1 Introduction
For the past few decades, we have focused our attention on the (bio)chemistry and reactivity of 2-amino-2-oxazolines developed either as synthons or potential bioactive heterocyclic derivatives. We have previously described the design and synthesis of biologically active derivatives based on the reactivity of the amidine group of 2-amino-2-oxazolines with various bis-electrophiles [1–4]. As with other amidines, the 2-amino-2-oxazoline moiety possesses two competing sites for potential ring annulation, leading to regioselectivity considerations. An empirical observation has emerged from our previous investigations, which indicates that, in such reactions, the endocyclic nitrogen is considered generally as the most nucleophilic and attacks the most electrophilic carbon of the bis-electrophile. A ring closure between the exocyclic nitrogen and the second electrophilic function generally concludes the synthesis [5,6].
As a continuation of our research for pharmaceutical tools, we herein report the structural characterization of the 2-(4,5-dihydro-5-phenoxymethyl-1,3-oxazol-2-ylamino)-7-(2-hydroxy-3-phenoxypropyl)hexahydro-2H,6H-pyrimido[6,1-b][1,3]oxazin-6-one (1), a serendipity structure of an adduct formed by a reaction of 5-(phenoxymethyl)-2-amino-2-oxazoline (2) with acrolein (Scheme 1). Pyrimido[6,1-b][1,3]oxazine derivatives are heterocyclic building blocks showing potential biological utilities, such as anti-human immunodeficiency virus (HIV), antibacterial, antifungal, antiamoebic, and anthelmintic properties [7–9].
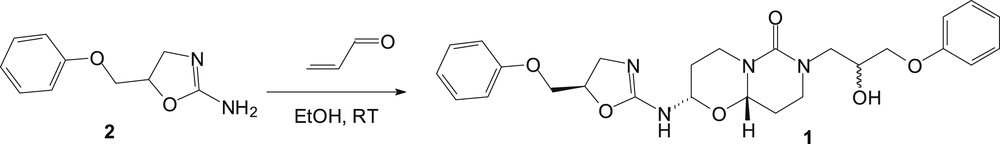
Synthesis of 2-(4,5-dihydro-5-phenoxymethyl-1,3-oxazol-2-ylamino)-7-(2-hydroxy-3-phenoxypropyl)hexahydro-2H,6H-pyrimido[6,1-b][1,3]oxazin-6-one (1).
2 Results and discussion
As a part of our research program on crystal structure analysis, the three-dimensional structure of this pyrimido[6,1-b][1,3]oxazin-6-one 1 has been achieved. Hence, after crystallization in a mixture of chloroform and methanol (4/1 v/v) at room temperature, the 2-(4,5-dihydro-5-phenoxymethyl-1,3-oxazol-2-ylamino)-7-(2-hydroxy-3-phenoxypropyl)hexahydro-2H,6H-pyrimido[6,1-b][1,3]oxazin-6-one (1) was surprisingly isolated as colorless needles. Title derivative was then submitted to spectroscopic analysis to confirm its molecular structure. The structure of 1 is depicted in Fig. 1. The proposed mechanism to access pyrimido[6,1-b][1,3]oxazin-6-one 1 is described in Scheme 2. Thus, the reaction proceeds via adduct formation first at the endocyclic nitrogen of 2-amino-2-oxazoline 1 [10,11] via a Michael reaction leading to intermediate I, and subsequently a ring closure takes place at the carbonyl carbon by the exocyclic nitrogen atom of I to form the ring-fused monoadduct II through a Dimroth-like rearrangement (Scheme 2). This first Michael addition is then followed by a second Michael reaction of acrolein at the exocyclic nitrogen atom of the oxazolopyrimidine II to give the intermediate III, which is then probably hydrolyzed into the pyrimidine IV in equilibrium with its tautomeric form V. We have previously described such hydrolysis in the opening of the oxazoline ring [4]. Condensation of the amino function of a second molecule of 2-amino-2-oxazoline 2 with the carbonyl of V led to the formation of the imine intermediate VI. Compound 1 is finally obtained through a second cyclization by addition of the hydroxyl of the initially formed ring onto the imine group of VI to complete the reaction sequence. Similar reactivity of a hydroxyl function has been previously described by a reaction of acrolein with adenosine/cytidine and adenine/cytosine, leading to the formation of diastereoisomers of the adducts, made up from two fused rings [12,13]. According to the X-ray data, compound 1 appears only as a mixture of 2S*-(4,5-dihydro-5R*-phenoxymethyl-1,3-oxazol-2-ylamino)-7-(2S*-hydroxy-3-phenoxypropyl)hexahydro-2H,6H-(9aS*)-pyrimido[6,1-b][1,3]oxazin-6-one and 2S*-(4,5-dihydro-5R*-phenoxymethyl-1,3-oxazol-2-ylamino)-7-(2R*-hydroxy-3-phenoxypropyl)hexahydro-2H,6H-(9aS*)-pyrimido[6,1-b][1,3]oxazin-6-one diastereoisomers and their respective enantiomers. Both diastereoisomers correspond, respectively, to the SRSS and SRRS absolute configurations in the order of the nomenclature or the corresponding RSRR or RSSR enantiomers. The only difference between the two stereoisomers is found for the hydroxyl function at position 2 of the 7-propyl side chain. From these crystallographic data, the diastereomers were formed in unequal amounts, that is, 75% and 25%, respectively, but they could not be separated (see Fig. 2).
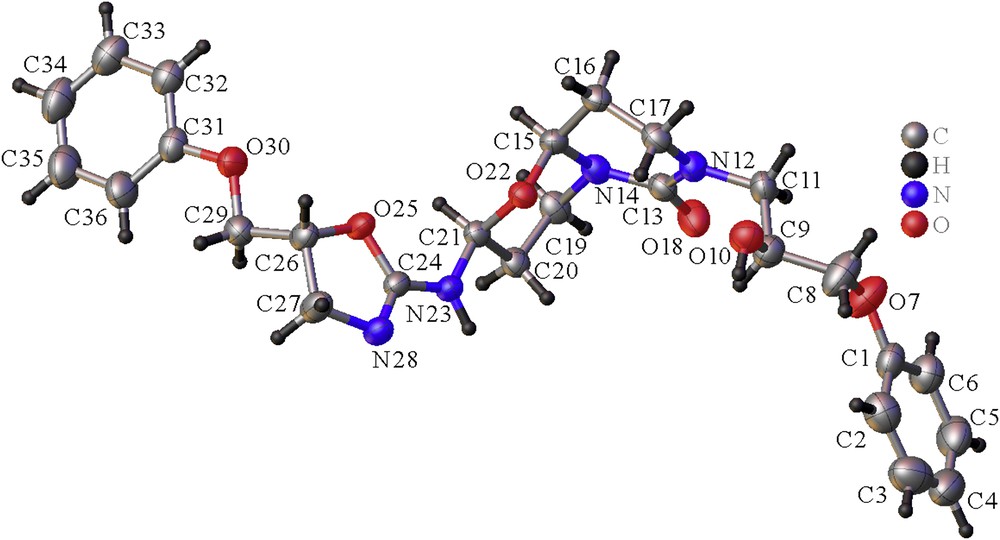
View of the asymmetric unit of 1, with the labeling scheme. The thermal ellipsoids are represented at 50% probability. The disordered OH group on C9 is not represented for clarity, and only the major diastereoisomer is represented here.
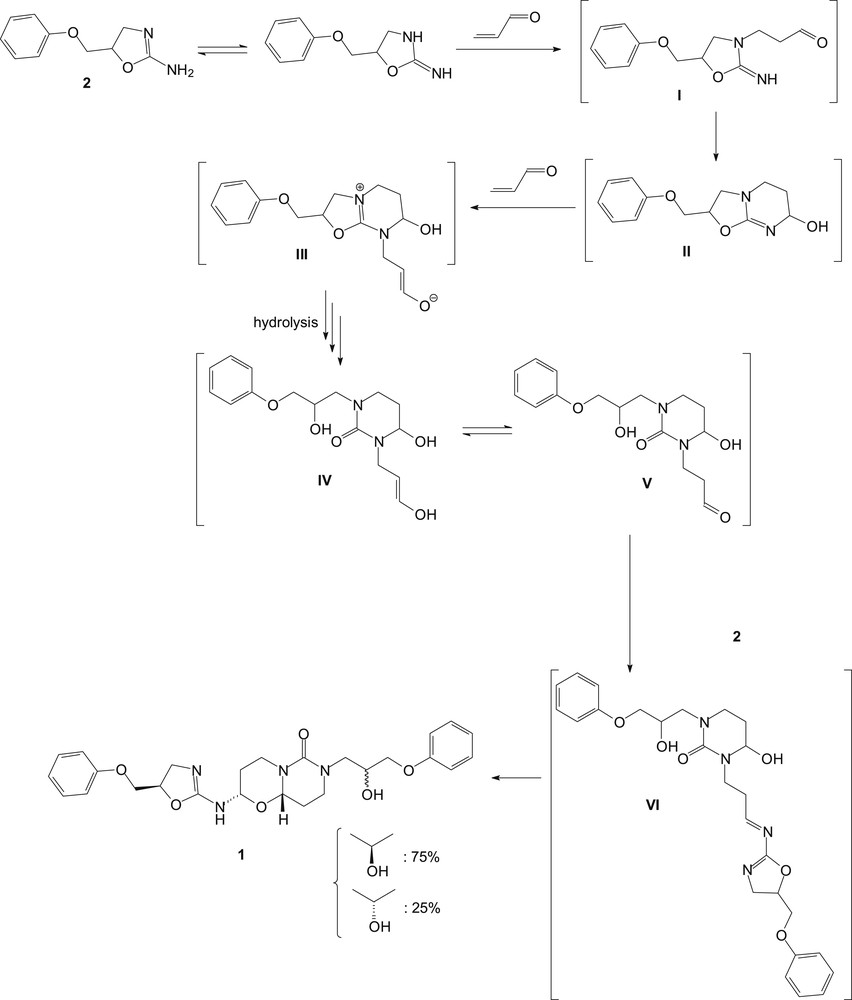
Hypothetical mechanism for the formation of 2-(4,5-dihydro-5-phenoxymethyl-1,3-oxazol-2-ylamino)-7-(2-hydroxy-3-phenoxypropyl)hexahydro-2H,6H-pyrimido[6,1-b][1,3]oxazin-6-one (1).
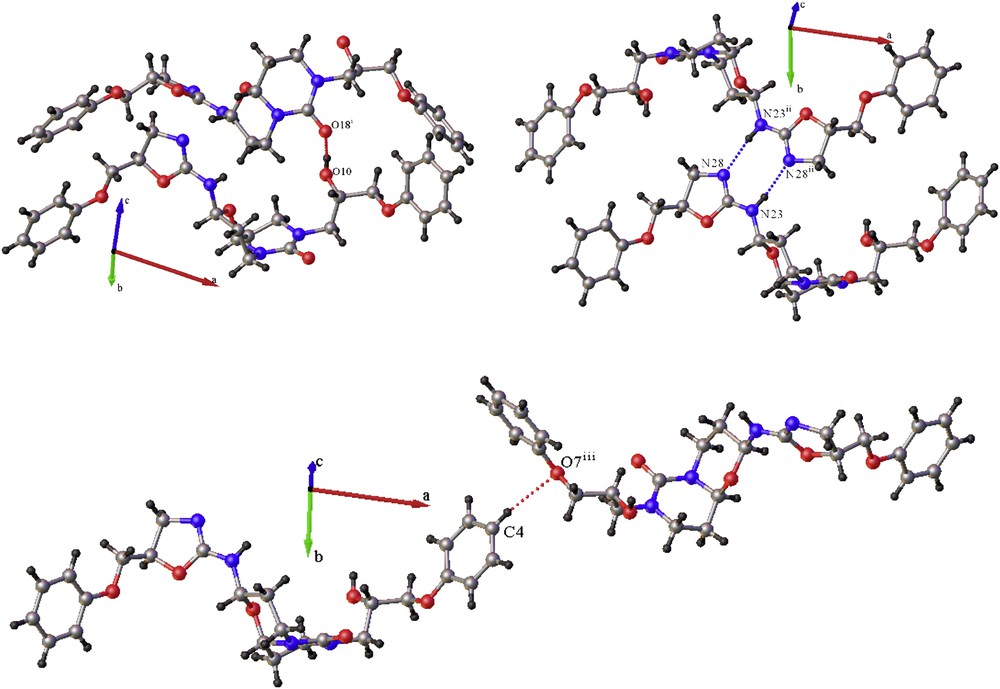
View of the principal intermolecular interactions in the crystal of pyrimido[6,1-b][1,3]oxazin-6-one 1 that ensure the crystal cohesion in (a) the c direction, (b) the b direction, and (c) the a direction. Symmetry codes: (i) x, ½ − y, ½ + z; (ii) −x, −y, 1 − z; (iii) 1 − x, −½ + y; 3/2 − z.
Title compound 1 crystallized at 183 K in the monoclinic system, space group P21/c with the unit cell parameters: a = 21.3337 (10) Å, b = 11.3712 (7) Å, c = 10.4936 (7) Å, β = 103.041 (3), and V = 2480.0 (3) Å3. Each asymmetric unit is constituted of one molecule corresponding to the formula C26H32N4O6 with four molecules in the unit cell (Z = 4) leading to a calculated density (Dc) of 1.330 g/cm3. The molecular structure of pyrimido[6,1-b]hydroxy-3-phenoxypropyl[1,3]oxazin-6-one 1 is depicted in Fig. 1.
The double bond C13O18 of the urea group is found at 1.242(2) Å, whereas the two CN bonds (C13N14 = 1.379 (2) Å and C13N12 = 1.356 (2) Å) of this same function were in agreement with the C(sp2)N distance [14]. The O bonds (C15O22 and C21O22) of the oxazine ring were noticed at 1.434 (2) and 1.456 (2) Å, respectively, whereas the other O bonds (C8O7, C29O30, C9O10, C9O10′, and C26O25) range from 1.425 (2) to 1.516 (6) Å. The intracyclic double bond C24N28 of the oxazoline is measured at 1.282 (2) Å, slightly smaller than those observed for C(sp2)N double bonds [14]. In addition, the C24N23 bond of this amidine function is found at 1.349 (2) Å due to the delocalization of the double bond in the amidine moiety of 2-amino-2-oxazoline. The CC bond lengths in the two phenyl rings lie in the range 1.361 (4)–1.391 (4) Å.
Compound 1 adopts a half-chair conformation for the pyrimidine ring while oxazine ring is a chair conformation. The torsion angles of this oxazine moiety O22C21C20C19 and O22C15N14C19 are −51.55 (18)° and 62.96 (18)°, respectively. In addition, the C1O7C8 and C31O30C29 of the phenoxy side chains are found at 119.7 (2)° and 117.22 (15)° (see Table 1).
Selected bond lengths (Å) and angles (°).
| Bond lengths | |||
| O(7)C(8) | 1.429 (3) | C(15)O(22) | 1.434 (2) |
| C(9)O(10) | 1.434 (3) | C(21)O(22) | 1.456 (2) |
| C(9)O(10′) | 1.516 (6) | N(23)C(24) | 1.349 (2) |
| N(12)C(13) | 1.356 (2) | C(24)N(28) | 1.282 (2) |
| C(13)O(18) | 1.242 (2) | O(25)C(26) | 1.461 (2) |
| C(13)N(14) | 1.379 (2) | C(29)O(30) | 1.425 (2) |
| Bond angles | |||
| O(22)C(21)C(20)C(19) | −51.55 (18) | C(1)O(7)C(8) | 119.7 (2) |
| O(22)C(15)N(14)C(19) | 62.96 (18) | C(31)O(30)C(29) | 117.22 (15) |
The crystal-structure cohesion is essentially ensured by intermolecular hydrogen bond between the OH group on C9 and the oxygen O18 from the carbonyl function propagating in the direction c, and between one amino and one imine group (N23 and N28, respectively) leading to a cyclic double hydrogen bond propagating in the b direction (Table 2). It is worth noting that both diastereoisomers, which correspond to two different conformations of the OH group on C9, lead nevertheless to a very similar H-bond between the hydroxyl and the carbonyl functions. This can explain why both diastereoisomers can accommodate in the same crystal lattice. The slightly weaker H-bond obtained for the SRRS diastereoisomer may explain why its proportion is smaller in the crystal leading to the 75/25 M ratio for the SRSS and SRRS diastereoisomers, respectively (Table 2). A weaker hydrogen-like interaction between an aromatic CH of the phenyl group (C4H4) with the oxygen O7 of another symmetrical equivalent O-phenyl group ensures the crystal cohesion in the c direction.
Hydrogen bonds for 1.
| DH⋯A | d(DH) (Å) | d(HA) (Å) | d(DA) (Å) | DHA (°) |
| O10H10⋯O18i | 0.84 | 1.937 | 2.714 (3) | 153.3 |
| O10′H10′⋯O18i | 0.83 | 2.069 | 2.748 (4) | 139.2 |
| N23H23⋯N28ii | 0.88 | 2.064 | 2.931 (3) | 168.1 |
| C4H4⋯O7iii | 0.95 | 2.583 | 3.514 (3) | 166.5 |
3 Experimental section
3.1 Preparation
The title compound 1 was prepared in moderate yield (55%) by a direct reaction of 1 equiv of 5-(phenoxymethyl)-2-amino-2-oxazoline 2 with 1 equiv of acrolein in ethanol at room temperature for 2 days. The precipitate formed during the reaction was then filtered and suitable crystals for X-ray diffraction analysis were obtained from a chloroform–methanol (4:1 v/v) solution by slow evaporation of the solvent at +20 °C.
3.2 X-ray crystallography
A single crystal of the title compound with dimensions 0.20 × 0.20 × 0.15 mm3 was chosen for X-ray diffraction study. The data were collected using a Rigaku R-axis rapid diffractometer equipped with micro-focus rotating anode Cu Kα radiation (λ = 1.5418 Å) mode at 183 (2) K. In the range of 2.13° < θ <72.31°, a total of 32,457 reflections were collected, of which 4802 were independent (Rint = 0.0501) and 4497 were observed with I > 2σ(I). The crystal structure was solved by direct methods and successive Fourier difference syntheses with the SHELXS program [15]. Refinement of the crystal structure was performed on F2 by weighted anisotropic full-matrix least-squares methods using the SHELXL program [15]. An absorption correction was performed by semiempirical methods using the SADABS program [15]. All parts of the program were used within the OLEX2 package [16]. All non-H atoms were refined anisotropically, and the positions of the H atoms were deduced from the coordinates of the non-H atoms to which they are linked, confirmed by Fourier synthesis and treated according to the riding model during refinement. H atoms were included for structure factor calculations, but not refined. The final full-matrix least-squares refinement gave R = 0.0501 and wR = 0.1584 for 4497 reflections with I > 2σ(I). The maximum and minimum difference peaks and holes were 0.239 and −0.302 eÅ−3, respectively. S = 1.188 and (Δ/σ)max = 0.000. The crystal data and refinement details are listed in Table 3.
Crystallographic data and structure refinement details.
| CCDC deposit number | 891807 |
| Chemical formula | C26H32N4O6 |
| Formula weight | 496.56 |
| Temperature (K) | 183 (2) |
| Wavelength (Å) | 1.54180 |
| Crystal size (mm) | 0.20 × 0.20 × 0.15 |
| Crystal system | Monoclinic |
| Space group | P21/c |
| a (Å) | 21.3337 (10) |
| b (Å) | 11.3712 (7) |
| c (Å) | 10.4936 (7) |
| α (°) | 90.00 |
| β (°) | 103.041 (3) |
| γ (°) | 90.00 |
| V (Å3) | 2480.0 (3) |
| Z | 4 |
| Dc (g/cm3) | 1.330 |
| F (000) | 1056 |
| Absorption coefficient (mm−1) | 0.786 |
| 2θ range (°) | 4.26–144.62 |
| Index ranges | −26 ≤ h ≤ 26; −13 ≤ k ≤ 13; −12 ≤ l ≤ 12 |
| Reflection collected | 32,457 |
| Independent reflections | 4802 [Rint = 0.0428] |
| Data/restraints/parameters | 4802/2/335 |
| Goodness-of-fit on F2 | 1.189 |
| R, wR indices [I > 2σ(I)] | 0.0501, 0.1584 |
| R, wR indices (all data) | 0.0569, 0.1878 |
| Largest differential peak and hole (eÅ−3) | 0.24, −0.30 |
Appendix A Supplementary data
The following is the supplementary data to this article:
Crystallographic data for the reported structure in this article have been deposited in the Cambridge Crystallographic Data Centre as supplementary publication no. CCDC-891807. Copies of available material can be obtained, free of charge, on application to the Director, CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (fax: +44-1223-336033 or e-mail: deposit@ccdc.cam.ac.uk).


