1. Introduction
Currently, low-grade and off-balance sheet deposits are becoming increasingly important in the mining and metallurgical industries. As a rule, they are characterized by refractory and hard-to-enrich ores with low metal content. Nowadays, the copper content of 0.6–0.8% in ores is considered profitable. The best enrichment technologies to date make it possible to extract metal with a 0.2–0.3% ore content. Nevertheless, as the deposit is being developed, ore with a copper content of 0.1–0.2% or less remains undeveloped. Consequently, during the development of deposits, a significant amount of metal always remains in the depths. In addition, significant quantities of copper are found in the dumps of copper mines and tailing dumps. Thus, the development of methods for additional recovery of non-ferrous metals from poor and off-balance ores and dumps is an important and urgent task in connection with the constant reduction of mineral reserves in rich deposits.
In the case of copper deposits, it is possible to concentrate the residual copper content on calcite materials (limestone, marble, etc.) in the form of basic copper carbonates, mainly malachite and azurite. This prevents further washing and metal loss. In this case, to intensify the processes of mineral formation and increase the geochemical mobility of copper ions, it is logical to use complexing substances. Here, organic complexing agents are preferable, since they are relatively harmless from an environmental point of view, and also have lower instability constants, which is important for the subsequent decomposition of copper ions into carbonates [1, 2].
Organic complexing agents can directly dissolve copper oxide minerals [3]. Citrate ions (as well as EDTA and tartrates) are commonly used for these purposes, as shown by [4, 5, 6, 7, 8]. However, citrates can form very stable complexes with metal ions, which is not very suitable for the subsequent secondary formation of minerals.
More positive results can be expected in systems that include the simplest hydroxy and amino acids (glycolic, lactic, etc.). These compounds can be considered elementary fragments of humic substances, which are largely involved in the migration of heavy metal ions in nature [9]. At the same time, humic acids are capable of forming fairly stable complexes with copper even in salt waters [10, 11].
Our earlier calculations showed that the formation reactions of malachite and azurite with the participation of organic ligands are thermodynamically probable [12].
In the case of lactic acid, as the temperature rises, the change in the Gibbs energy increases, and the reverse reaction becomes more preferable. Deng [13] used this circumstance in their work, where lactic acid was used to dissolve copper from malachite ore at temperatures up to 70 °C.
This research aimed to study the kinetic aspects of the deposition of copper ions on marble from a solution of copper lactate, with the formation of malachite. The process under study is of practical importance for the development of methods for extracting off-balance metal in deposits and dumps of spent copper.
2. Materials and methods
Kinetic experiments were carried out to determine the deposition rate of copper ions at various temperatures. Marble bars 1 × 1 × 8 cm in size were placed in an initial solution of copper lactate with a volume of 1 L with a concentration of 0.0001, 0.0002, and 0.0003 mol/L. Thus, the surface area on which the chemisorption of copper ions took place was precisely known. This approach made it possible to consider the reaction of mineral formation on the marble surface as a heterogeneous interaction and to apply some aspects of the theory of adsorption. Copper lactate was synthesized by dissolving basic copper carbonate Cu2[CO3](OH)2 in an equivalent amount of lactic acid (80 wt%). The solution was stirred using a magnetic stirrer, and the temperature (20, 30, and 40 °C) was maintained with an accuracy of ±1 °C. The experiments were carried out continuously for several weeks. During the experiment, deposits of basic copper carbonates formed on the marble blocks and the walls of the flask. The analysis of the copper content in the solution (1 mL probe) was carried out by the method of absorption photometry by the dithiocarbamate method [14, 15].
2.1. Determination method
To a probe of the test solution containing no more than 150 μg of copper, 1 mL of a 20% potassium–sodium tartrate solution and 1 mL of a 0.1 mol/L solution of ethylenediaminetetraacetic acid disodium salt (complexone III, Trilon B) are added, neutralizing the solution with ammonia to pH 8.5 and adding 5 mL of 0.1% sodium diethyldithiocarbamate solution. The resulting solution is extracted with two portions of carbon tetrachloride in a separatory funnel, shaking each time for about 1 min. The resulting extracts are placed in a 25 mL volumetric flask, made up to the mark with solvent, stirred and photometric at 436 nm (blue filter) using carbon tetrachloride as a reference solution.
2.2. IR spectroscopy method
Samples for IR spectroscopic studies were prepared in the form of tablets compressed from 2 mg of the test substance and 300 mg of potassium bromide KBr, preliminarily crushed and dried at 150 °C. The sample was mixed and crushed in an agate mortar, after which it was pressed at a force of 8 tf, obtaining a tablet with a diameter of 13 mm and a thickness of 1 mm. The spectra were recorded on an FSM-1201 (Infraspek Ltd, Russia) in the transmission mode in the range from 400 to 4000 cm−1 with a spectral resolution of 4 cm−1 at room temperature (25 °C). The results obtained are shown in Table 1.
Concentration of copper in lactate solutions during the kinetic experiments, mol/L
| Time, days | Temperature 20 °C | Time, days | Temperature 30 °C | Time, days | Temperature 40 °C | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.0001 | 0.0002 | 0.0003 | 0.0001 | 0.0002 | 0.0003 | 0.0001 | 0.0002 | 0.0003 | |||
| 1 | 6.85 × 10−6 | 1.37 × 10−5 | 2.07 × 10−5 | 1 | 7.91 × 10−6 | 1.26 × 10−5 | 2.32 × 10−5 | 1 | 7.77 × 10−6 | 1.54 × 10−5 | 2.36 × 10−5 |
| 3 | 6.73 × 10−6 | 1.45 × 10−5 | 2.06 × 10−5 | 3 | 7.25 × 10−6 | 1.24 × 10−5 | 2.34 × 10−5 | 3 | 7.77 × 10−6 | 1.44 × 10−5 | 2.25 × 10−5 |
| 5 | 6.35 × 10−6 | 1.37 × 10−5 | 1.92 × 10−5 | 5 | 7.13 × 10−6 | 1.23 × 10−5 | 2.20 × 10−5 | 5 | 7.46 × 10−6 | 1.34 × 10−5 | 2.10 × 10−5 |
| 8 | 6.77 × 10−6 | 1.31 × 10−5 | 1.24 × 10−5 | 8 | 6.15 × 10−6 | 1.17 × 10−5 | 1.83 × 10−5 | 7 | 7.70 × 10−6 | 1.26 × 10−5 | 1.94 × 10−5 |
| 10 | 6.94 × 10−6 | 1.02 × 10−5 | 9.89 × 10−6 | 10 | 4.61 × 10−6 | 1.12 × 10−5 | 1.48 × 10−5 | 9 | 6.77 × 10−6 | 1.23 × 10−5 | 1.80 × 10−5 |
| 12 | 5.56 × 10−6 | 9.43 × 10−6 | 8.53 × 10−6 | 12 | 4.18 × 10−6 | 1.01 × 10−5 | 1.52 × 10−5 | 11 | 6.63 × 10−6 | 1.07 × 10−5 | 1.77 × 10−5 |
| 14 | 5.44 × 10−6 | 7.61 × 10−6 | 5.47 × 10−6 | 15 | 3.06 × 10−6 | 1.01 × 10−5 | 1.41 × 10−5 | 14 | 5.21 × 10−6 | 1.16 × 10−5 | 1.66 × 10−5 |
| 16 | 5.49 × 10−6 | 6.44 × 10−6 | 6.75 × 10−6 | 17 | 3.09 × 10−6 | 9.50 × 10−6 | 1.32 × 10−5 | 16 | 4.32 × 10−6 | 1.13 × 10−5 | 1.75 × 10−5 |
| 18 | 5.11 × 10−6 | 5.32 × 10−6 | 5.20 × 10−6 | 19 | 3.07 × 10−6 | 9.50 × 10−6 | 1.26 × 10−5 | 18 | 3.73 × 10−6 | 1.14 × 10−5 | 1.88 × 10−5 |
| 21 | 4.77 × 10−6 | 3.73 × 10−6 | 3.66 × 10−6 | 22 | 4.23 × 10−6 | 9.32 × 10−6 | 1.28 × 10−5 | 21 | 2.11 × 10−6 | 1.13 × 10−5 | 1.77 × 10−5 |
| 23 | 4.68 × 10−6 | 3.06 × 10−6 | 3.97 × 10−6 | 26 | 1.98 × 10−6 | 8.96 × 10−6 | 1.25 × 10−5 | 23 | 1.55 × 10−6 | 1.15 × 10−5 | 1.70 × 10−5 |
| 25 | 4.64 × 10−6 | 3.02 × 10−6 | 3.21 × 10−6 | 29 | 3.57 × 10−6 | 9.84 × 10−6 | 1.45 × 10−5 | 26 | 1.48 × 10−6 | 1.20 × 10−5 | 1.77 × 10−5 |
| 26 | 4.56 × 10−6 | 2.69 × 10−6 | 2.95 × 10−6 | 31 | 2.87 × 10−6 | 8.49 × 10−6 | 1.55 × 10−5 | 28 | 1.86 × 10−6 | 1.14 × 10−5 | 1.63 × 10−5 |
| 33 | 3.07 × 10−6 | 9.36 × 10−6 | 1.66 × 10−5 | 30 | 1.83 × 10−6 | 1.13 × 10−5 | 1.66 × 10−5 | ||||
| 36 | 3.00 × 10−6 | 9.40 × 10−6 | 1.57 × 10−5 | 32 | 1.97 × 10−6 | 1.14 × 10−5 | 1.74 × 10−5 | ||||
| 38 | 3.15 × 10−6 | 9.40 × 10−6 | 1.66 × 10−5 | 35 | 1.10 × 10−6 | 1.16 × 10−5 | 1.55 × 10−5 | ||||
| 37 | 1.04 × 10−6 | 1.16 × 10−5 | 1.62 × 10−5 | ||||||||
| 39 | 1.52 × 10−6 | 1.18 × 10−5 | 1.66 × 10−5 | ||||||||
| 42 | 1.28 × 10−6 | 1.17 × 10−5 | 1.65 × 10−5 | ||||||||
| 44 | 1.21 × 10−6 | 1.24 × 10−5 | 1.61 × 10−5 | ||||||||
| 46 | 1.69 × 10−6 | 1.16 × 10−5 | 1.74 × 10−5 | ||||||||
3. Results and discussion
The kinetic curves of the deposition of copper ions on marble (Figure 1) at the initial stage are characterized by latent behavior, followed by a gradual smooth increase. The amount of precipitated copper ions decreases with increasing temperature, which indicates the presence of a reverse reaction in the system or side reactions, for example, hydrolysis of dilute copper solutions with the formation of a colloidal suspension of poorly soluble substances. As a rule, this happened in experiments at elevated temperatures and low concentrations at the final stages, when copper was adsorbed on the oxidation products of the lactate ion.
Kinetic curves for copper deposition on marble from lactate solution. From top to bottom: at 20 °C (a), at 30 °C (b), at 40 °C (c). Initial concentration: diamonds—0.0001 mol/L, squares—0.0002 mol/L, circles—0.0003 mol/L.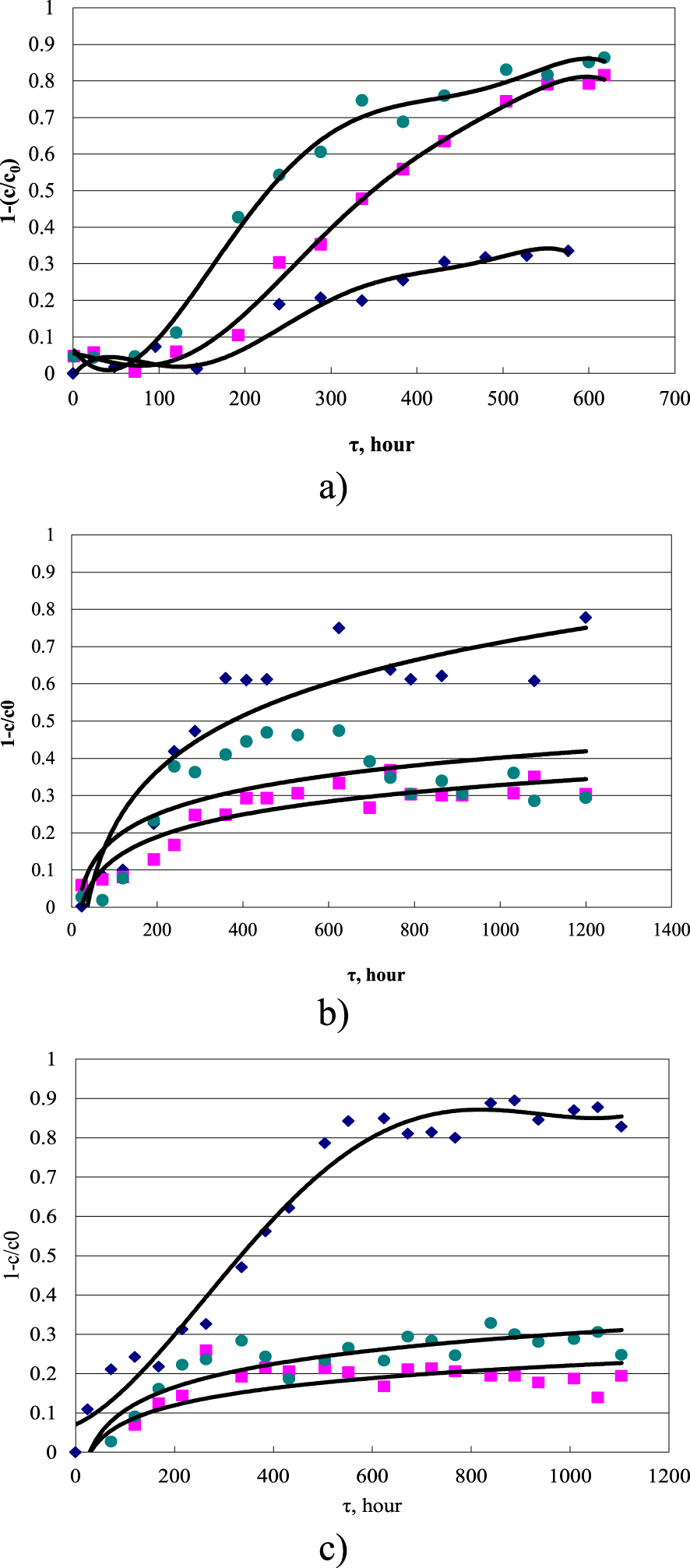
Since the rate of accumulation of products upon dissolution of copper compounds obeys the ratios dc∕dt < 0 and d2 c∕dt2 < 0, the processes under consideration correspond to a simple chemical reaction with a low order of reaction concerning the final product, which slowly approaches equilibrium concentrations. Under the experimental conditions, the reaction proceeding is heterogeneous, and because of the low dissolution rates of copper compounds, the interface area can be considered constant, and the concentration of the complexing agent in the solution also remains at a constant level. Thus, the concentration of the starting materials does not have a decisive effect on the kinetics of the process (that is, they enter the kinetic equation in the form of constants).
Formally, the reaction kinetics can be described by the reaction product, that is, the concentration of copper in the solution, which is included in the kinetic equation as the difference between the initial concentration of copper in the solution, which is greater than the true equilibrium concentration of copper:
| (1) |
When the equation is differentiated, all the constant values (initial concentration [Cu]init and rate constant k) are converted in a new constant C: dv∕dt = d[Cu2+]∕dt + C, and the concentration of copper in the solution [Cu2+] remains the influencing variable. Linearization of the resulting equation using the logarithm gives an array of reaction rates (Figure 2).
Logarithmic kinetic curves for copper deposition on marble from lactate solution at 20 °C. From top to bottom: initial concentration 0.0001 mol/L, 0.0002 mol/L, 0.0003 mol/L.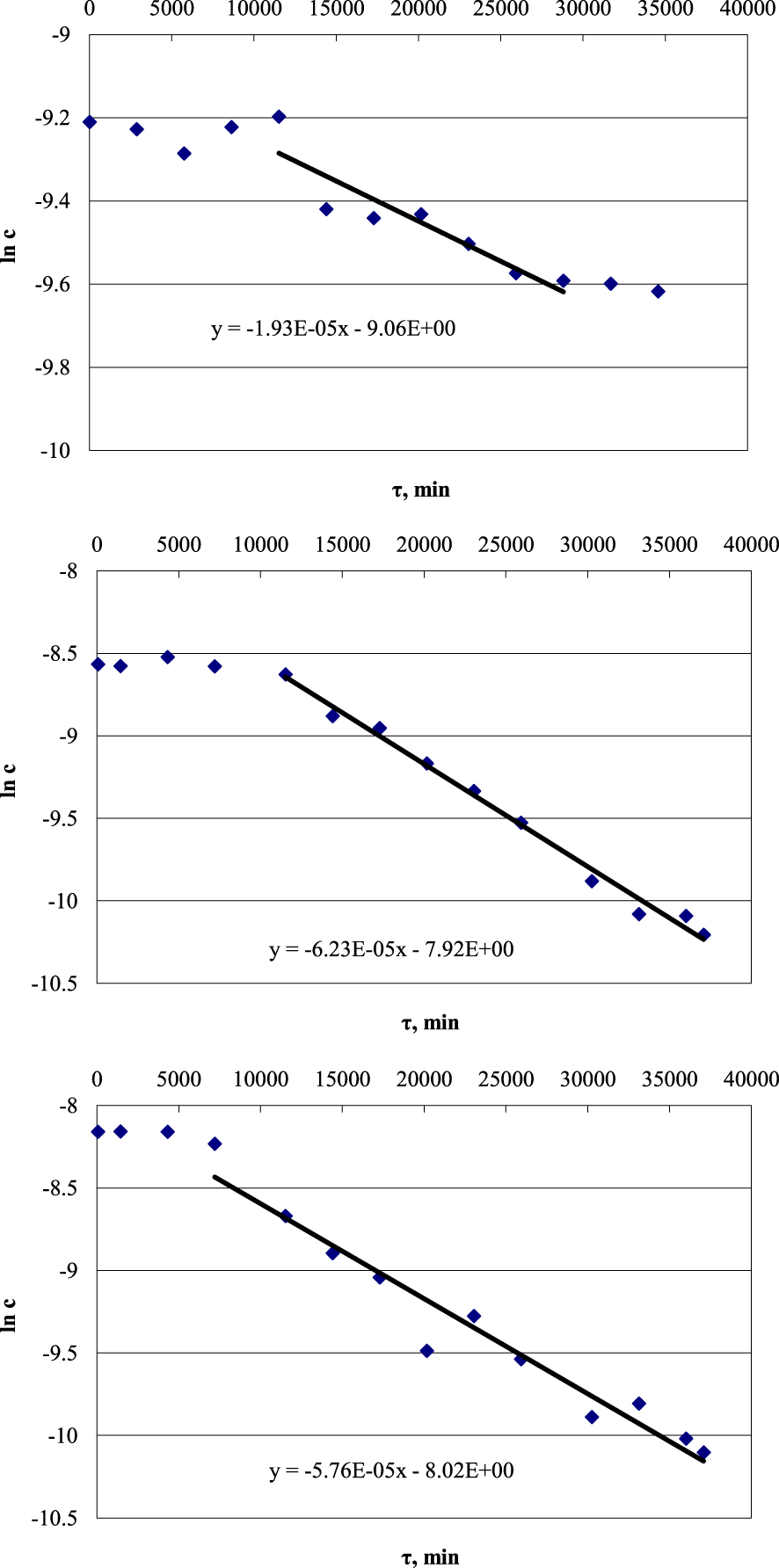
For solutions containing copper lactate, mineral formation at 40 °C is slightly faster than at 20 °C. In this investigated range, the dependences remain rectilinear, which indicates the gradual formation of a microcrystalline precipitate. The reaction rate constants, in this case, are 4.19 × 10−6 s−1 at 20 °C and 5.86 × 10−6 s−1 at 40 °C.
Based on the limiting value of chemisorption in these experiments, it is possible to calculate the relative value of chemisorption 𝛾 =𝜒 ∕𝜒∞ (where 𝜒 is the value of chemisorption per unit mass of the sorbent; 𝜒∞ is the limiting value of chemisorption). The time dependence of the relative chemisorption in the 𝛾– coordinates is shown in Figure 3.
Relative value of chemisorption of copper ions on the marble: diamonds—at 20 °C, triangles—at 30 °C, squares—at 40 °C.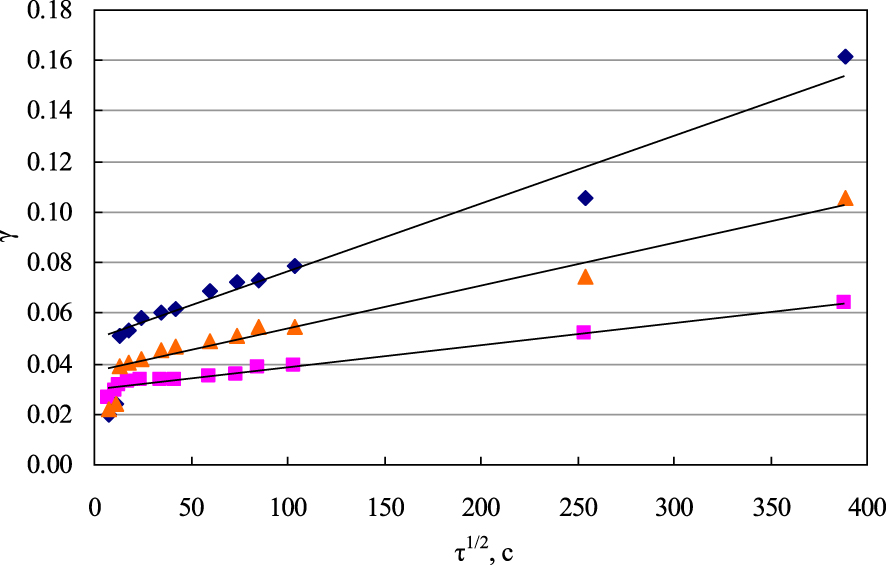
In the range from small to medium 𝛾 values, the sorption value is proportional to the square root of time, and experimentally this dependence is satisfactorily applicable up to 𝛾 = 0.6–0.7, that is, it approximates most of the kinetic curve. This area is used for kinetic measurements, in particular, to determine the time to reach the relative sorption value 𝛾 = 0.5, denoted as 𝜏0.5, which is used in calculating the diffusion coefficients.
However, in this case, as can be seen from Figure 3, the linear dependence observed in the experiments does not come from the origin of coordinates, and only the initial section, far from the equilibrium conditions, is directly proportional to the square root of time. In addition, during the period of kinetic experiments, the degree of the reaction did not exceed a few percent, which indicates the relatively low rates of copper chemisorption in this system. Low rates of processes in this system make it possible to characterize it using an effective diffusion coefficient.
For any transport mechanism, the rate of the process can be expressed by the diffusion equation with some effective value of the diffusion coefficient. The diffusion process through a flat surface following Fick’s second law is described by the equation
| (2) |
| (3) |
The comparatively low rates of processes in these systems make it possible to characterize them using the effective diffusion coefficient. For the system under consideration, the effective values of the diffusion coefficient are: 1.39 × 10−12 m2∕s at 20 °C, 5.62 × 10−13 m2∕s at 30 °C, and 1.45 × 10−13 m2∕s at 40 °C. The order of the values obtained is characteristic of diffusion processes occurring in solids. The activation energy of the precipitation reaction is 12.8 kJ/mol.
The sediments of basic copper carbonates formed during the experiments were investigated by IR spectroscopy. For comparison, Figure 4 shows the IR spectra of natural copper carbonates, malachite and azurite, from the abandoned mine of the Spassky deposit (Abay district of the Karaganda region, Kazakhstan).
Infrared spectra of the natural malachite (above) and azurite (below) samples (Spassk mine, Central Kazakhstan).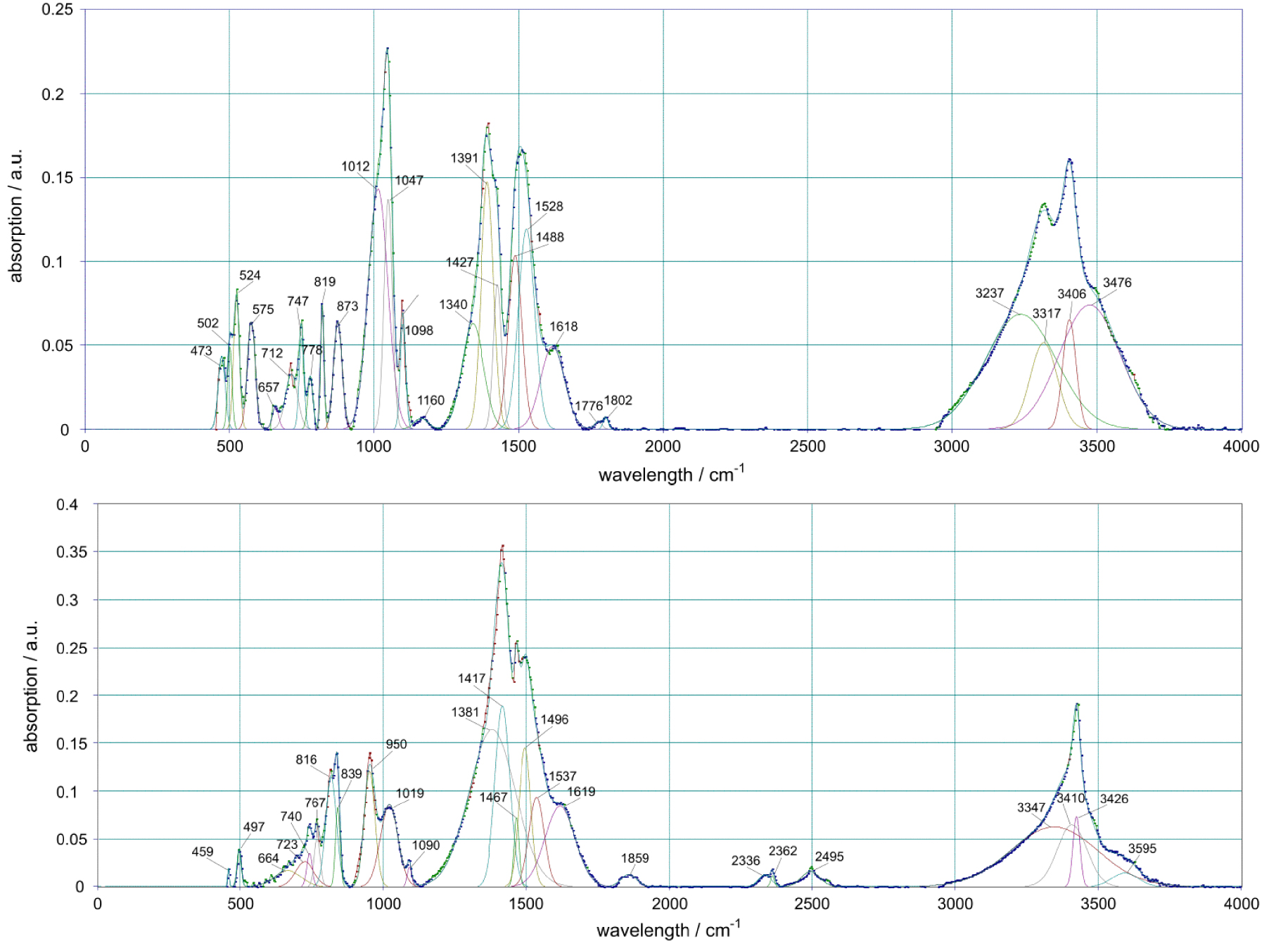
The positions of individual absorption bands in the IR spectra of natural samples of malachite and azurite are in good agreement with the known experimental data [17, 18, 19, 20, 21, 22]. The spectrum of malachite at 1510 cm−1 shows a line of montmorillonite. The absorption bands of the samples in the range 2000–4500 cm−1have a complex structure due to the presence of OH groups in symmetrically nonequivalent positions. The stretching vibrations of the hydroxyl group in the region of 3500 cm−1 are less affected than others by neighboring atoms and usually coincide in various minerals. Azurite carbonate peak at 1400 cm−1 is split into a triplet due to a rather strong deviation of the structure of the carbonate ion from the structure of an isosceles triangle. At the same time, a doublet is observed for malachite, since the carbonate group in its structure has a higher symmetry. Further, in the region of 800–1200 cm−1, azurite and malachite have a group of lattice vibrations, which also includes vibrations of copper–oxygen polyhedra. The remaining group of lattice vibrations at 400–600 cm−1 is represented by a series of peaks, since the lines included in it are differentiated in frequency due to a significant difference in the reduced masses of the atomic pairs of copper and the atoms surrounding it.
In the IR spectrum of the sediment formed from copper lactate on marble (Figure 5), one can see absorption bands of malachite against the background of rather strong fluctuations of free water and a group of powerful peaks in the area of 1400–1600 cm−1, which can be attributed to carboxylate ion and others fluctuations in carboxylic acid residues. Thus, the lactate ion is partly included in the composition of the sediment. Water can also be contained in the form of crystalline hydrates.
Infrared spectra of the sediments from copper lactate on marble at 20 °C (above) and 30 °C (below).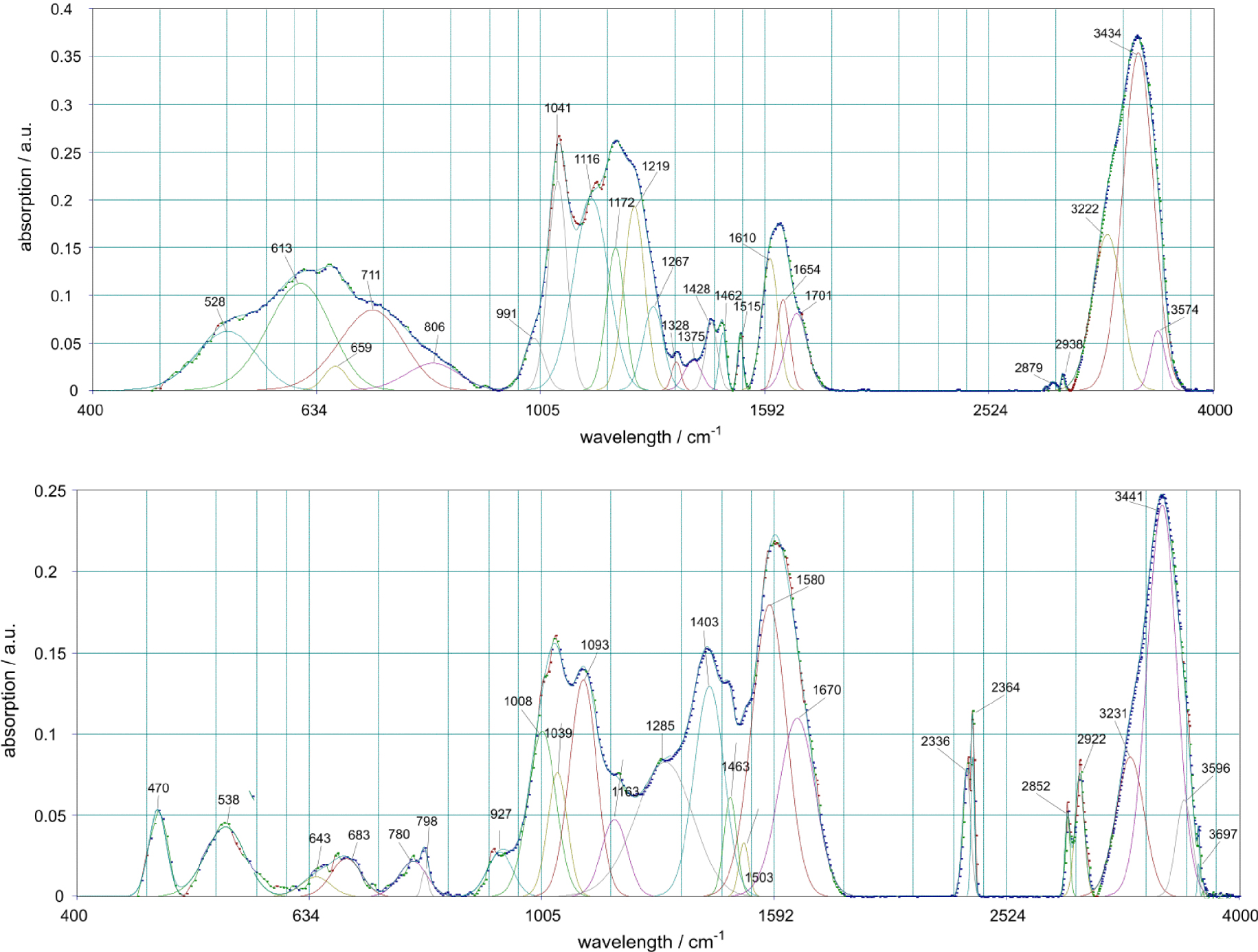
Thus, the researches carried out show that the formation of basic copper carbonates from lactate solutions on calcite proceeds satisfactorily. In these processes, deposits are formed corresponding to malachite. At the same time, due to comparable reaction rates, the carbonate material has time to dissolve, and a precipitate is formed in the entire volume of the solution.
4. Conclusion
The results obtained show the possibility of using environmentally friendly organic complexing agents to increase the geochemical migration of copper ions. According to kinetic measurements, the release of malachite from copper lactate complexes on calcite is of relatively high value. A growing layer of oxidized carbonate copper minerals is gradually formed on carbonate materials, which can subsequently be processed by known beneficiation methods.
Based on the experimental data obtained on the rate constants, it is possible to estimate the growth rate of basic copper carbonates when interacting with organic complexing agents. In accordance with the obtained reaction rate constants, the growth time of a 1 mm thick sediment layer is of the order of 400–500 days in the case of copper lactate. As a result, the concentration of copper in carbonate materials increases compared to the original rocks, copper losses in dumps are reduced and environmental safety is increased.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding
This work was performed under financial support of the Ministry of Education and Science of the Republic of Kazakhstan (Project No. AR09259337/GF).




 CC-BY 4.0
CC-BY 4.0