results can be found in the literature. Some groups of scientists confirmed the adsorption of feritin, cytochrome c and peroxidase [28–30], while others claim that non-specific bonding is negligible and the share of such interactions is less than 5% [16]. Some recent detailed studies confirmed lipase adsorption on o-MWCNTs using techniques, such as XPS, TEM, CD and AFM [26,31–34], and the found quantities of adsorbed protein were similar and in accordance with the results obtained in the present work.
3.2 Influence of enzyme loading on immobilized enzyme activity
Due to the property of lipase to form aggregates and to adsorb in multilayers, the immobilized preparation with the highest enzyme loading does not necessarily represent the best biocatalyst. The best measure of the immobilization efficiency is catalytic activity and for this reason, the obtained preparations were tested in the hydrolytic reaction of p-nitrophenylpalmitate and the results are shown in Fig. 4.

The influence of the initial enzyme concentration and immobilization time on the activity of the immobilized preparations.
It is interesting to observe the differences in the way that the initial enzyme concentrations and enzyme loadings changed the activity of the immobilized preparations in the two performed experimental sets. Without coupling agents, the maximum activity of 220 IU per g of immobilized enzyme was achieved with an initial concentration of 3 IU cm−3 while further increase had no effect, which is in agreement with the enzyme loading. On the other hand, under the covalent promoting conditions, activity increased over the entire tested range with a maximal value of 560 IU per g of immobilized enzyme. For initial concentrations of 1 and 3 IU cm−3, the activities were similar, although the enzyme loadings were much higher under the covalent promoting conditions (Fig. 2). At the highest tested concentration (5 IU cm−3), a significant difference between the experimental sets was observed, demonstrating the advantages of using activated o-MWCNTs. For the adsorption, the activity insignificantly increased (from 210 for 3 IU cm−3 to 220 for 5 IU cm−3), as was expected since adsorption had reached the maximum. On the other hand, under the covalent promoting conditions, the small increase in the enzyme loading of 10 μg per mg CNTs doubled the activity, enabling immobilized activities above 550 IU g−1 of support, which clearly indicated that the activated support had by far better properties for application in lipase-catalyzed reactions.
Better insights into the effects of the support on the activity of immobilized enzyme molecule can be gained by analysis of specific activities, expressed in IU per mg of enzyme on the support surface (Fig. 5). In general, higher specific activities were achieved for the enzyme immobilized on o-MWCNTs, which is in accordance with the established rule that immobilization by adsorption is less invasive and enables higher flexibility of enzyme conformation and usually high retained activity. On the other hand, covalent immobilization imparts rigidification of the enzyme conformation, which is beneficial with respect to the stability of the immobilized enzyme, but often leads to a reduction in the activity retained after immobilization [35]. However, taking into account all the obtained results, the activated o-MWCNTs seem to be the support of choice since they provide significantly higher overall activity immobilized on the support.

The influence of the initial enzyme concentration and immobilization time on the specific activity of the immobilized preparations.
The effect on the specific activity of the enzyme concentration offered in the immobilization on o-MWCNTs was typical, since the highest activity (22 IU mg−1) was achieved at the lowest offered concentration, while a further increase in the offered lipase concentration resulted in a steep decrease of specific activity to approximately 5 IU mg−1. Such results are common for lipase adsorption and are ascribed to the previously mentioned aggregation and multilayer adsorption [31,36]. The aggregated molecules would likely be denatured and could also exert unfavorable mass transport effects by restricting the access of the substrate to the active site, which lowers the specific activity of the enzyme. On the other hand, a more complex effect was observed during immobilization on the activated o-MWCNTs. Increasing the enzyme concentration from 1 to 3 IU cm−3 constantly decreased the specific activity from 7.7 to 6 IU mg−1 enzyme, probably due to similar phenomena as previously described for adsorption. It was also noticeable that the immobilization time is an important factor for specific activity since as the immobilization progresses, the activity falls even more. Then, an increase in the initial concentration to 5 IU cm−3 increased the specific activity, as did prolongation of this activation since the rise in the specific activity lasted for 1 h during the immobilization process. Since the bonding is based on a chemical reaction between the enzyme amino groups and the activated carboxylic groups of nanotubes, it is plausible that with the highest initial enzyme concentration and prolonged immobilization, the reactive groups were more frequently in the vicinity to collide and form amide bonds. On the one hand, strong covalent bonds may restrict the movement of the catalytically important chains and cause partial inactivation of the immobilized enzyme that retained about 20% of the native activity, but on the other hand, the covalently bonded enzyme probably stabilized the previously adsorbed enzyme by fixing it into the pores of the o-MWCNTs thereby preventing desorption hence resulting in a higher activity compared to the adsorbed preparations. Although the enzyme loading still increased after 2 h of immobilization, the activity and the specific activity of the immobilized enzyme decreased, which means that activity is no longer a function of loading after 63 μg of enzyme per mg of o-MWCNTs.
3.3 FT-IR analysis
The FT-IR spectra of oxidized MWCNTs (a), free lipase (b) and the lipase immobilized under the covalent promoting conditions (c) are shown in Fig. 6. Direct confirmation of the oxidation process are the peaks at 3438 and 1730 cm−1 in the spectrum of o-MWCNT that arise from the stretching vibrations of –OH and –CO bonds from the carboxylic groups, respectively. Moreover, as a consequence of the oxidation treatment, a peak appears at 1386 cm−1 that originates from sulfate groups [21]. Further confirmation of the oxidation process is the new peak at 1097 cm−1 that is assigned to stretching vibrations of –C–O bonds and the successful re-hybridization of carbon from sp2 to sp3 [33]. The spectrum of free lipase is typical for a protein, with the most prominent band at 1636 cm−1 (amide I) that originates from –CO stretching and –NH bending vibrations and the band at 1123 cm−1 from the carbohydrate moiety of the enzyme [37]. Appearance of some peaks and the disappearance of others in the spectrum of the immobilized enzyme confirmed successful immobilization. Since the amide bond established between the protein and the o-MWCNTs cannot be detected due to strong amide I band, a true indicator of covalent linking is the disappearance of the band at 1730 cm−1after immobilization. The appearance of peaks at 2964, 2919 and 2851 cm−1 confirms the presence of protein since they originate from the asymmetric and symmetric stretching vibrations of methylene and methyl groups, respectively [21]. In addition to covalent binding, the shift of the –OH stretching band (from 3439 to 3435 cm−1), amide I band (from 1636 to 1631 cm−1) and protein carbohydrate moiety band (from 1123 to 1113 cm−1) to lower frequencies in the spectrum of the immobilized enzyme confirmed the existence of non-specific interactions of the protein with the surface of the o-MWCNTs [38].
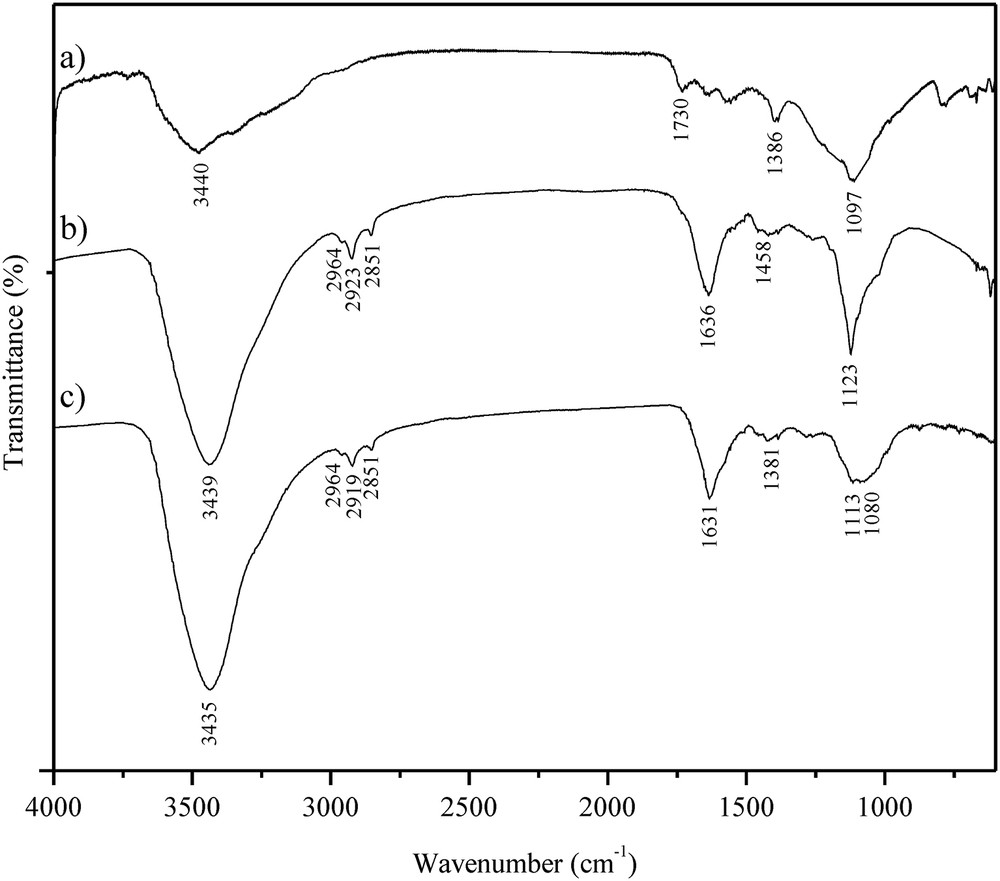
FT-IR spectra of the (a) o-MWCNTs, (b) Candida rugosa lipase and (c) immobilized enzyme.
3.4 Thermogravimetric analysis
The TG curves of (a) raw and (b) oxidized MWCNTs, (c) CRL adsorbed and (d) immobilized via EDC on o-MWCNTs are presented in Fig. 7. Insignificant weight loss was observed for the raw MWCNTs. The weight loss for o-MWCNTs was larger in the temperature range from 150 to 700 °C, which indicates successful oxidative treatment. The first weight loss at around 150 °C corresponds to degradation of surface hydroxyl groups, while the weight loss at higher temperatures originates from the decomposition of –COOH groups [39]. As a consequence of the attached enzyme, the TG curves of the immobilized preparations had a slightly different shape. Both curves (c) and (d) evince the same trend up to 500 °C. A weight loss up to around 150 °C derives from the residual water (curves c and d) or weakly bonded molecules left after EDC activation (curve d). The further steep decline from 150 to 500 °C is assigned to thermal decomposition of lipase and the larger percentage weight loss for the lipase immobilized under covalent promoting conditions probably corresponds to the increased amount of the attached protein [40]. A small, but pertinent, weight loss for lipase immobilized under covalent promoting conditions that starts at 500 °C indicates the degradation mechanism of the adsorbed and EDC-activated immobilized enzyme were different. Hence, such behavior may support the combined bonding mechanism that the enzyme is, both physically and chemically bonded under covalent promoting conditions. Since enzymes, as organic molecules, decompose mainly at temperatures lower than 500 °C, the weight loss thereafter could be assigned to high temperature condensation/cyclization reactions in the vicinity of the covalently bonded group residues, which results in the formation of low residual condensed materials that decompose between 500 and 605 °C.
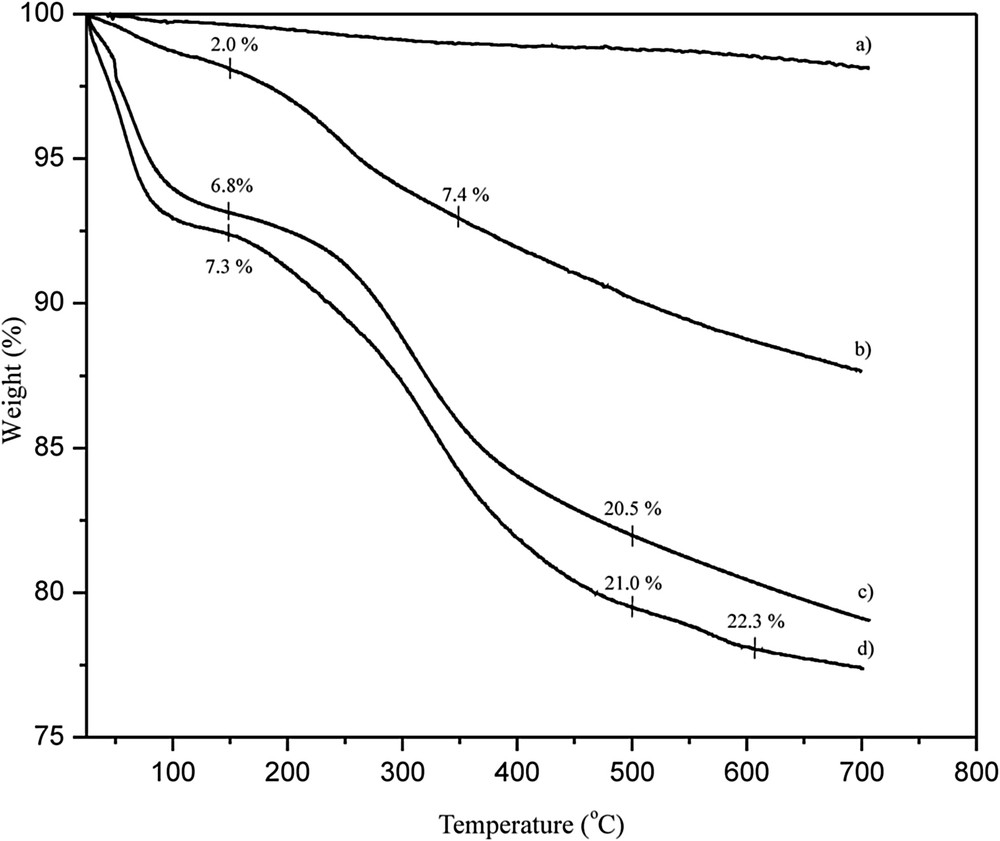
TG curves of the (a) raw-MWCNTs, (b) o-MWCNTs, (c) CRL adsorbed on o-MWCNTs and (d) CRL attached under covalent promoting conditions.
4 Conclusions
In this study, an efficient method for the two-step activation of MWCNTs is presented and the obtained derivatives were evaluated for the immobilization of lipase from C. rugosa with respect to protein loading capacity and catalytic activity of the immobilized enzyme. It was observed that the first step of activation, oxidation, of MWCNT surface, led to a decreased protein-loading capacity of the nanotubes. However, it was proved that further activation with EDC and NHS, besides the primary advantage of enabling conditions that promote covalent immobilization, also enabled significant increase in protein loadings and the overall immobilized enzyme activity per mg of support. The higher activity of the biocatalysts obtained under covalent promoting conditions is very useful for their potential use on the industrial scale, as well as from the perspective of the possibilities of re-use.
Acknowledgments
The authors are grateful to the Ministry of Education and Science of Serbia for the financial support (projects No. 172013, III 46010 and 172049).


