1 Introduction
Peloids or thermal muds are hot cataplasm formed essentially by clay minerals (bentonite, kaolinite, illite associated with carbonate, quartz, and other minerals) with seawater, thermal water, or medicinal water. Pelotherapy is the local or generalized application of thermal muds for recovering rheumatism, arthritis, and bone–muscle traumatic damages. It is considered as one of the popular procedures, used in wellness and rehabilitation centers. The history of balneotherapy dates way back to the ancient times. The use of thermal and medicinal waters and peloids for health and cosmetic face care is a Tunisian traditional activity, which is practiced in traditional “Hammam” (a collective bath) and in Tunisian spa resorts. The medicinal water has multiple virtues and it is recognized that Tunisia has exceptional thermal springs.
During the past few years a number of scientific studies on the thermophysical properties of peloids have been carried out all over the word (Greece, Italy, France, Spain, etc.) [1–3], However, in Tunisia, although the number of spas, clay deposit, and thermal water are widely spread all over the country, the number of studies and scientific articles dealing with pelotherapy are limited in number [4,5]. Jamoussi and co-workers have been interested in the suitability of some Tunisian clay to be used as semisolid health care paste and cosmetic products. Very recently, heavy metals' mobility during maturation of silty-clay peloid has been carried out [6] Therefore, more characterizations and investigations are needed.
In this article, we present an experimental investigation of the mineralogical and thermal characterization of peloids prepared from two Tunisian clays and medicinal waters.
2 Materials and methods
2.1 Materials
Two clay samples were used in this work. They were collected from Tunisian formations: Sample CZ was collected from Zaghouan, one of the richest areas in the country for this type of clays, and CS clay was from Siliana. The samples were wet sieved (<63 μm) and dried at 80 °C for 24 h. The thermal waters were collected from two sources designated as A (Ain Atrous in Korbos region) and B (Ain El Eryan in Megrine region).
2.2 Preparation of peloids
The peloid was prepared by dispersing 45 g of CS or CZ dried powder in 75 g of water A or B and mixing them at 10,000 rpm for 120 min. The mixtures were left for 7 days for maturation. Four peloid samples were obtained: CZ + A, CZ + B, CS + A, and CS + B.
2.3 Characterization
2.3.1 Mineralogy and geochemistry
Mineralogical analyses of the samples were carried out by X-ray diffraction using PAN analytical powder diffractometer with Cu Kα radiation at 40 kV and 40 mA. All samples were scanned in the 2θ range of 3°–70°.
The chemical composition for major, minor, and trace elements of the <63-μm sieved samples was determined using a ParkinElmer optima inductived coupled plasma apparatus after digestion with a mixture of HCl + H2SO4 + HNO3.
2.3.2 Granulometry
The granulometric distribution of samples was measured by means of a Laser granulometer Microtrac S3500.
2.3.3 Cation-exchange capacity
Cation-exchange capacity of clays was determined using Tucker's method [7].
2.3.4 Specific heat
The specific heat has been determined for each clay in dry powder form using a differential scanning calorimetry (Shimadzu) using a mass 10 mg of clay minerals with a heating rate of 10 °C/min.
2.3.5 Determination of cooling rate curves
The paste was introduced in a closed 50 mL cylindrical Teflon container and was heated at temperature constancy (70 °C), and then it was introduced in a thermostatic bath at 35 °C. The temperature of the paste was measured every 60 s until the temperature of the clay paste and the thermostatic bath attain equilibrium [8,9].
2.3.6 Plasticity index
The Atterberg limits (WL, liquid limit; WP, plastic limit; PI, plasticity index) were determined following the norm NP 143-1969 using a standard Casagrande cup.
3 Results and discussion
3.1 Physicochemical characteristic of clays
3.1.1 Mineralogy and geochemistry
The mineralogical composition of the two samples is shown in Table 1 and Fig. 1. CZ sample is rich in calcite and presents a very limited quantity of quartz. On the other hand, CS sample showed high amounts of calcite (33%), quartz, and a small amount of dolomite. Concerning clay fraction, CZ presents a higher amount of clay minerals, which is composed of 41% of smectite and 39% kaolinite. For CS clay, the proportion of clay fraction is about 53%, which is composed of 33% and 20% kaolinite. The two clays present a high quantity of kaolinite (between 20% and 39%).
Mineralogical composition of the studied clays (in %).
| Samples | Smectite | Kaolinite | Calcite | Quartz | Dolomite |
| CS | 33 | 20 | 33 | 11 | 4 |
| CZ | 41 | 39 | 14 | 6 | – |
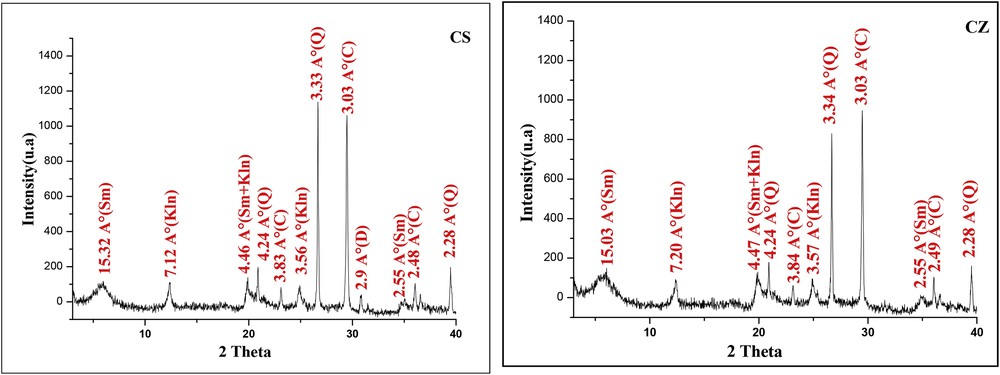
X-ray diffraction patterns of the samples. I = illite, Sm = smectite, Kln = kaolinite, Q = quartz, C = calcite, D = dolomite.
3.1.2 Major and trace element composition
In the field of pelotherapy, the application of clays is directly related to their chemical and mineralogical composition.
The chemical analysis values for the two samples are represented in Table 2 and show that for both clays SiO2, Al2O3, Fe2O3, MgO, and CaO were the elements constituting the major composition. The Si concentration was found in the two clays with the same quantity. According to Carretero and Pozo [10], high amounts of Si mean that the clay could be used in skin tissue reconstruction. Al is the second element found in highest amounts in the samples. On the other hand, the identified trace element concentrations presented in Table 2 show no significant differences between studied samples. Clay selection for peloid applications must be safe. For that reason, Cd, Pb, and As should be essentially absent, as they are known to be toxic to humans and environmentally hazardous. Mo, Ni, V, Cr, and Cu have been reported to have less toxicity effects than Cd, Pb, and As, but should be limited in pharmaceutical formulations. Tl and Te have also been classified as unwanted for cosmetic products, including clays and for pelotherapy. In addition, Ba, Se, Zn, and Sb were considered as impurities in cosmetic products. Both samples CS and CZ exhibit acceptable trace element concentrations for official regulations because the measured concentrations of trace metals were found to be in the range of other healing muds.
Chemical composition and trace element content in the studied clays with safety concerns according to European and USA pharmacopoeias.
| Samples | CZ | CS | Acceptable limit (ppm) |
| Major element (%) | |||
| SiO2 | 40.65 | 44.02 | |
| Al2O3 | 1.93 | 10.57 | |
| Fe2O3 | 6.91 | 6.46 | |
| CaO | 12.14 | 1.29 | |
| MgO | 2.05 | 1.83 | |
| Na2O | 0.26 | 0.18 | |
| K2O | 1.45 | 1.16 | |
| SO3 | 1.46 | 1.64 | |
| LIO (loss in ignition) | 1.73 | 2.21 | |
| Trace element (ppm) | |||
| As | 1.1 | 3 | <8 |
| Cd | 9.2 | 8 | – |
| Pb | 0.3 | 1 | <50 |
| Cr | 19 | 20 | 1100 |
| Cu | 5.8 | 4 | 130 |
| Mn | 161.5 | 120.1 | – |
| Ni | 9.1 | 5.6 | 60 |
| V | 71.6 | 6.5 | – |
| Ba | 76 | 74 | 1300 |
| Sr | 224.4 | 183.9 | – |
| Zn | 109.3 | 94 | 200 |
3.1.3 Grain size analysis
The two samples were characterized by very low percentage of sand fraction (better than 1.6%, Table 3 and Fig. 2), thus the composition is being extremely preferable for use as medicinal paste [11,12]. On the other hand, the particle size of CZ presents a bimodal distribution with two maxima at 11 and 5 μm and the particle size distribution of CS is very narrow, with a maximum around 10 μm. With regard to the clay fraction, CZ showed the highest percentage of particles inferior to 2 μm and CS having the lowest, and the two clays showed a wide range of particle sizes.
Geotechnical parameters of clays.
| Parameter (%) | CZ | CS |
| Cation-exchange capacity (mmol/100 g) | 40 | 37 |
| Liquid limit | 59 | 65 |
| Plastic limit | 27 | 28 |
| Plastic index | 32 | 37 |
| Granulometry | ||
| Sand (2 mm–63 μm) (%) | 1.3 | 6.1 |
| Silt (63–2 μm) (%) | 88.7 | 84 |
| Clay (<2 μm) (%) | 10 | 9.9 |
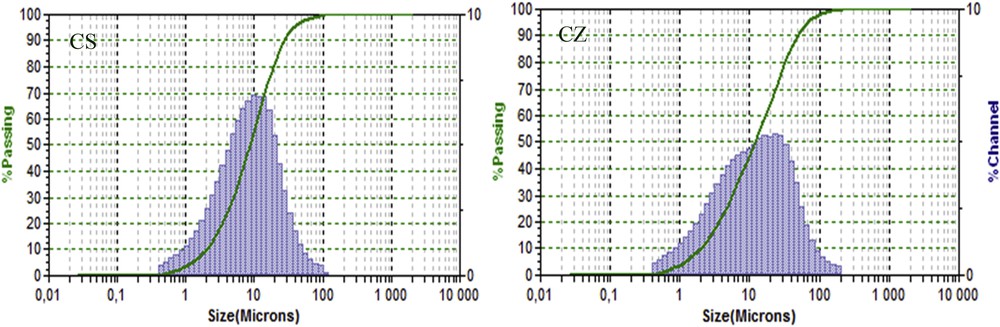
Distribution of particle size and cumulative weight percentage (green curves) of the studied clays.
3.1.4 Cation-exchange capacity
The two studied clays showed a greatest cation-exchange capacity (40 mequiv/100 g for CZ and 37 mequiv/100 g for CS) (Table 3). These values agree with the mineralogy composed essentially of smectitic clay.
3.1.5 Plasticity index
The PIs of the samples are between 32 and 37 (Fig. 3 and Table 3). CS sample has a higher value of PI than CZ sample. This is mainly due to the higher value of the liquid limit (Table 3). Both studied samples were grouped in high plasticity clays because liquid limit is ≥50% [13]. The plastic limit WP of the samples was 27% and 28%. Referring to Jenkins classification [14], the samples were considered with high plasticity because their PI is ≥15%.
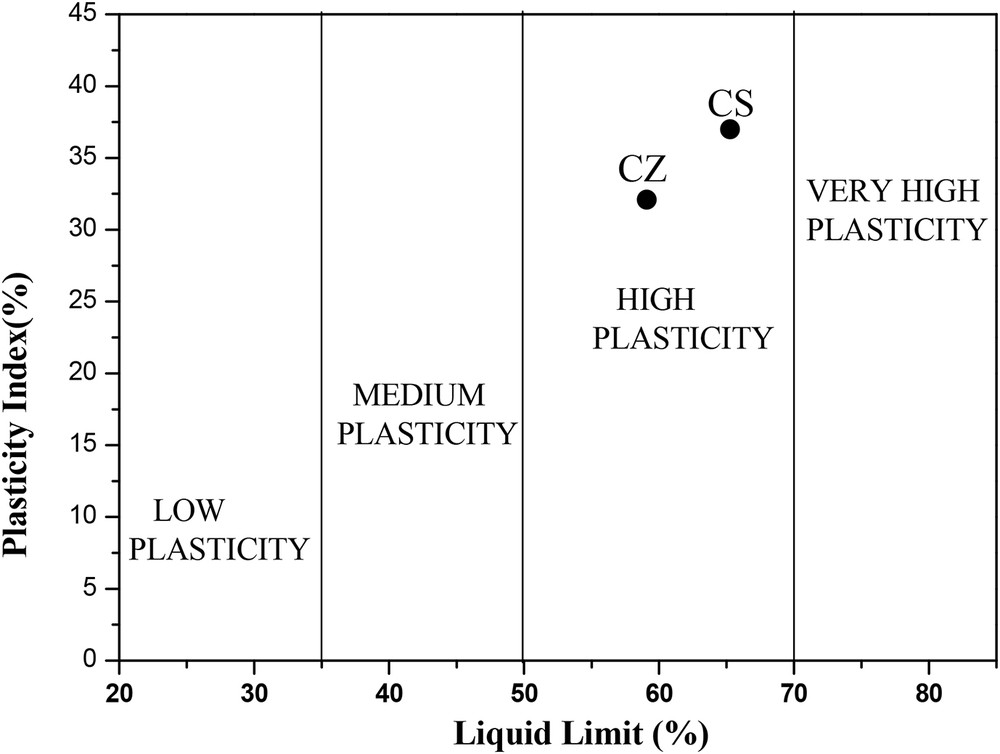
Casagrade's chart [12] for the evaluation of the plasticity of the samples.
3.2 Thermal behaviors of peloids
3.2.1 Presentation and classification of thermal waters
The chemical composition and some physical properties of the two mineral waters are presented in Tables 4 and 5, respectively. On the basis of their fixed residues, water B could be classified as rich mineral content water because solid residue was better than 1500 mg/L and A as medium mineral content water (500–1500 mg/L). The two waters are slightly acid and hard [15].
Chemical composition of the two minerals waters.
| A (Ain Atrous water) | B (Ain El Aryan) | |
| Cl− (ppm) | 4970.00 | 7923.00 |
| SO42− (ppm) | 2110.13 | 1116.00 |
| HCO3− (ppm) | 597.00 | 536.80 |
| Ca2+ (ppm) | 89.70 | 124.02 |
| Mg2+ (ppm) | 240.76 | 162.94 |
| K+ (ppm) | 89.70 | 124.02 |
| Na+ (ppm) | 2953.20 | 3776.60 |
| F− (ppm) | 3.20 | 2.70 |
| CO32− (ppm) | 106 | 133.4 |
Physical properties of the two minerals waters.
| A | B | |
| pH | 6.7 | 6.6 |
| Solid residue (110 °C) (mg·L−1) | 1230 | 1570 |
| Conductivity (μS/cm) | 15.8 | 19.7 |
On the basis of the chemical composition, thermal water takes its name from the element or group of elements from which it is composed. Although determining what kind of ionic composition there is in the water, first we have to take account of the prevalent anion (or anions) and then of the cation. When an ion is >20 mequiv/L, this gives the water its name. According to prevalent ionic composition, mineral waters are classified as bicarbonate waters/salt waters or with sodium chloride/sulfurous waters, arsenical ferruginous waters/sulfated waters Thus, referring to Tickel's classification [16] (Fig. 4), both waters A and B could be classified as sodium chloride-rich sulfated waters; however, water A contains more sulfate and bicarbonate. In addition both waters are not calcic. The presence of chloride and sulfate confers to the anti-inflammatory activity of these waters [15].
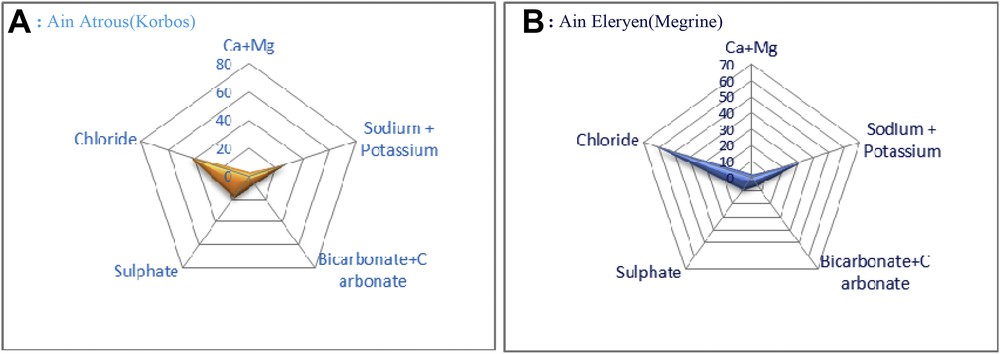
Tickel's diagram of mineral–medicinal waters: (a) Ain Atrous (region of Korbos) and (b) Ain El Eryan (region of Megrine) [16].
3.2.2 Specific heat
The thermal characteristic of clay mineral–forming peloids is related to their specific heat and cooling kinetics. The average of specific heat of the two clays in dry powder form has been determined using differential scanning calorimetry curves of the two samples for temperature ranging from 35 to 45 °C, they were between 868 J/kg·K for CS to 1044 J/kg·K for CZ clay. These values are in accordance with that reported for montmorillonite clay in the literature (Table 6).
A list of some Cp values of some clays cited in the literature.
| Cp (J/kg·K) | Temperature (°C) | Clay | References |
| 1720–4630 | 44–64 | Sepiolites | [17] |
| 440–660 | Ca–montmorillonite | ||
| 1510–1990 | NaCa montmorillonite | ||
| 895–900 | 36–45 | Na-activated magnesium bentonite | [18] |
| 873–892 | Mg-bentonite and sepiolite | ||
| 751–776 | Palygorskite | ||
| 1245–1264 | Sepiolite | ||
| 1044 | 35–45 | 33% Smectite and 20% kaolinite | Our study |
| 868 | 41% Smectite and 39% kaolinite |
The variance of specific heat between the two clays can be explained by the difference in clay fraction between the two samples (80% for CZ as compared to 53% for CS) as shown in the literature, the specific heat capacity increases with increase in clay content [17]. On the other hand, these values are in accordance with that reported in the literature for specific heat capacity of powder clays determined by differential scanning calorimetry measurement [18]. It was established that specific heat of clay in dry powder form decreases in the following order: the Cp of sepiolite is superior to that of montmorillonite and to that of illite and kaolinite [18].
For peloids that represent a mixture of clay and water, the specific heat capacity of the system is given, in a first approximation, by the sum of those of their components according to the general Eq. (1) [19]:
| (1) |
| (2) |
The calculated Cp values of the paste are, respectively, 2110 and 1696.5 J/kg·K for CS and CZ clay and they are in accordance with that of peloids suitable for use in pelotherapy [19].
3.2.3 Cooling kinetics of the peloids
The cooling kinetic is defined as the capacity of paste to slowly release heat to the body. Heat loss from the peloid to the body is defined by the Newton law and is represented by the following Eq. (3):
| (3) |
| (4) |
It can be seen that different samples presented acceptable cooling kinetic. The t37 parameter is defined as time required for the paste to cool from 45 to 37 °C. These parameters for different pastes prepared with the two clays and mineral waters have been calculated from kinetic cooling rate curves and are represented in Fig. 5. It could be seen that peloid prepared in water A, which contains less solid residues and cations, presents the slowest cooling kinetic. This trend could be explained by the fact that specific heat (Cp) of pure water is 4.18 J·K−1·g−1 and decreased with salinity and its content in cations [20].
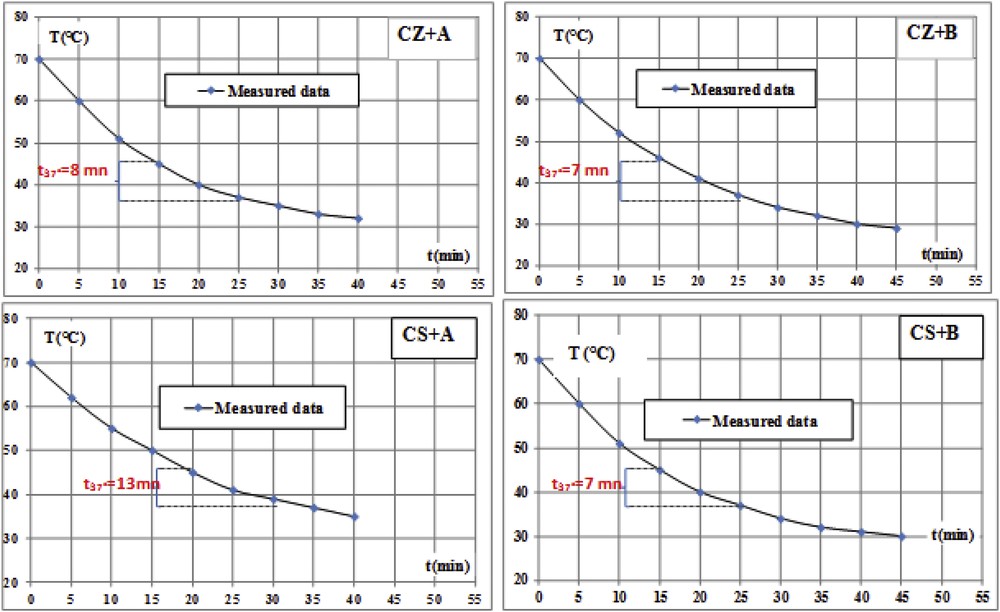
Kinetic cooling curves of CZ and CS clays prepared with waters A and B.
4 Conclusion
The main objective of this study was the characterization of the physicochemical characteristics of two Tunisian clays and thermal waters for potential use as curative pastes. They were characterized in terms of mineralogy, granulometry, plasticity, adsorptive property, heat capacity, and cooling behavior. The two samples are suitable for application as curative pastes for the reasons below.
- 1. The two samples contain smectite and kaolinite as major clay fractions and calcite and calcite + dolomite + quartz as associate minerals; however, CZ contains more clay fraction with a proportion of 80%. These compositions explain the high cation-exchange capacity and plasticity, which are suitable for curative application. On the other hand, the two samples are characterized by their very low percentage of sand fraction (>1.6%). These compositions are preferable for this application.
- 2. The chemical analysis values for the two samples proves that Si is the principal major element and that both samples CS and CZ exhibit acceptable trace element concentrations for official regulations because the measured concentrations of trace metals were found to be in the range of other healing muds.
- 3. Concerning their thermal characteristics, it was shown that pastes prepared with thermal water containing less solid residues and cations present preferable thermal capacity.
Acknowledgments
This work was supported by the Tunisian Ministry of Higher Education and Scientific Research of Tunisia.


