1 Introduction
One of the most valuable synthetic methods to prepare symmetric and nonsymmetrical biaryls is a palladium-catalyzed Suzuki coupling reaction of aryl halides with arylboronic acids. So many efforts in the catalysis research have been allocated to extend efficient, safe, and reusable palladium catalysts [1]. Nowadays, the immobilization of palladium active sites onto supports to produce heterogeneous and reusable catalysts has attracted much attention [2,3]. Different inorganic, organic, and hybrid porous systems, such as zeolites [4], metal–organic frameworks (MOFs) [5], and polymers [6], have been applied for supported palladium catalysts. Catalyst-based layered double hydroxides (LDHs) have gained considerable attention because, in addition to their readily dispersed characteristic in a reaction solution with an intrinsically high surface area rending, they are heterogeneous and can be easily recovered from the reaction mixture. Thus, a lot of LDH-supported catalysts with high catalytic activities have been extended and used in different organic synthesis, including transesterification reactions [7], hydrogenation and oxidation reactions [8], polymerization of alkene oxide [9], aldol condensation [10], and synthesis of heterocyclic compounds [11].
In this study, in continuation of our research on the stabilization of palladium nanoparticles (NPs) on the novel supports [12–14], we present LDH/Tris/Pd using LDH as a support and Tris as a coupling linker. Tris with three hydroxyl groups in a molecule can chelate to palladium, producing a novel and efficient catalyst.
2 Experimental setup
2.1 Chemicals and instruments
All commercial materials were purchased from Merck companies and used without further purifications. Fourier transform infrared (FT-IR) spectra were recorded using a Shimadzu 435-U-04 FT spectrophotometer (KBr pellets). Scanning electron microscopy (SEM) was performed using field emission SEM instrument (SIGMA, Germany). Transmission electron microscopy (TEM) was performed in an inert atmosphere (N2) in the temperature range 0–1000 °C at a heating rate of 10 °C min−1 using Zeiss-EM10C-100 kV. The qualitative analysis of LDH/Tris/Pd was performed using energy-dispersive X-ray (EDX) spectroscopy. EDX analysis of the prepared catalyst was performed on an FESEM instrument (SIGMA). TGA was performed on a TA instruments (model 951 DUPONT) apparatus. Ultrasonication was done in a 2200 ETH-SONICA ultrasound cleaner with a frequency of 45 KHz.
2.2 Preparation of LDHs
CaAl LDH material was provided by the coprecipitation method in a one-pot reaction [15]. An aqueous solution containing a molar ratio of 2:1 from mixed metal solutions, calcium nitrate tetrahydrate (Ca(NO3)2·4H2O) and aluminum nitrate nonahydrate (Al(NO3)3·9H2O) dissolved in deionized (DI) water, was prepared. Then, under vigorous stirring, at 65 °C, the mixed metal solution was slowly added to a 2 M sodium hydroxide solution to maintain a constant pH of approximately 10 or 11. After completion of the addition, the LDH was aged for 18 h at 65 °C. In the next step, the obtained white powder was separated by centrifugation, washed three times with DI water, and dried overnight at 100 °C.
2.3 Preparation of LDH coated with (3-chloropropyl)triethoxysilane (LDH/(CH2)3-Cl)
LDH/(CH2)3-Cl was synthesized via surface reaction of LDH by using 3-chloropropyltrimethoxysilane. LDH (1.0 g) and toluene (50.0 mL) were mixed into a 250 mL round-bottom flask. Then 2.0 mL of 3-chloropropyltrimethoxysilane was added and the mixture was refluxed at 110 °C with continuous stirring for 12 h. The resulting LDH/(CH2)3-Cl was collected and washed with toluene and EtOH several times and dried at 70 °C until complete dryness.
2.4 Anchoring of Tris onto LDH/(CH2)3-Cl surface (LDH/Tris)
A mixture of 3.0 g of Tris and 50 mL of EtOH was stirred at 80 °C. Then, 1.0 g of LDH/(CH2)3-Cl was added to the flask. The reaction was conducted for 48 h. The crude product was filtered off and washed with ethanol three times to remove all unreacted Tris. The final product (white solid) was dried under vacuum overnight.
2.5 Synthesis of LDH/Tris–supported palladium NPs (LDH/Tris/Pd(0))
LDH/Tris (0.5 g) was added to a flask containing a solution of PdCl2 (2 mmol, 0.34 g) in CH3CN (10 mL) and stirred for 24 h at 50 °C. After cooling, the resulting product was collected on a filter, washed four times with acetone (8 mL), and dried in an oven at 70 °C. Moreover, with the use of NaBH4, as a reducing agent, Pd(II) reduced to Pd(0): the obtained catalyst was dispersed in 20 mL CH3CN, aqueous solution of NaBH4 (0.2 g in 20 mL) was added to a round-bottom flask, and the mixture was stirred at room temperature for 5 h. Then, with the use of centrifuge, black solid catalyst was collected, washed with DI water (3 × 4 mL) and CH3CN (4 mL), and finally dried under vacuum for 18 h. The concentration of palladium in LDH/Tris/Pd(0), which was 13.52 wt %, was determined by inductively coupled plasma optical emission spectrometry ( ICP-OES).
2.6 General procedure for the Suzuki–Miyaura coupling reaction using LDH/Tris/Pd
A mixture of ArX (1 mmol), PhB(OH)2 (1.1 mmol), K2CO3 (2 mmol), catalyst (0.05 mol % Pd), and H2O/EtOH (1:1, 5 mL) were placed in a 25 mL Schlenk tube and the mixture was stirred at room temperature. After the completion of the reaction monitored by thin-layer chromatography (n-hexane/acetone 10:4), the catalyst was separated by centrifugation, and then the reaction mixture was washed three times with ethyl acetate. The combined organic layer was dried over magnesium sulfate and evaporated in a rotary evaporator under reduced pressure to obtain the biaryl product.
3 Results and discussion
The catalyst was prepared using a simple and efficient method and characterized by FT-IR, X-ray diffraction (XRD), SEM, EDX, TEM, and X-ray photoelectron spectroscopy (XPS).
In this study, we used NaBH4 as a reducing agent and reduced Pd(II) to Pd(0) to prepare LDHs/Tris/Pd. After the addition of NaBH4, the yellow color of the Pd(II) solution immediately changed into black, which indicated the formation of Pd NPs (Scheme 1).
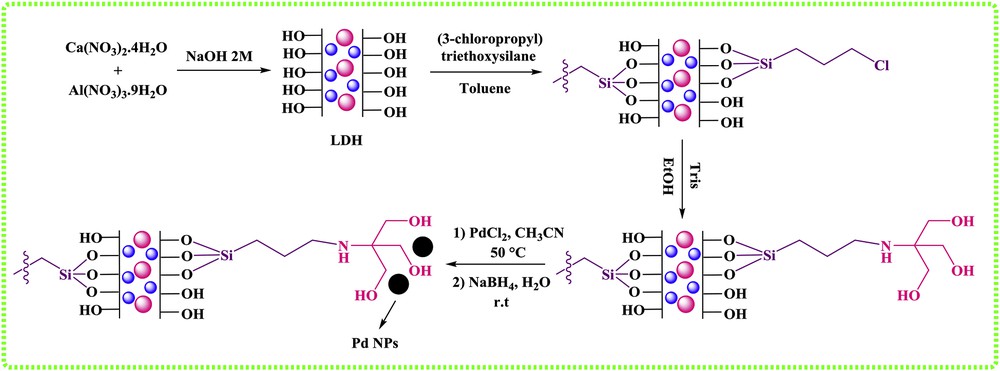
Synthetic route of LDH/Tris/Pd.
Conversion of Pd(II) to Pd(0) in the resulted Pd-supported NPs was confirmed by the UV–vis spectrum. The disappearance of the peak at 420 nm, which belongs to Pd(II) species, ascertains the formation of the Pd(0) catalysts (Fig. 1).
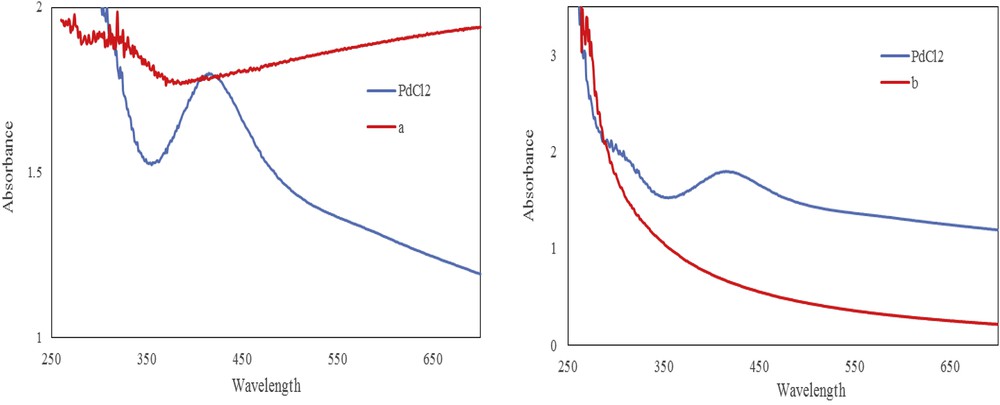
UV–vis spectrum of the (a) PdCl2 and (b) LDH/Tris/Pd(0).
3.1 Characterization of the prepared LDH/Tris/Pd
The FT-IR spectra of the LDH, LDH/(CH2)3-Cl, LDH/Tris, and LDH/Tris/Pd are shown in Fig. 2, peak assignments appeared as “X value”. The broad observed peaks, in all samples, shown at 3400–3650 and 535–580 cm−1 were attributed to the Al-OH/H2O stretching and metal–oxygen vibrations, respectively. The appearance of the strong peak at 1385 cm−1 imputed to the interlayer NO3− anion is the other notable finding for these figures. In Fig. 2b observed peaks at 1057 and 1138 cm−1 attributed to Si–O–M and Si–O–Si confirm that the (3-chloropropyl)triethoxysilane have functionalized the surface of the LDH. The successful reaction of Tris with LDH/(CH2)3-Cl was confirmed with observed peaks at 1353, 1385, and 3423 cm−1 attributed to stretching vibration of C–N, C–O, and N–H groups (Fig. 2c). The reduction in the intensity of OH groups at 3641 cm−1 in Fig. 2d demonstrated the coordination of Pd NPs on the surface of the functionalized LDH.
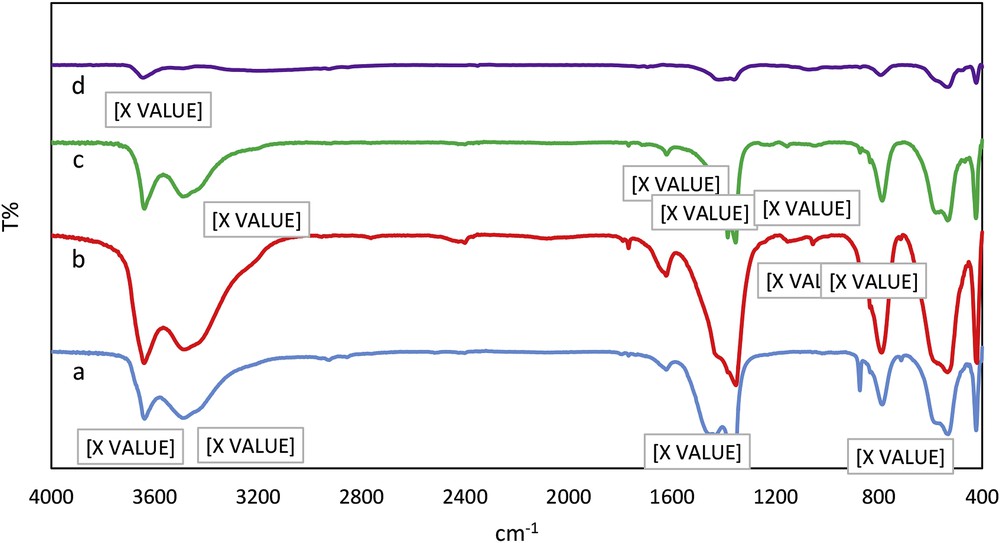
FT-IR spectra of (a) LDH, (b) LDH/(CH2)3-Cl, (c) LDH/Tris, and (d) LDH/Tris/Pd.
To check the thermal degradation property of LDH/Tris/Pd, the TGA of the catalyst was performed. Catalyst started to decompose at less than 150 °C attributed to the loss of the trapped H2O and interlayer anions. The weight loss at a higher temperature (150–300 °C) could be mainly imputed to the evaporation and subsequent decomposition of (3-chloropropyl)triethoxysilane groups. The final degradation temperature occurred at 400–700 °C, ascribed to the release of Tris. The maximum mass loss is 39.10%, which was attributed to the strong interaction among LDH, Tris, and Pd. As a result, the catalyst was stable up to 600 °C and could be safely used in organic reactions at high temperatures (Fig. 3).
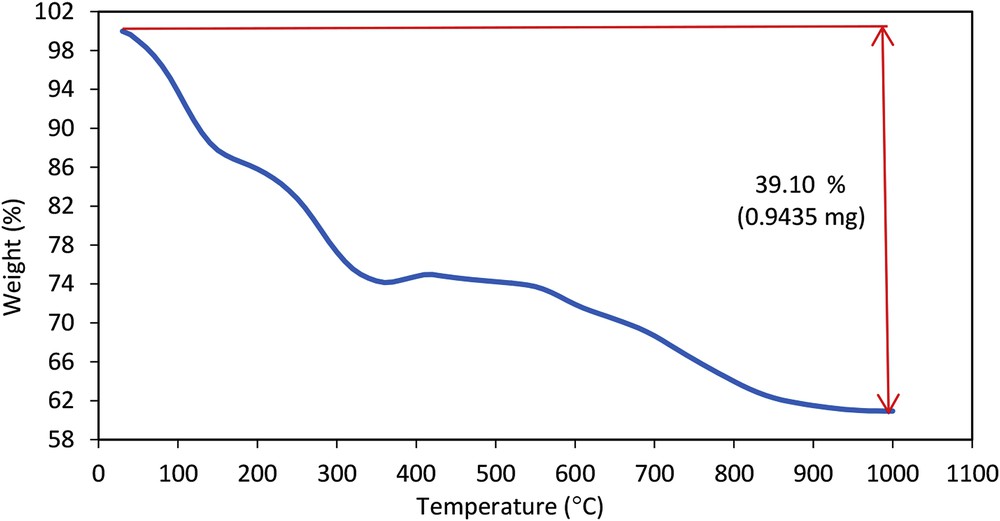
Thermogravimetric diagram of the LDH/Tris/Pd.
Fig. 4 shows the XRD patterns of LDH and LDH/Tris/Pd. The successful syntheses of CaAl-LDHs are established by comparison of the XRD pattern of synthesis LDH with those previously reported [15,16]. The observed tetragonal phase of LDH/Tris/Pd indicated that the coordinating Tris did not change the crystallinity of the LDH. The appearance of the peaks related to palladium in 2θ = 39.8°, 47.5°, 68.4°, and 81.7° showed the formation of Pd(0) NPs in LDH/Tris/Pd.
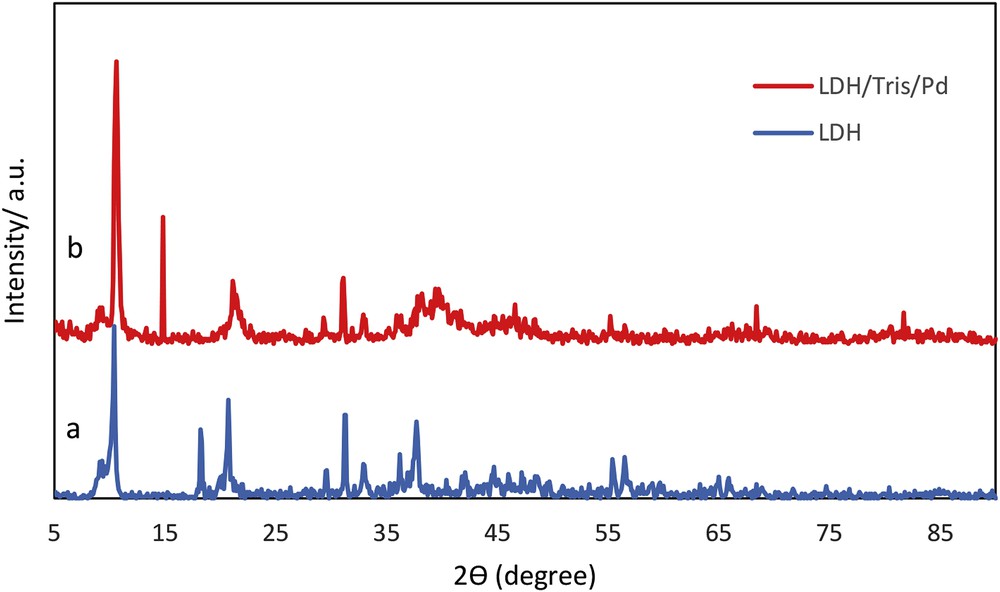
XRD pattern of (a) LDH and (b) LDH/Tris/Pd.
The surface morphology of the LDH/Tris/Pd was characterized by SEM and TEM. SEM results show separate hexagonal structure attributed to LDH/Tris/Pd. On the other hand, TEM images of LDH/Tris/Pd demonstrated that palladium NPs are bonded to the organic matrix in the catalyst (Fig. 5).
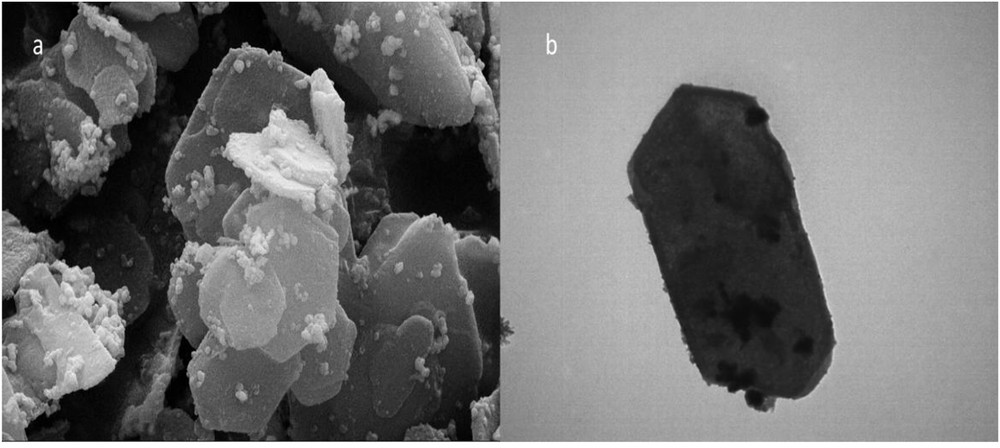
FESEM image of (a) LDH/Tris/Pd and (b) TEM image of LDH/Tris/Pd.
The LDH/Tris/Pd was analyzed by EDX and the outcome was acceptable with expected elements such as Ca, Al, Si, C, O, N, and Pd. EDX analysis of the catalyst is shown in Fig. 6.
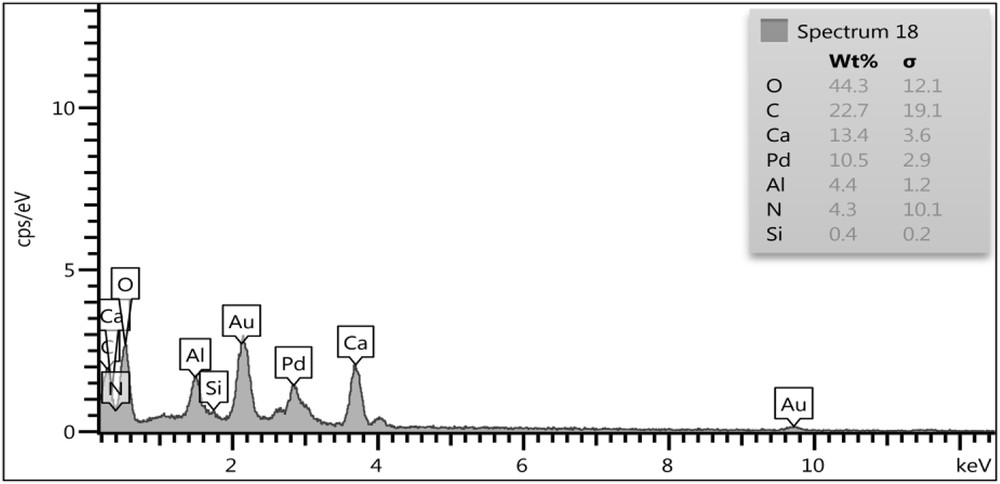
EDX spectrum of LDH/Tris/Pd.
The surface turnover of the LDH/Tris/Pd was investigated by XPS analysis. The elemental peaks are dominated by O, Ca, Al, and C of LDH/Tris/Pd (Fig. 7a). The presence of C, N, and Si in the LDH/Tris/Pd sample was confirmed by the characteristic peaks for C 1s at binding energy (BE) of about 284.8 eV, N at BE of about 398 eV, and Si 2p at BE of about 102.5 eV (Fig. 7b–d). In Pd 3d XPS spectra of LDH/Tris/Pd, more intense peaks (335 and 340 eV) were assigned to metallic palladium, Pd(0) (Fig. 7e). In addition, the characteristic peaks of C 1s included three peaks at 284.5 eV (CC bonding), 286.3 eV (CN bonding), and 289.1 eV (CN+ bonding) indicating that the Tris was successfully introduced onto the LDH surface by covalent linkages (Fig. 7b).
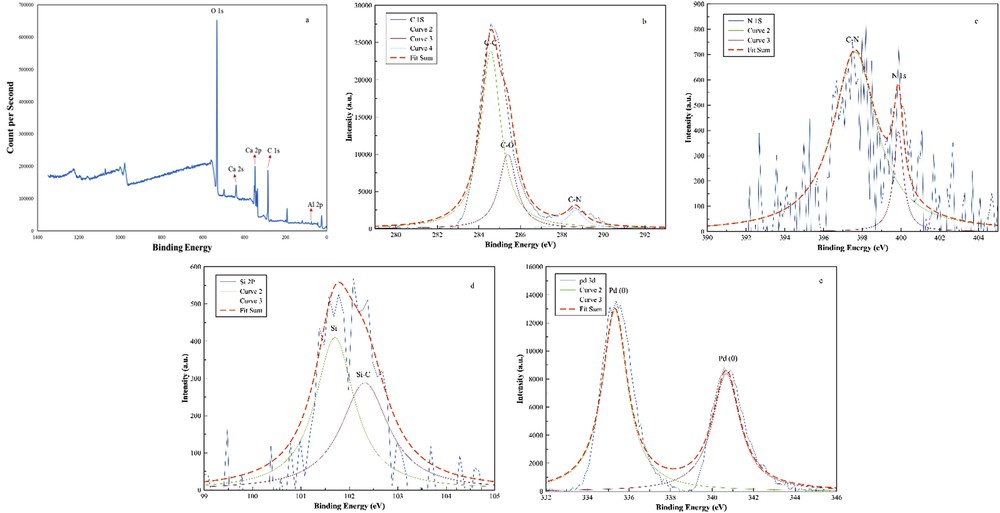
XPS spectra of (a) LDH/Tris/Pd, and high-resolution XP spectrum of (b) C 1s, (c) N 1s, (d) Si 2p, and (e) Pd 3d.
The concentration of palladium in LDH/Tris/Pd, which was 13.52 wt %, was determined by ICP-OES.
3.2 Evaluation of the catalytic activity of LDH/Tris/Pd through the Suzuki–Miyaura coupling reaction
To check the catalytic activity of LDH/Tris/Pd, we used the as-prepared supported catalyst for the Suzuki–Miyaura coupling reaction. In the first step, we determined the best reaction conditions, studying the effect of media upon the reaction. As a model reaction, the coupling reaction of iodobenzene with phenylboronic acid in H2O/EtOH (1:1) in the presence of LDH/Tris/Pd was chosen. A variety of parameters such as solvent, base, temperature, and catalyst loading impressed the coupling rate.
First, we have studied the model reaction using K2CO3 in different solvents and observed that H2O/EtOH (1:1) was the best solvent. The use of other organic solvents (Table 1, entries 1–5) was not useful to the process. In the following, we tried to find the effect of different organic and inorganic bases on this reaction (Table 1, entries 5–11). Results indicated that K2CO3 was the most efficient and other bases were less effective. In addition, the effect of temperature on this model reaction was studied and it was found that room temperature was the best condition (Table 1, entries 5 and 12–14). To investigate the effect of the catalyst amount, the catalyst studies were carried out in the presence of different amounts of the catalyst and results indicated that 0.05 mol % was the best choice (Table 1, entries 5 and 15–17). Thus, the optimum conditions selected are iodobenzene (1 mmol), phenylboronic acid (1.1 mmol), LDH/Tris/Pd (0.05 mol %), H2O/EtOH (5 mL, 1:1), K2CO3 (2 mmol), and rt.
Optimization studies of the reaction of 4-iodotoluene with phenylboronic acid in the presence of LDH/Tris/Pd.a
| Entry | Solvent | Pd (mol %) | Base | Temperature | Time (min) | Yieldb (%) |
| 1 | Toluene | 0.05 | K2CO3 | rt | 180 | 50 |
| 2 | CH3CN | 0.05 | K2CO3 | rt | 180 | 60 |
| 3 | EtOH | 0.05 | K2CO3 | rt | 150 | 65 |
| 4 | H2O | 0.05 | K2CO3 | rt | 180 | 50 |
| 5 | H2O/EtOH | 0.05 | K2CO3 | rt | 40 | 98 |
| 6 | H2O/EtOH | 0.05 | DABCO | rt | 120 | 65 |
| 7 | H2O/EtOH | 0.05 | HMTA | rt | 180 | 50 |
| 8 | H2O/EtOH | 0.05 | Et3N | rt | 120 | 70 |
| 9 | H2O/EtOH | 0.05 | Na2SO4 | rt | 240 | 40 |
| 10 | H2O/EtOH | 0.05 | Na3PO4 | r.t | 210 | 40 |
| 11 | H2O/EtOH | 0.05 | Na2CO3 | rt | 180 | 45 |
| 12 | H2O/EtOH | 0.05 | K2CO3 | 40 | 40 | 85 |
| 13 | H2O/EtOH | 0.05 | K2CO3 | 60 | 40 | 85 |
| 14 | H2O/EtOH | 0.05 | K2CO3 | 80 | 40 | 80 |
| 15 | H2O/EtOH | 0.04 | K2CO3 | rt | 60 | 90 |
| 16 | H2O/EtOH | 0.03 | K2CO3 | rt | 90 | 87 |
| 17 | H2O/EtOH | 0.06 | K2CO3 | rt | 40 | 98 |
a Reactions were carried out using 1 mmol of iodobenzene, 1.1 mmol of phenylboronic acid, and 2 mmol of base in the presence of LDH/Tris/Pd (0.05 mol % of Pd).
b Yields are given for isolated products.
Using the optimized reaction conditions (Table 1, entry 5), a variety of aryl iodides, bromides, and chlorides with various substituent groups were examined in the Suzuki–Miyaura coupling reaction (Table 2). According to Table 2, aryl halides containing both electron donating and electron withdrawing groups reacted with phenylboronic acid and afforded the corresponding biaryl products in high yields. It was found when substituted located at para- or meta-position, the yield was better than ortho-position due to steric effects.
Suzuki–Miyaura cross-coupling reaction of aryl halides with phenylboronic acid.a
| Entry | Aryl halide | Product | Time (min)/yieldb |
| 1 | 20/95 | ||
| 2 | 40/98 | ||
| 3 | 35/98 | ||
| 4 | 10/92 | ||
| 5 | 60/75 | ||
| 6 | 30/90 | ||
| 7 | 40/99 | ||
| 8 | 75/87 | ||
| 9 | 120/66 | ||
| 10 | 40/90 | ||
| 11 | 25/70 | ||
| 12 | 20/77 | ||
| 13 | 150/60 | ||
| 14 | 120/88 |
a Reaction conditions: ArX (1 mmol), phenylboronic acid (1.1 mmol), K2CO3 (2 mmol), H2O/EtOH (5 mL, 1:1), and catalyst (0.05 mol % Pd).
b Isolated yields.
In view of economical purposes and green chemistry, recyclability and reusability of the catalyst were investigated in the reaction of phenylboronic acid with 1-iodo-3-nitrobenzene. At the end of the reaction, catalyst was separated by centrifugation, washed three times with H2O and hot EtOH, dried in an oven, and used for another batch of the reaction. The results indicated that this catalyst can be successfully reused six times without any significant loss in activity (Fig. 8).
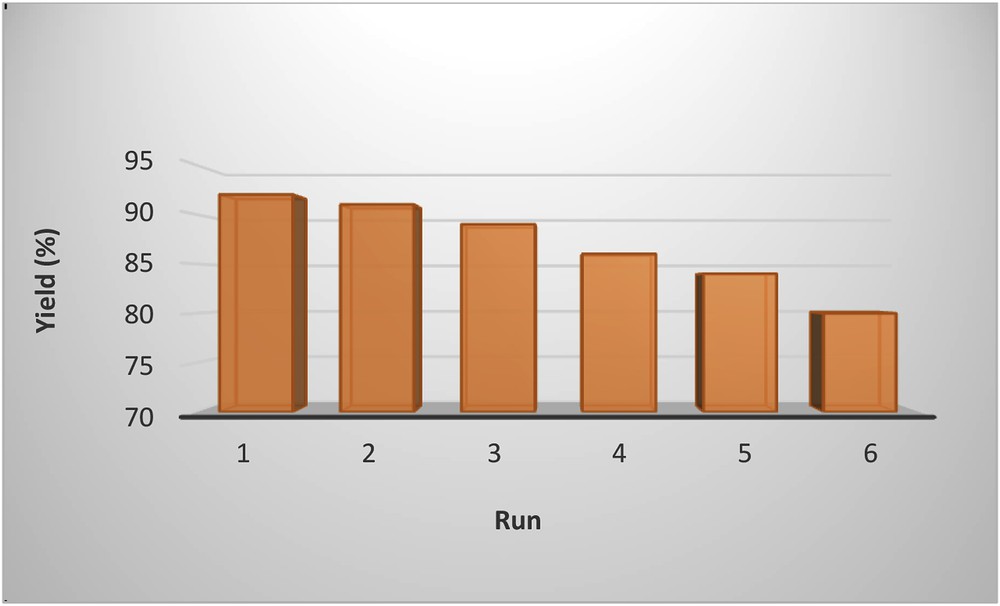
Recyclability test of LDH/Tris/Pd.
Another important parameter of a catalyst, which was studied, is heterogeneity of the catalyst. For this purpose, the phenomenon of leaching was studied by ICP-OES analysis of the resulting reaction solution mixture. It was shown that palladium content of the used catalyst was the same as that of the fresh one (13.48%). In addition, the TEM image of the catalyst after recycling did not show a considerable change in the morphology, which clearly indicated that LDH/Tris/Pd is recyclable and stable under the reaction conditions (Fig. 9).

TEM image of LDH/Tris/Pd after six uses.
The filtration test for the Suzuki–Miyaura reaction between bromobenzene and phenylboronic acid using LDH/Tris/Pd as a catalyst was performed to check the leaching of Pd during the reaction. A catalytic run was started as for a standard reaction, and after reaction for 30 min (the reaction was completed in 60 min), corresponding to 50% conversion, the reaction mixture was stopped and centrifuged to afford a clear filtrate. Then the mixture without the solid catalyst was treated as a standard catalytic run for another 30 min, and the conversion did not proceed significantly.
4 Conclusions
LDH/Tris/Pd was successfully synthesized and used in the Suzuki–Miyaura coupling reaction. The structure of the catalyst was exactly characterized by various techniques. The results indicated that the stability of the catalyst was much improved and LDH/Tris/Pd was an effective and recyclable catalyst for coupling reactions in aqueous media. Low cost, good stability, excellent workup, and reusability are some of the most notable advantages of catalysts.
Acknowledgments
The authors wish to thank the Bu-Ali Sina University and the Center of Excellence in Development of Environmentally Friendly Methods for Chemical Synthesis (CEDEFMCS) for financial support to this research.


