1 Introduction
Agricultural food production consumes about 70–80% of the total pesticides used [1]. It was estimated that 99.7% of the applied pesticides are released into the environment [2]. These large amounts of pesticides could jeopardize humans and other living organisms.
Azole fungicides (imidazoles and triazoles) show activity against a broad spectrum of fungi and inhibit fungal lanosterol-14R-demethylase, thus preventing and curing fungal infections [3,4]. Besides agriculture usage, they can be applied in material protection (wood, concrete, paints and roofs) [5,6].
Tebuconazole (IUPAC name: (RS)-1-p-chlorophenyl-4,4-dimethyl-3-(1H-1,2,4-triazol-1-ylmethyl)pentan-3-ol; Chemical Abstracts name (±)-α-[2-(4-chlorophenyl)ethyl]-α-(1,1-dimethylethyl)-1H-1,2,4-triazole-1-ethanol) is a triazole fungicide (Fig. 1).

Structure of tebuconazole.
It is used to treat fungi in cereals, peanuts, oilseed rape, soya beans, grapevines, coffee, tomatoes and potatoes [7]. Tebuconazole decreases ergosterol biosynthesis, which is a key component of fungal cell membranes [8]. The half-life of tebuconazole in soil was found to be 49–610 days under aerobic conditions [9] and thus, frequent application could lead to its accumulation in soils [9]. After application, tebuconazole may be a risk for soil ecosystems, groundwater, surface water and aquatic organisms [10,11]. In addition, tebuconazole is classified as a possible human carcinogen [12]. It is one of the contaminants resistant to conventional wastewater treatment [3,5]. Due to its persistence and the risk it poses to the environment, there is a need for the development of enhanced methods that could eliminate tebuconazole and other triazole fungicides from soil and water.
Different methods and materials have been used to remove tebuconazole from soil and water. A high-pressure mercury lamp was applied for tebuconazole photodegradation in different soils [13,14]. It was found that the photodegradation was influenced by the organic matter and clay content of the soil, as well as by soil moisture. Tebuconazole was more rapidly photodegraded under neutral than under acid and alkaline soil conditions. The degradation half-life of tebuconazole in soil is 10–22 min. A microbiological approach was also used. Screening of degrading bacteria was performed in bioreactors and the strains having the ability to degrade 51% of the initial tebuconazole concentration were identified as Enterobacter sakazakii and Serratia sp [8]. In another study, four strains that had high efficiency, high tolerance and good genetic stability were found, separated and purified from their natural environment. Their degradation efficiency for tebuconazole ranged from 89.9% to 92.2% [15]. Moreover, an invention related to the application of a glutathione-S transferase protein, prepared by cloning and recombinant production, in the degradation of tebuconazole was disclosed [16].
Advanced oxidation processes (AOP) are all characterized by the production of OH radicals and include processes such as Fenton, photo-assisted Fenton and photocatalysis [17]. Thus, the Fenton reaction was used for the treatment of an aqueous solution containing six pesticides including tebuconazole. The pesticide degradation was found to be from 60 to 100%, with mineralization up to 55% [18]. Photocatalysis was also applied for the degradation of tebuconazole. Tandem ZnO/Na2S2O8 was used as a photosensitizer/oxidant for the photodegradation of eight pesticides including tebuconazole in leaching water at the pilot plant scale under natural sunlight [19]. TiO2 was also used as a photocatalyst for tebuconazole degradation [5,20]. High resolution accurate mass liquid chromatography (HR-LC-MS) and gas chromatography–mass spectroscopy (GC–MS) identified nine transformation products proceeding through tert-butyl chain cleavage, hydroxylation, oxidation and dechlorination pathways.
Electrochemical methods are often used for pesticide degradation [21]. It should be noted that the voltammetric behavior of tebuconazole was investigated by differential pulse voltammetry (DPV) and cyclic voltammetry (CV) using a developed mercury meniscus-modified copper solid amalgam electrode [22]. The electrochemical behavior of tebuconazole was also investigated on Bi-doped PbO2 electrodes [23]. Another study showed that supported Ag and Au nanoparticles may be employed in sustainable environmental remediation, as they can be used at room temperature in aqueous solutions without the use of an additional stimulus, such as UV light [24]. The electrochemical behavior of tebuconazole on a gold electrode was not hitherto studied. The electrocatalytic properties of gold electrodes in the electrooxidation of numerous organic molecules are well known. For example, the oxidation of malic acid on a gold electrode proceeded in the region where the electrode was covered by gold oxide [25]. The electrochemical behavior of biological compounds, such as glucose, hormones and therapeutic drugs, was frequently investigated on gold electrodes and a layer of gold oxide formed on the surface of the gold electrode exhibited a great catalytic effect on their oxidation [25–27].
The aim of this work was the study of the catalytic influence of the surface of the gold electrode on the degradation of tebuconazole in 0.05 M NaHCO3. First, tebuconazole was characterized on the gold electrode by performing CV and SWV. The data obtained were used for its quantitative determination and for the elucidation of the conditions for the catalytic elimination of tebuconazole. The products formed during the electrochemical treatment of tebuconazole were analyzed by GC–MS analysis and a possible mechanism of the degradation is proposed.
2 Materials and methods
2.1 Chemicals
Tebuconazole (purity higher than 98.8%) was supplied by DR Ehrenstorfer GmbH. All other chemicals were of p.a. grade (Merck).
2.1.1 Preparation of the electrolyte
Tebuconazole is partly soluble in water and very well soluble in alcohols. A stock solution of tebuconazole was prepared by completely dissolving 36 mg of the pesticide in 1 dm3 of 18 MΩ cm deionized water and aliquots of this solution were taken and added to an electrochemical cell. For higher concentrations, tebuconazole is partly soluble in water and in this work the use of methanol for its dissolution was avoided.
2.2 Apparatus and preparation of electrode surfaces
Standard equipment was used for the cyclic voltammetry measurements and the three-electrode electrochemical cell was previously described in detail [27]. Polycrystalline gold (baer gold, surface area 0.5 cm2), which served as the working electrode, was polished with diamond paste, cleaned with a mixture of 18 MΩ cm deionized water and sulfuric acid and further cleaned with 18 MΩ cm deionized water in an ultrasonic bath. A gold wire was used as the counter electrode and a saturated calomel electrode (SCE) as the reference electrode. All potentials are given vs. SCE. The working electrode was checked prior to each experiment by cycling the potential scan between −0.55 and 1.0 V in the supporting solution (0.05 M NaHCO3; pH = 8.4) at a scan rate of 50 mV s−1 until unchanged CV characteristics of the Au electrode were obtained. Subsequently, the electrode was transferred to an electrochemical cell containing tebuconazole and the electrochemical measurements were performed. The electrolytes were deoxygenated by purging with nitrogen. All experiments were performed at room temperature using a PGZ 402 Volta Lab (Radiometer Analytical, Lyon, France). The square wave voltammetry measurements were performed using a pulse height of 100 mV, frequency of 2 Hz, step size of 5 mV, scan rate of 10 mV s−1 and an accumulation time of 0.2 s at 0.0 V.
2.3 GC–MS analysis
The samples were analyzed using a GS-MS system (Agilent 6890 gas chromatograph with an Agilent 5975N mass spectrometer). Briefly, an HP-5MS column (30 m × 0.32 mm I.D., 0.25 μm film thickness) was used with helium as the carrier gas, operating at a constant pressure of 150 kPa. The injection system was operated at 250 °C. The GC oven was set at a start temperature of 70 °C (2 min), heated to 150 °C at a rate of 25 °C min−1–200 °C at a heating rate of 3 °C min−1 and then to 280 °C at a heating rate of 20 °C min−1 that was held for 10 min. The mass spectrometer was operated in the electron impact ionization mode. The temperatures of the transfer line, ion source, and quadrupole were set to 280, 230 and 150 °C, respectively. The mass spectrometer (MS) was operated in SCAN/SIM.
3 Results and discussion
3.1 Cyclic voltammetry of tebuconazole
The catalytic role of the surface of a gold electrode in the electroactivity of tebuconazole was first examined by CV. The CVs (sequential scans, 1–10) of tebuconazole on a gold electrode in 0.05 M NaHCO3 alongside the voltammetric response of the Au electrode in a blank solution (dotted line) are presented in Fig. 2.
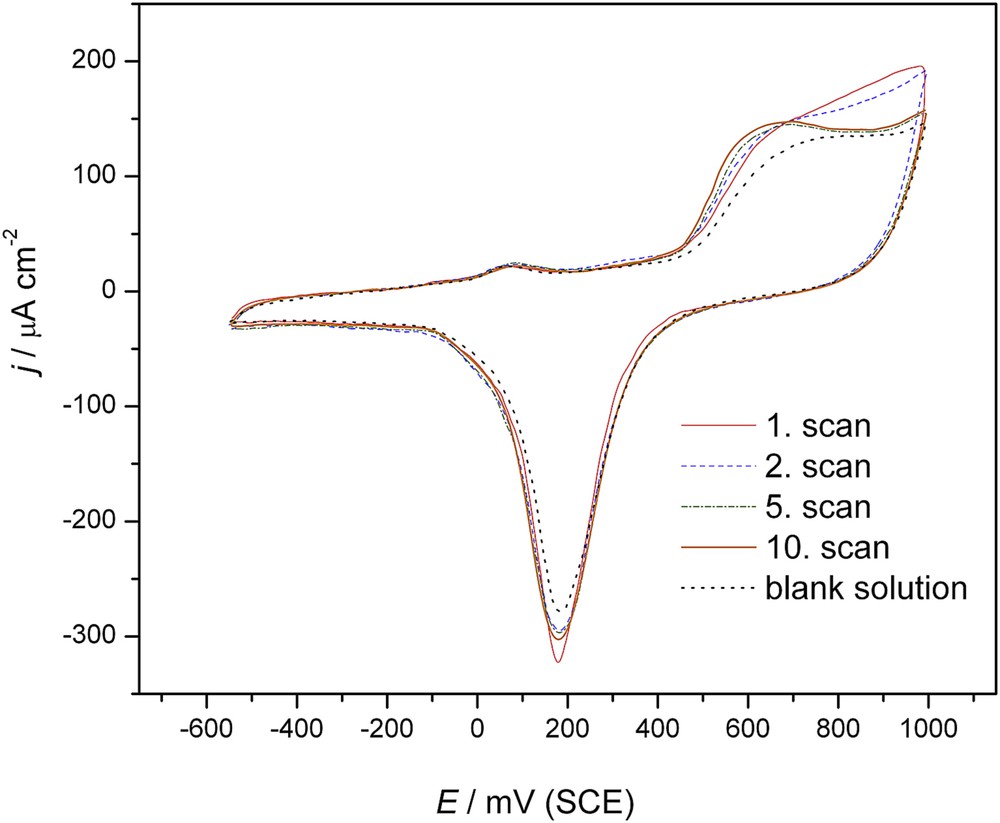
Cyclic voltammogram of the Au electrode using 0.05 M NaHCO3 (---) and after the addition of 0.76 μmol dm−3 tebuconazole (scan 1–10), scan rate: 50 mV s−1.
The cyclic voltammogram of the electrode in the first scan changed in the presence of tebuconazole compared to that of the electrode in the blank solution in that the current slightly increased in the anodic direction in the area prior to the oxide formation with an apparent increase in the region of the oxide formation. Namely, the oxidation of tebuconazole prior to the oxide formation was correlated with the AuOH formation on the gold surface since the chemisorption of OH– creating the AuOH layer occurred at −0.1 to 0.3 V (in phosphate buffer, pH = 7), showing different voltammetric waves depending on the surface orientation [28]. At a clean gold electrode, cycling brought about a steeper increase in the beginning of Au oxidation and completion of oxide monolayer formation [29]. At high potentials, a layer of gold oxide was formed on the surface of the gold electrode, as is presented by the dotted lines in Fig. 2. Its catalytic effect on tebuconazole oxidation is obvious, giving much higher anodic currents from 0.4 to 1 V (first scan, full line in Fig. 2). The oxide reduction peak also increased compared to that of the Au electrode in the blank solution, indicating an apparent reduction of the species formed in the anodic scan.
During cycling of the electrode in a solution of tebuconazole, the voltammograms were modified between the 1st and the 10th cycle. The anodic currents slightly increased with the consecutive scans before the oxide formation shifting the anodic peak towards less positive potentials. A decrease in the peak height in the region of the oxide formation from 600 to 1000 mV was observed with all successive scans compared to the first scan. The oxide reduction peak also increased with consecutive scans. This indicates that the oxidation products formed in the area before oxide formation block the electrode surface in the area of the oxide formation, giving the tendency of decreasing currents. It seems that all these species were successfully reduced with the reduction of gold oxide, giving a clean electrode surface for the next anodic scan. This enabled the increase in the anodic currents and the shift of the anodic peak towards less positive potentials, suggesting the absence of strong adsorption of tebuconazole and its products in the area before gold oxide formation. All these indicate that the Au electrode played a catalytic role in the reaction.
The voltammetric behavior of tebuconazole using a solid copper amalgam was investigated and Britton–Robinson buffer/methanol (1:1, v/v) of pH 6.4 was found to be the most suitable supporting electrolyte for its determination [17]. For the Bi-doped PbO2 electrode, NaClO4 (pH 7) was used as the electrolyte [23] and 0.05 M NaHCO3 for the gold electrode. Both electrolytes were carefully chosen because they do not undergo electrochemical oxidation, i.e., they do not generate other oxidant species that could change the mechanism of degradation of tebuconazole. For all three different electrodes, the pH value of the electrolyte was close to neutral, representing the best choice for the maximum efficiency of tebuconazole oxidation.
In any case, the use of phosphate buffers at different pH values (2–10) for the gold electrode was tested and evidenced the much lower oxidation ability of tebuconazole, even at pH 7. This indicates the stronger adsorption of tebuconazole and oxidation products than was obtained when using 0.05 M NaHCO3.
The CVs on Au at different scan rates (v) in 0.05 M NaHCO3 containing 0.76 μmol dm–3 tebuconazole are demonstrated in Fig. 3. It is obvious that the currents become higher with increasing scan rate. The relationship between the peak current and v1/2 is displayed in Fig. 3 (inset a). The observed linearity indicates that the oxidation of tebuconazole was a diffusion controlled process. This slope is close to the theoretical one of 0.5 for an ideal diffusion-controlled electrode process.
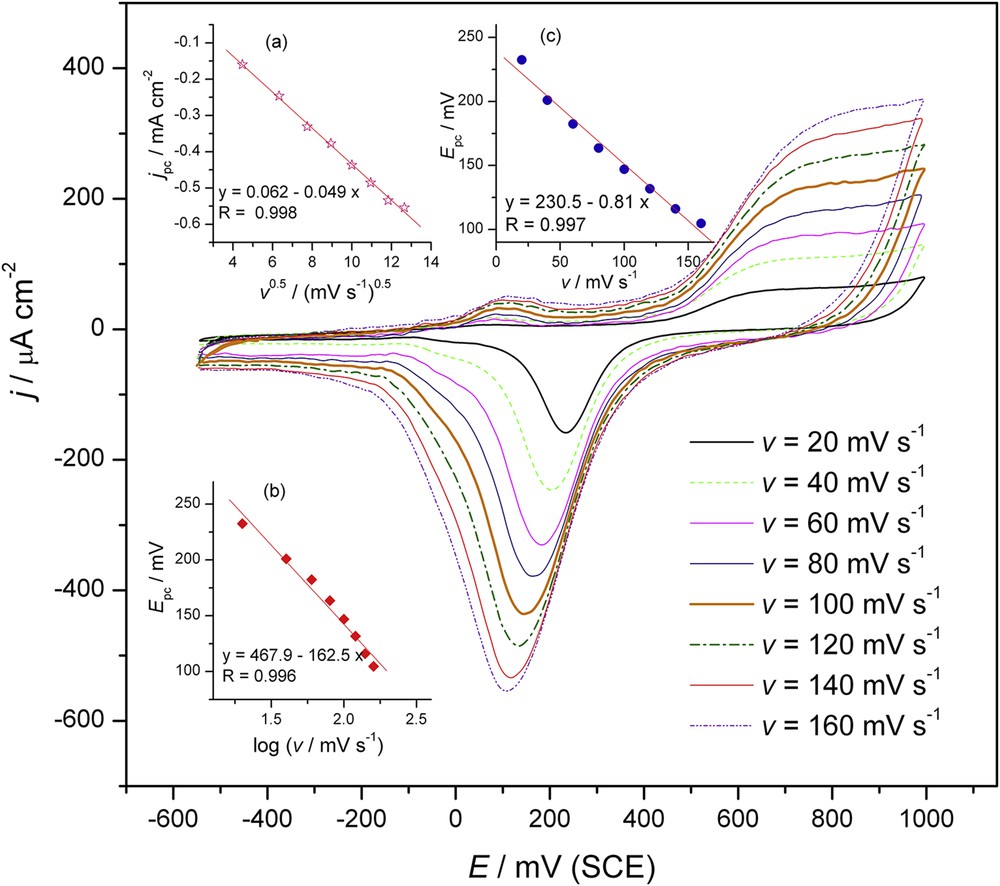
CVs of 0.76 μmol dm−3 of tebuconazole on a gold electrode using 0.05 M NaHCO3 for scan rates: 20, 40, 60, 80, 100, 120 and 140 mV s−1. Insets: plots of peak current density vs. v1/2 (a), peak potential shift vs. log of scan rates (b) and peak potential vs. v (c).
According to Bard and Faulkner [30], the value of the cathodic peak potential increases and the peak is shifted in the negative direction with increasing scan rate. Thus, the straight line relationship observed for the dependency of the cathodic peak potentials on the log of the scan rates, Fig. 3 (inset b), suggested that the oxidation of tebuconazole was an irreversible electrode process.
In order to determine the kinetic parameters of the electron-transfer process for the oxidation of tebuconazole on the Au electrode, cyclic voltammetric experiments were performed at different scan rates. The Laviron theory for irreversible processes was applied in order to calculate the number of electrons transferred and the heterogeneous electron-transfer rate constant (k°) according to the Equation (1) [31]:
| (1) |
Thus, the value of αn can be easily calculated from the slope of the plot of Ep vs. log v. In this system, the slope was 162.5. Furthermore, the number of electrons (n) transferred in the electrooxidation of tebuconazole was calculated to be 0.85 (approximately equal to 1) since the value of α is 0.5. The value of k° can be determined from the intercept of the previous plot if the value of E° is known. The value of E° can be obtained from the intercept of the Ep vs. v curve (Fig. 3, inset c) and the value was calculated to be 230.5 mV. From this, k° was calculated to be 1.58 s–1.
The well developed oxidation peak of tebuconazole was further investigated in order to test the dependency of peak height versus tebuconazole concentration under the experimental conditions presented in Fig. 4. Between two consecutive concentrations, the gold electrode was cleaned as described in the Experimental part. The CVs for the set of concentrations of tebuconazole (0.076, 0.19, 0.38, 0.57 and 0.76 μmol dm–3) are displayed in Fig. 4. The dependence of the anodic peak current density on the concentration of tebuconazole, showing a linear relationship in the potential range from 0.45 to 1.0 V with an excellent correlation coefficient (R = 0.998), is presented in the inset to Fig. 4. The Limit of Detection (LOD) and Limit of Quantification (LOQ) were calculated according to the equation k × SD/b, where k = 3 for LOD and 10 for LOQ, SD is the standard deviation of the intercept and b is the slope of the calibration curve [32]. The CV values of LOD and LOQ were 0.064 and 0.21 μmol dm–3, respectively.
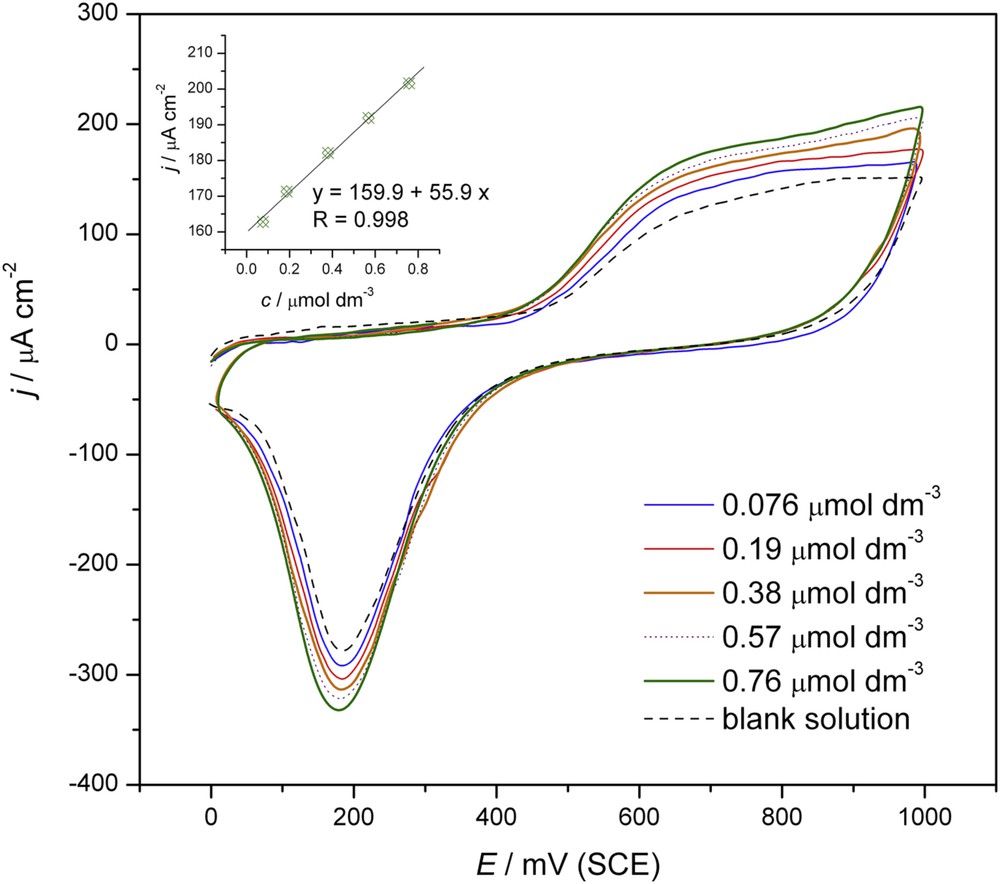
CVs for a set of higher concentrations of tebuconazole (0.076, 0.19, 0.38, 0.57, 0.76 μmol dm−3), scan rate: 50 mV s−1, only first scan is presented. Inset: plot of the current density vs. concentration at 0.9 V.
According to potentiodynamic measurements, it is clear that the gold electrode was very sensitive to the tebuconazole molecules in the examined range of concentrations.
3.2 Square wave voltammetry of tebuconazole
Cyclic voltammetry revealed the apparent electrochemical activity and concentration dependency of tebuconazole at pH 8.4 in 0.05 M NaHCO3 solution and thus, the possibility for its quantitative determination by square wave voltammetry.
Deposition times of 10 s and 2 s, deposition potentials of 0.1 and 0.2 V, and a pulse amplitude of 50 mV were also tested in the SWV experiments but better results were obtained under the conditions presented in Fig. 5.
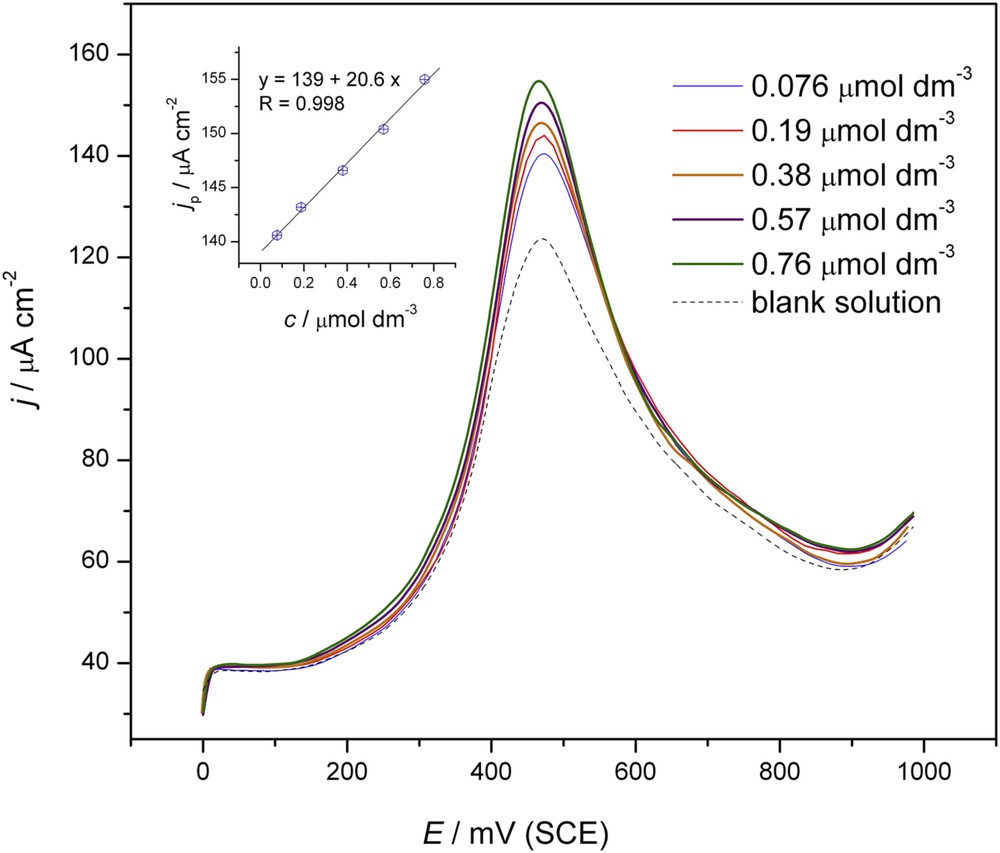
Square wave anodic stripping voltammograms of tebuconazole (0.076, 0.19, 0.38, 0.57, 0.76 μmol dm−3) obtained with the Au electrode (---) using 0.05 M NaHCO3. Accumulation time: 0.2 s at E = 0.0 V; step size 5 mV, pulse size 100 mV, frequency 2 Hz, scan rate 10 mV s−1. Inset: linear dependency of the anodic peak currents vs. concentration of tebuconazole.
SW voltammograms recorded while increasing the amount of tebuconazole for the set of concentrations of 0.076, 0.19, 0.38, 0.57, 0.76 μmol dm–3 showed a linear increase in the anodic peak currents with the concentration (inset in Fig. 5) with an excellent correlation coefficient (R = 0.998). The linear relationship was used to evaluate the limit of detection and the limit of quantization. The values of the LOD and LOQ were 0.045 and 0.15 μmol dm–3, respectively.
Using a developed mercury meniscus-modified copper solid amalgam electrode with DPV determination of tebuconazole, a limit of detection of 0.2 μmol dm–3 was reached [22]. This suggests that the gold electrode was highly sensitive compared to the developed mercury meniscus-modified copper solid amalgam electrode. Both electrodes contributed to the development and application of new methods for the simple and fast determination of the highly toxic pollutant tebuconazole.
Recovery studies were also performed to judge the accuracy of the CV and SWV methods by performing six measurements of tebuconazole concentrations as presented in Table 1. By comparing the obtained results for CV and SWV methods, it could be concluded that the SWV method was more accurate for the electroanalytical determination of tebuconazole.
Determination of tebuconazole by CV and SWV.
| Parameter | CV | SWV |
| Range/μmol dm–3 | 0.076–0.76 | 0.076–0.76 |
| Regression equationa | ||
| Slope | 55.9 | 20.6 |
| S.D. of slope | 2.121 | 0.66 |
| Intercept | 159.9 | 139.0 |
| S.D. of intercept | 0.986 | 0.307 |
| Regression coefficient | 0.998 | 0.998 |
| Recovery/% | 99.0 | 99.7 |
| R.S.D./% | 1.175 | 0.366 |
| LOD/μmol dm−3 | 0.064 | 0.045 |
| LOQ/μmol dm−3 | 0.21 | 0.15 |
a Y = a + bC, where C is the concentration of tebuconazole, μmol dm−3; Y is the current per area unit, μA cm−2. R.S.D.: relative standard deviation.
3.3 Electrochemical degradation of tebuconazole on the gold electrode followed by GC–MS
Tebuconazole as a highly toxic pollutant is stable for more than 28 days at pH 5, 7, and 9 [33]. It requires the development of new, fast procedures for pesticide degradation. The catalytic role of the Au electrode in the oxidation of tebuconazole and the data obtained from the CV and SWV experiments offered the experimental conditions for testing the degradation possibility of the pesticide. The CVs (first scan, after 20 min and after 60 min) of tebuconazole on the gold electrode in 0.05 M NaHCO3 are presented in Fig. 6a. The described phenomena in Fig. 2 were present during continuously cycling giving apparently higher reaction currents and a potential shift to the negative values by approximately 70 mV after 60 min of continuous cycling compared to those observed in the first cycle (Fig. 6a). This confirmed that gold electrodes are a good catalyst for tebuconazole degradation, i.e., they are not poisoned at all during 60 min of cycling. The same effect was observed in the SWV and SW voltammograms presented in Fig. 6b. The phenomena observed by the both techniques suggest changes in the molecule structure of the pesticide.
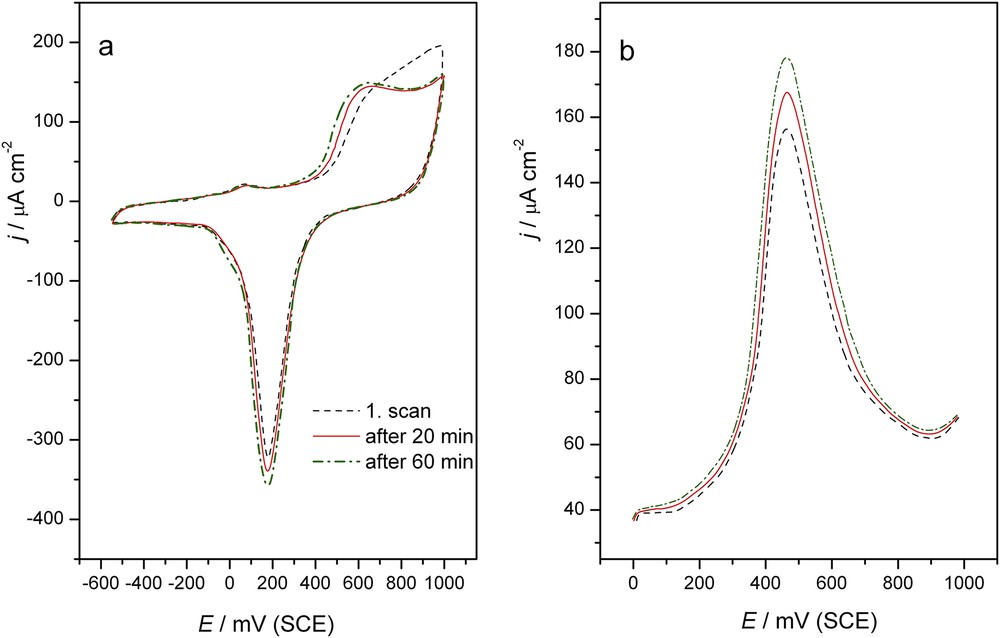
a) Cyclic voltammogram of the Au electrode using 0.05 M NaHCO3 after the addition of 0.76 μmol dm−3 tebuconazole (first scan, after 20 min and after 60 min), scan rate: 50 mV s−1 b) Square wave anodic stripping voltammograms of tebuconazole (0.76 μmol dm−3) obtained with the Au electrode (---) using 0.05 M NaHCO3. Accumulation time: 0.2 s at E = 0.0 V; step size 5 mV, pulse size 100 mV, frequency 2 Hz, scan rate 10 mV s−1.
The electrochemical degradation of tebuconazole was followed by GC–MS analysis. The total disappearance of the primary tebuconazole compound (m/z 307, retention time 25 min) was achieved after 60 min. Concurrently with the disappearance of the ion m/z 307, the formation of several peaks with different retention times and m/z ratios were registered as a result of the evident degradation of tebuconazole. The first major degradation product after 20 min of treatment, with a retention time of 16 min, was identified at m/z 83, which corresponded to simple C–C cleavage α to the tertiary OH group, yielding the triazolylmethylene cation (product A, route a). The most abundant degradation product after 60 min of treatment, with a retention time of 12 min and m/z 125, was identified as the p-chlorobenzyl cation, arising from C–C cleavage β to the tertiary OH group in the primary molecule (product B, route b). These products were previously detected during studies of tebuconazole degradation [6,23]. The proposed main electrodegradation products of tebuconazole are given in Fig. 7.

The proposed main electrodegradation products of tebuconazole.
Alternative, non-precious metal electrodes were used to electrooxidize tebuconazole, e.g., Bi-doped PbO2 prepared and characterized by very complicated and expensive procedures, as presented in the literature in detail [23]. The advantage of the Au electrode is that it must only be properly cleaned prior to tebuconazole degradation. For the Bi-doped PbO2 electrode, NaClO4 was used as the electrolyte and for the gold electrode, 0.05 M NaHCO3 was the medium for tebuconazole degradation. In both cases the electrolytes were carefully chosen because they do not undergo electrochemical oxidation, i.e., they do not generate other oxidant species that could change the mechanism of tebuconazole degradation.
4 Conclusions
The catalytic role of the Au electrode in the oxidation of tebuconazole was observed in 0.05 M NaHCO3 by CV and SWV. The investigated process is irreversible and diffusion controlled. The linear relationships (currents vs. concentrations) in the range of 0.076–0.76 μmol dm–3 were obtained by both methods but the SWV method was more accurate.
Based on the catalytic role of the Au electrode for tebuconazole oxidation in CV and SWV experiments, the conditions for the degradation of pesticide were achieved. After 20 min of continuous cycling, the first major degradation product was identified at m/z 83, attributed to the simple cleavage of the C–C in the α position to the tertiary OH group, yielding the triazolylmethylene cation. The most abundant degradation product obtained after 60 min of treatment, with m/z 125, was identified as the p-chlorobenzyl cation, which arises from cleavage of the C–C bond in the β position to the tertiary OH group in the primary molecule. The total disappearance of the primary tebuconazole compound (m/z 307) was achieved after 60 min of continuous potential cycling, thereby promoting gold electrodes as the catalyst for the degradation of environmental pollutants.
Acknowledgements
The work was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia (Grants No. ON172013 and ON172060).


