1. Introduction
Plants emit complex mixtures of volatile compounds that may contain up to a hundred different molecules [1]. These volatile organic compounds (VOCs) strongly influence ecological interactions between plants and insects, as the latter highly rely on olfaction to perceive their external environment [2, 3]. VOCs can be used by plants to attract pollinators and seed dispersers, and can also participate to the defense against herbivores, for example by recruiting natural enemies [1]. Each plant species, and at a finer scale each population or even genotype, emits VOCs in particular amounts and ratios, such that not two ever exhibit strictly identical VOC blends [4, 5]. These differences are used by phytophagous insects to identify and select their host plants. From an evolutionary perspective, there thus is a strong selection pressure on insects to develop efficient ways to detect host-specific blends, as their survival and reproduction depend on it [6]. Although attraction can be induced by one or a few taxonomically characteristic compounds, such as observed with specific VOCs of Brassicaceae [7, 8, 9], Asteraceae [10], Rosaceae [11] or Alliaceae [12], insects of several orders have been shown to possess olfactory receptors able to detect ubiquitous VOCs such as fatty acid derivatives, phenylpropanoids and isoprenoids [13, 14]. Studies have shown that host recognition is mostly based on the detection of a host-specific combination of either specific or ubiquitous VOCs, rather than on the detection of a single host-specific compound. Indeed, many examples in Diptera [15, 16], Lepidoptera [17, 18, 19] or Hymenoptera [20] show that combinations of VOCs can be significantly more attractive than individual compounds. Furthermore, individually repulsive VOCs can become attractive once in mixtures [21, 22, 23] and attractive mixtures can become repulsive if VOC relative proportions change [20].
Although VOCs are known to play a key role in distance host location [14], they can also act synergistically with contact compounds and influence insect behavior once in contact with the plant [24, 25]. Furthermore, it has been shown in many species such as Bombyx mori [26], Drosophila melanogaster [27], Bactrocera dorsalis [28, 29], Anopheles arabiensis and A. coluzzii [30], that certain VOCs alone or in mixtures can stimulate female oviposition on their own and thus be important in the contact phase of the host selection process. As an example, Müller and Hilker [31] tested the effect of VOCs on the behavior of the monophagous chrysomelid Cassida stigmatica, by comparing Tanacetum vulgare petioles wrapped with perforated filter paper tubes to dummy petioles. They observed that walking duration was longer on T. vulgare petioles than on dummy ones, showing that VOCs alone can influence host assessment and thus trigger contact exploration behaviors. However, in most examples where the effect of VOCs on host selection was investigated, the distance effect (i.e. attraction of the insect to the oviposition site) and contact effect (i.e. stimulation of exploration or oviposition once in contact) are poorly distinguished, which does not allow to understand precise cues involved at each step of the host selection behavioral sequence.
In the cabbage root fly, Delia radicum, receptive females exhibit a stereotypical host exploration behavior before accepting a host plant as a suitable oviposition site [32], which involves several types of cues, both physical and chemical. Physical cues such as color, shape or texture [33, 34, 35], as well as contact chemical cues [36, 37, 38, 39] have been extensively studied. Other studies have shown the involvement of plant VOCs in mediating the female behavior [40, 41]. For example, allyl isothiocyanate has been shown to be involved in long-range attraction [41, 42] while other VOCs such as dimethyl disulfide (DMDS) [43] or cis-3-hexenyl acetate [44, 45] influence oviposition. Both simultaneously perceived volatile and contact cues synergistically mediate host plant acceptance [46]. In parallel, studies have shown contrasts in attractiveness between different host species [47, 48] and others have characterized VOCs emitted by these species [47, 49, 50, 51]. However, to our knowledge, no studies have both characterized the compounds emitted by plants in the host range of D. radicum and identified those involved in either attracting females or stimulating their host exploration behavior prior to oviposition. In this study, we aimed to (i) identify VOCs involved in host recognition by females of the cabbage root fly Delia radicum, and (ii) determine their involvement in either female attraction at distance or stimulation of host pre-ovipositional exploration behavior. Experiments were conducted on three host species of D. radicum, namely Brassica oleracea, B. rapa and Sinapis alba, which are known to be contrasted both in terms of attractiveness [47, 48] and oviposition [52]. At a finer scale, two cultivars of B. rapa and S. alba, also contrasted for oviposition [52], were also included. These contrasts suggest fine-tuned detection and recognition abilities in D. radicum females, but the influence of volatiles on these discrimination abilities remains unknown. First, headspace collection of VOCs emitted by the different host plants was performed and the compounds present in the blends were identified by gas chromatography coupled to mass spectrometry (GC-MS). In parallel, gas chromatography coupled to electro-antennography detection (GC-EAD) was performed to identify, among all VOCs present in the blends, those that are detected by the females’ antennae. Finally, the behavioral effect of the different compounds detected, both taken individually and in combination, was tested using an artificial leaf with synthetic VOCs emissions.
2. Materials and methods
2.1. Plants
Experiments were carried out with five different cultivars, all of which being commercial: two cultivars of Sinapis alba (“Verte” and “Sarah”), two cultivars of Brassica rapa ssp. pekinensis (“Richi” and “Tabaluga”) and one cultivar of B. oleracea (“Parthenon”). Single seeds were sown in individual 3.5 × 3.5 × 5 cm clumps filled with potting soil (Premier Tech Horticulture) and plants were grown in a climatic chamber at optimum conditions (20 ± 1 °C, 16L:8D and 70 ± 10% RH) where they were watered twice a week with a nutritive solution [53]. One week before the experiments started, plants were repotted individually in 7 × 7 × 6.5 cm plastic pots containing the same substrate. All plants were at the 3–4 true leaves stage when used for experiments.
2.2. Insects
Females of D. radicum originated from a laboratory rearing initially constituted from a field population collected in the field in 2019 (Pleumeur-Gautier, Brittany, France). Flies were reared on rutabagas as described in [54] and fed with a milk powder: yeast: sugar (1:1:1) mixture. One week after emergence, females were considered fertilized and used for experiments until they were 12 days old. Females were identified based on the sexual dimorphism in the size of the eye-spacing, which is much more pronounced in females than in males [55].
2.3. Volatile collection
Volatiles from the five cultivars tested were collected using dynamic headspace. The aboveground part of an individual plant was enclosed in a PET bag (35 × 43 cm, Alfapac), in which an air flow was maintained by two pumps (KNF, Neuberger) connected with PTFE tubing. The airflow was purified by an activated charcoal filter placed between the upstream pump and the bag. The entrance and exit flow rates were regulated by flowmeters at respectively 300 and 200 ml⋅min−1. Volatiles (from plants or an empty bag as control) were collected during 24 h on a cartridge filled with 30 mg of Porapak Q (80:100 mesh, Sigma-Aldrich) placed downstream from the sampling bag. After sampling, all cartridges were kept in the dark at −20 °C until further use. Six to eight replicates were performed per cultivar.
2.4. Volatile identification and quantification
Samples were analysed at the “Platform for Chemical Analyses in Ecology” (PACE), with the support of the LabEx CeMEB (Centre Méditerranéen pour l’Environnement et la Biodiversité, Montpellier, France). Sampling cartridges were solvent desorbed using 200 μl of hexane (⩾98.5%; Carlo Erba reagents) and stored at −20 °C until analysis. Separation and semi-quantitative analyses of volatiles peaks were achieved using a Shimadzu QP2010Plus gas chromatograph coupled to a quadrupole mass spectrometer (GC-MS, Shimadzu Scientific Instruments). The GC was equipped with an Optima 5-MS fused silica capillary column (length: 30 m; diameter: 0.25 mm; film thickness: 0.25 μm, Macherey-Nagel), with helium as carrier gas (1 ml⋅min−1). The temperature ramp started at 40 °C hold for 1 min, then increased to 220 °C at 12 °C⋅min−1, and finally held at 220 °C for 2 min. One microliter of sample was injected into a splitless injector set at 200 °C. Mass spectra were recorded in electronic impact mode (EI) at 70 eV over a m∕z mass ranging from 38 to 350. The temperature of the transfer line and the ion source were set to 250 °C. Data were processed according to Kidyoo et al. [56] using the MZmine™ software version 2.53 [57]. The control samples analyzed were used to subtract potential contaminant compounds from the plant samples. Compound identification was based on computer matching of mass spectra with a database (NIST 2005 MS library, Wiley 9th edition), on retention times and mass spectra of external synthetic standards. The retention times of a series of n-alkanes (nC8 to nC20 alkane solution, 04070, Sigma Aldrich®) were used to calculated the retention index of compounds and compare with those reported in the literature [58]. Peak areas were used for statistical analyses.
2.5. Electrophysiological recordings
Elutions from all samples of the same cultivar were pooled together and then concentrated under nitrogen flow for the recordings. Electroantennography assays were performed using D. radicum females only. Each female was immobilized with forceps, its head was cut and separated from the thorax, then put into an adapted glass capillary. It was obtained from glass capillary (length: 76 mm, diameter: 1.12 mm; World Precision Instrument) pulled and cut using a vertical micropipette-puller (P-30 model, World Precision Instruments). The capillary was previously filled with an electrolytic solution of Ringer (6.0 g⋅l−1 NaCl, 0.4 g⋅l−1 KCl, 0.3 g⋅l−1 CaCl2, 3 g⋅l−1 NaHCO3) and connected to a reference electrode. The tip of the antenna was in contact with the recording electrode, also filled with the electrolytic solution, at the opposite side of the reference electrode. A stimulus controller (CS-55 Syntech Ockenfels) was set to provide a continuous flow of purified and humidified air, passing through a glass tube to send the VOCs one by one to the antenna at a flow rate of 245 ml⋅min−1. Electroantennography assays were performed using coupled gas chromatography-electroantennographic detection (GC-EAD). A volume of 2 μl of the solution was injected in a 8890 GC system gas chromatograph (Agilent) equipped with a flame ionization detector (FID), a splitless injector kept at 200 °C and an Optima 5-MS capillary column (length: 30 m; diameter: 0.25 mm; film thickness: 0.25 μm). Helium was used as carrier gas (1 ml⋅min−1). The temperature ramp was set as above. The effluent was split into two deactivated fused silica capillary columns (100 cm × 0.25 mm), one leading 10% of the outlet flow to a FID set à 300 °C and a second one leading 90% of the outlet flow into a heated EAD transfer line kept at 200 °C (Syntech Ockenfels). Antennal electrical responses to volatile compounds were tested and digitized using an IDAC board (acquisition controller IDAC-2; Syntech Ockenfels). Data were processed with a PC-based interface and software package (GcEad 1.2.5, Syntech Ockenfels). Electrophysiological measurements were conducted on 8–12 females per cultivar. A compound was considered olfactively active in D. radicum females when it elicited a clear depolarization response in at least half of the females tested.
2.6. Behavioral experiments
The effect of the VOCs detected by the females’ antennae on their behavior was assessed using a dual-choice setup with artificial leaves (Supplementary Figure A). Leaves were made of a 10 cm green-paper straw (Utopia®) on which a green-paper limb (Cultura®) was stapled at 6 cm from the straw bottom. To reproduce a plant waxy cuticle, which is mandatory for D. radicum to lay eggs [34] and thus to induce an exploration behavior, each leaf was briefly immersed in a 1 l glass beaker containing 800 ml of water and 40 g of paraffin wax heated at 85 °C in a water bath. Leaves were then placed individually in a Petri dish containing a 7 mm layer of river sand. Two dishes each containing one leave were placed in a 30 cm cubic nylon cage (BugDorm®, Taichung, Taiwan). A 2 × 2 cm filter paper was placed on a wooden rod inserted in the paper straw of each leaf, so that the filter paper overlaid the artificial leaf without touching it. On one filter paper, 10 μl of mineral oil containing a single VOC or a VOC mixture determined by the authors in the course of the present work and some earlier ones was deposited (each VOC at 1 μg∕μl), whereas the second filter paper with only 10 μl of mineral oil served as control. After impregnating the filter paper with volatiles for 2 min, it was placed in the cage. Immediately thereafter, one female was released and the experiment started. Every 5 min during 90 min, it was recorded (i) whether the female was on a leaf and (ii) whether it was showing an exploration behavior. As preliminary experiments showed that VOCs alone were not sufficient to induce oviposition in these experimental conditions, the number of eggs laid was not recorded. Seven experiments were conducted, one with each single VOC detected by the females antennae, and three with VOC mixtures characterizing the three plant species studied (hereafter called “B. oleracea like”, “S. alba like” and “B. rapa like”). All experiments took place at 20 ± 2 °C, 16L:8D, and 60 ± 10% RH, and 10–15 replicates were performed per experiment.
2.7. Statistical analyses
All analyses were performed using the R software version 4.1.3 [59]. Volatile profiles were compared multivariately using a redundancy analysis (RDA) on quadratic-root transformed and autoscaled data [60], with species and cultivar as independent factors. The effect of these factors was tested using a permutation F test with 9999 permutations (R package “vegan” [61]). Pairwise comparisons between species and cultivars were performed also using permutation F-tests and p-values corrected with the False Discovery Rate method [62]. Analyses were based on the mean area of the extracted VOCs per plant. In each behavioral experiment, the probability of being present on a leaf or showing an exploration behavior was compared between treatments (control vs. VOC or control vs. VOC mixture) using Wald tests applied on Generalized Linear Mixed Models (GLMM, distribution: binomial, link: logit), in which treatment, time and their interaction were considered as fixed factors and the cage as a random factor. Estimated Marginal Means (EMMeans) were computed for interpretation (R package “emmeans” [63]). Three analyses were performed per experiment: one on the probability for the fly of being attracted to the artificial leaf (test of the attractiveness of the treatments at distance, i.e. pre-contact effect), one on the probability of triggering an exploratory behavior (test of the ability of the treatments to stimulate an exploration behavior at contact, i.e. post-contact effect) and one on the global probability of exploring the leaf (integration of the two previous effects, i.e. test of the global ability of the treatments to lead to an exploration behavior) (Supplementary Table I).
3. Results
3.1. VOC emission profiles are characteristic of species but not of cultivars
GC-MS analyses allowed the detection and identification of 12 different compounds, 10 being terpenes (eight monoterpenes and two sesquiterpenes) and the two others being ethylacetophenone and hexenyl acetate (Figure 1a). Significant differences in volatile profiles were observed between species (F = 12.66, p < 0.001). An almost significant difference in volatile profiles was observed between cultivars of S. alba but not B. rapa. (S. alba: p = 0.065, B. rapa: p = 0.363) (Figure 1b). Each species was characterized by a specific profile. The specific signature of B. oleracea consisted of α-farnesene and all monoterpenes. The specific signature of S. alba mostly consisted of α-farnesene and hexenyl acetate (with all VOCs but hexenyl acetate emitted in greater amounts in cv. Sarah compared to cv. Verte). The specific signature of B. rapa mostly consisted of β-myrcene, β-ocimene, β-caryophyllene and ethylacetophenone (with all VOCs except β-caryophyllene emitted in greater amounts in cv. Richi compared to cv. Tabaluga).
Volatile Organic Compound (VOC) emission profiles of five cultivars of three Brassicaceae species. (a) Heatmap of VOCs emitted by each cultivar (mean value per cultivar). The emission level of each compound is compared between cultivars and represented by a color gradient from light yellow (low) to dark red (high). (b) Score plot of the redundancy analysis performed on the VOCs emitted by individual plants (orange: Brassica rapa (cv. Tabaluga and Richi); blue: Sinapis alba (cv. Verte and Sarah); green: B. oleracea (cv. Parthenon)). All species differed significantly from each other (respectively B. rapa vs. S. alba: p = 0.003; B. rapa vs. B. oleracea: p = 0.005; S. alba vs. B. oleracea: p = 0.008).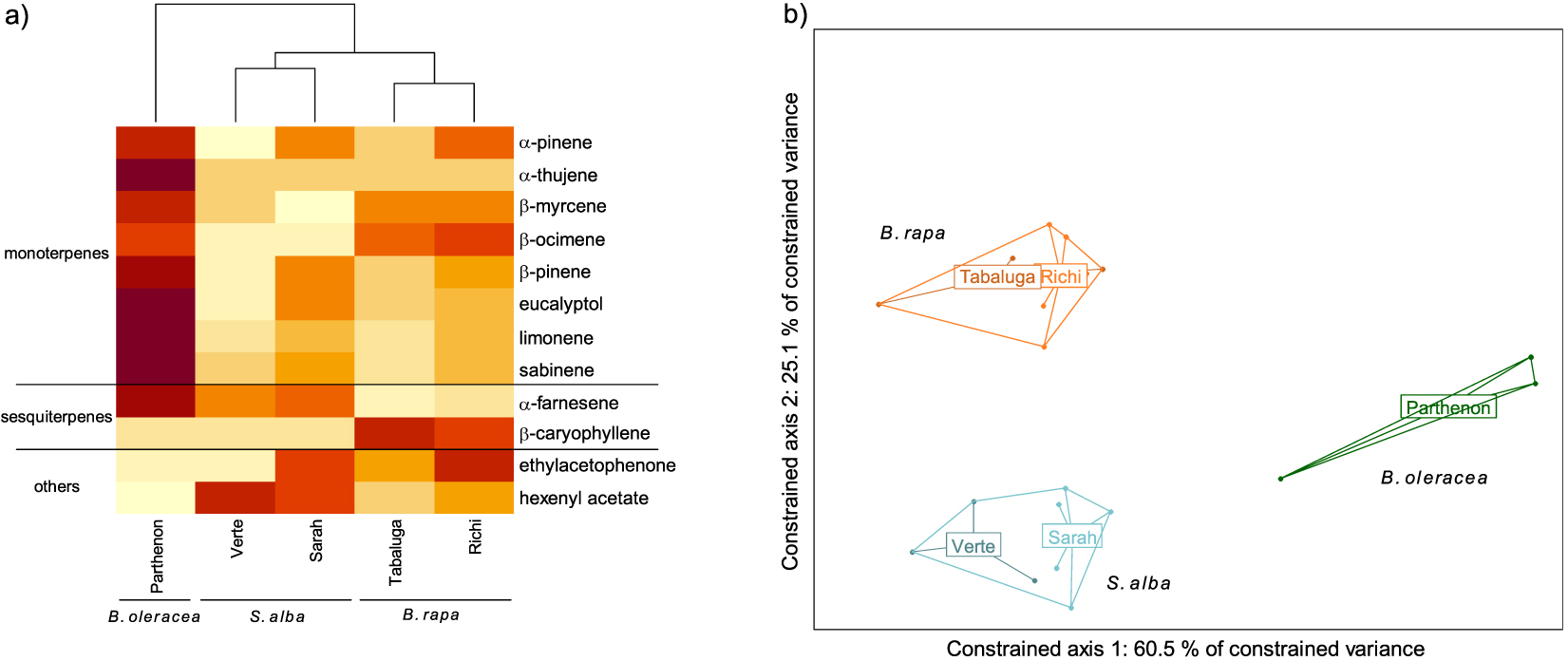
3.2. Four compounds among those present are detected by D. radicum females
GC-EAD analyses revealed that females responded to four compounds present in the VOC blends of the different cultivars: two monoterpenes (β-ocimene and sabinene) and two sesquiterpenes (α-farnesene and β-caryophyllene) (Figures 2, 3a). The response to these compounds was confirmed with synthetic compounds [64]. Four females also responded to β-myrcene but only with VOC blends from cv. Tabaluga. This compound was more abundant in the blend from cv. Parthenon than from cv. Tabaluga (Figure 1a), and since it is detected more intensely by the female antennae of D. radicum with increasing dose [64], it should also have been detected by GC-EAD in the VOC mixture from cv. Parthenon. This compound was therefore removed from the following analyses, as its detection by female antennae was considered inconsistent and unreliable in the present experiment. A statistical analysis based on the detected compounds only, still highlighted interspecific differences in volatile profiles (F = 14.77, p < 0.001), but none at the intraspecific scale (S. alba: p = 0.103, B. rapa: p = 0.338) (Figure 3b). A species-specific signature was shown again (Figure 3a), with a blend of β-ocimene, sabinene and α-farnesene for B. oleraceae (Figure 2a), sabinene and α-farnesene for S. alba (Figure 2b) and β-ocimene, sabinene and β-caryophyllene for B. rapa (Figure 2c).
Examples of antennal responses of Delia radicum females to Volatile Organic Compounds (VOCs) from headspaces of three Brassicaceae species. (a) Brassica oleracea (cv. Parthenon), (b) Sinapis alba (cv. Sarah) and (c) B. rapa (cv. Richi). Grey circles show true antennal responses, with the respective VOCs identified on the chromatogram.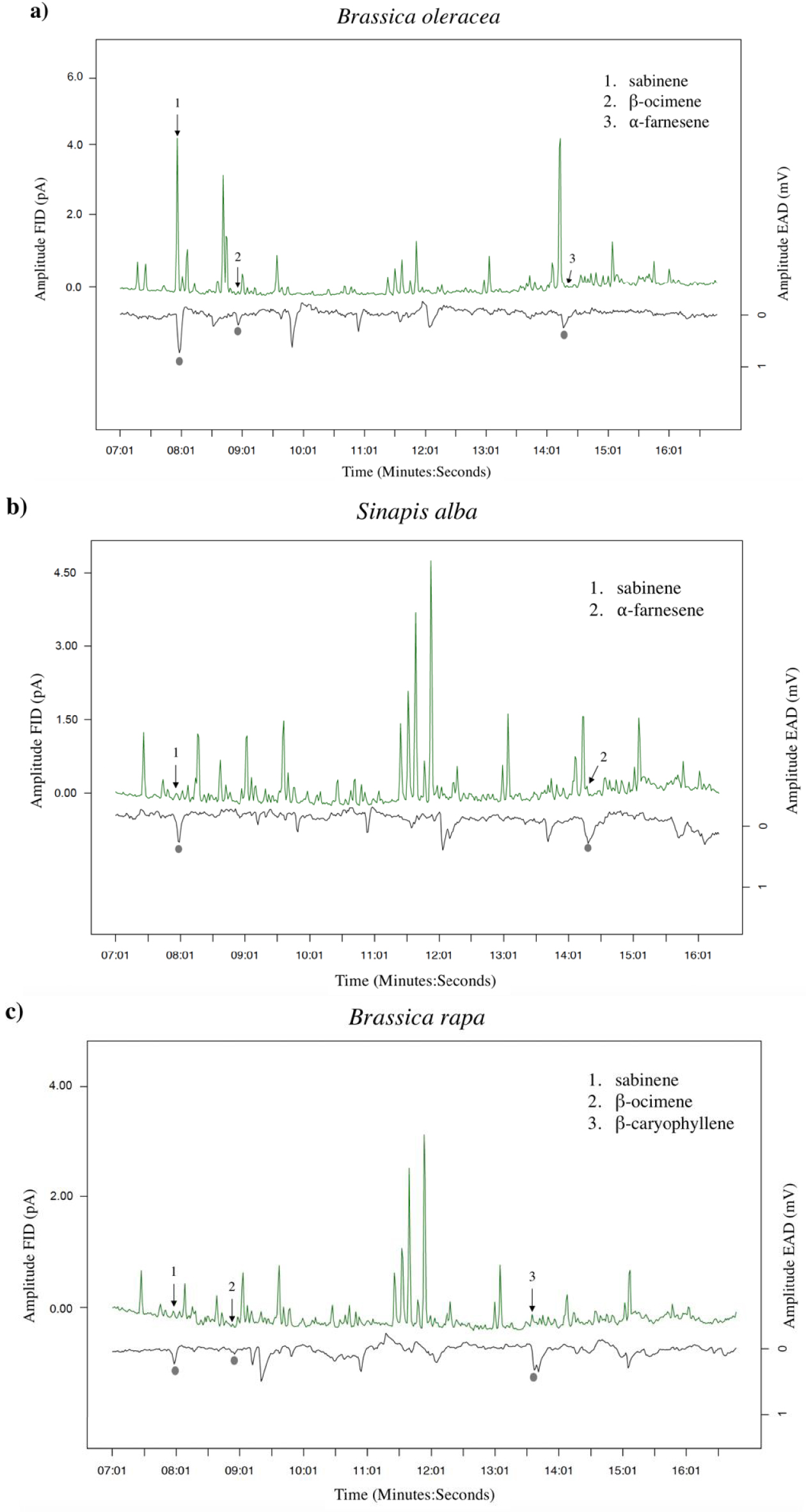
Volatile Organic Compounds (VOCs) emitted by three Brassicaceae species and detected by antennae of Delia radicum females. (a) Heatmap of VOCs emitted by each cultivars and detected by females (mean value per cultivar). The emission level of each compound is compared between cultivars and represented by a color gradient from light yellow (low) to dark red (high). (b) Score plot 1–2 of the redundancy analysis performed on the VOCs emitted by individual plants (orange: Brassica rapa (cv. Tabaluga and Richi); blue: Sinapis alba (cv. Verte and Sarah); green: B. oleracea (cv. Parthenon)). All species differed significantly from each other (respectively B. rapa vs. S. alba: p = 0.003; B. rapa vs. B. oleracea: p = 0.004; S. alba vs. B. oleracea: p = 0.004).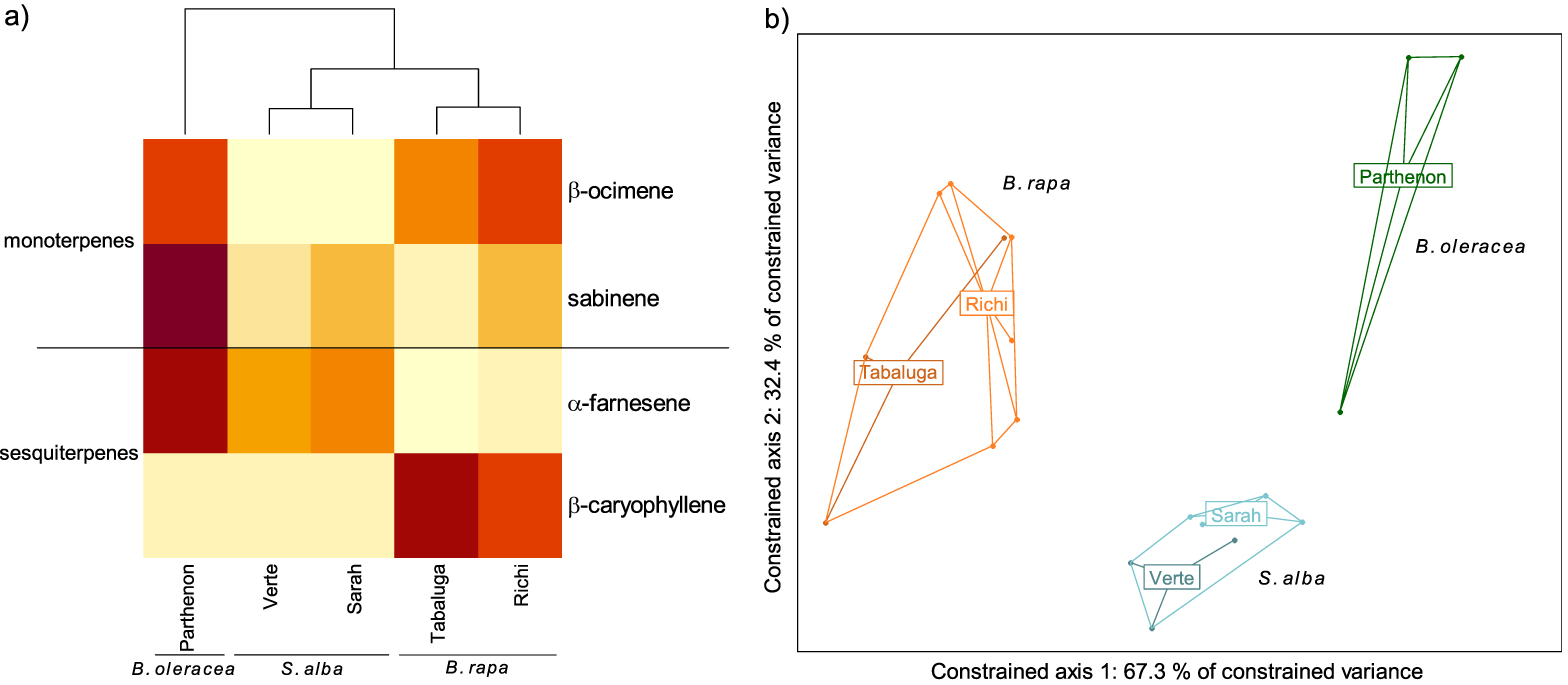
3.3. VOCs have biological effects mostly when combined, with effects being either at distance or at contact
Following results of the GC-EAD recordings, four VOCs were retained for behavioral experiments (β-ocimene, sabinene, α-farnesene and β-caryophyllene) as well as the mixtures β-ocimene + sabinene + α-farnesene (“B. oleracea like”), sabinene + α-farnesene (“S. alba like”) and β-ocimene + sabinene + β-caryophyllene (“B. rapa like”). When tested alone, one of the four VOCs (sabinene) did induce neither pre- or post-contact effects and two (β-ocimene, α-farnesene) seemed to induce repulsion as their associated probability for the fly of being attracted to the leaf was lower than that of the control (Figure 4a). None of these three compounds induced a higher global probability of exploration when considered alone (Figure 4c). Only β-caryophyllene induced attraction (Figure 4a), but not stimulation of exploratory behavior at contact (Figure 4b). Overall, β-caryophyllene led to increase the global probability of exploration, but this probability was very low (Figure 4c). Contrary to most single VOCs, all VOC mixtures increased the probability of triggering to an exploratory behavior and the global probability of exploration, significantly or nearly (Figure 4b,c). However, VOC mixture had contrasted effects: the “B. rapa like” mixture induced significant pre-contact effect but not post-contact one, the “B. oleracea like” had opposite effect and the “S. alba like” induced significant repulsion but a high stimulation of a post-contact exploratory behavior (Figure 4).
Behavioral response of Delia radicum females to the VOCs emitted by three Brassicaceae species and eliciting an antennal response, when tested alone (left of the dotted line) or in mixtures (right of the dotted line). (a) Mean probability (± SE) of being attracted to the leaf (pre-contact effect). (b) Mean probability (± SE) of triggering to an exploratory behavior (post-contact effect). (c) Global probability (± SE) of exploration (global effect). Black dots indicate the presence of the VOC in the treatment tested. NS: p > 0.1, .: p < 0.1, *: p < 0.05, **: p < 0.01, ***: p < 0.001. N = 10–15 per experiment.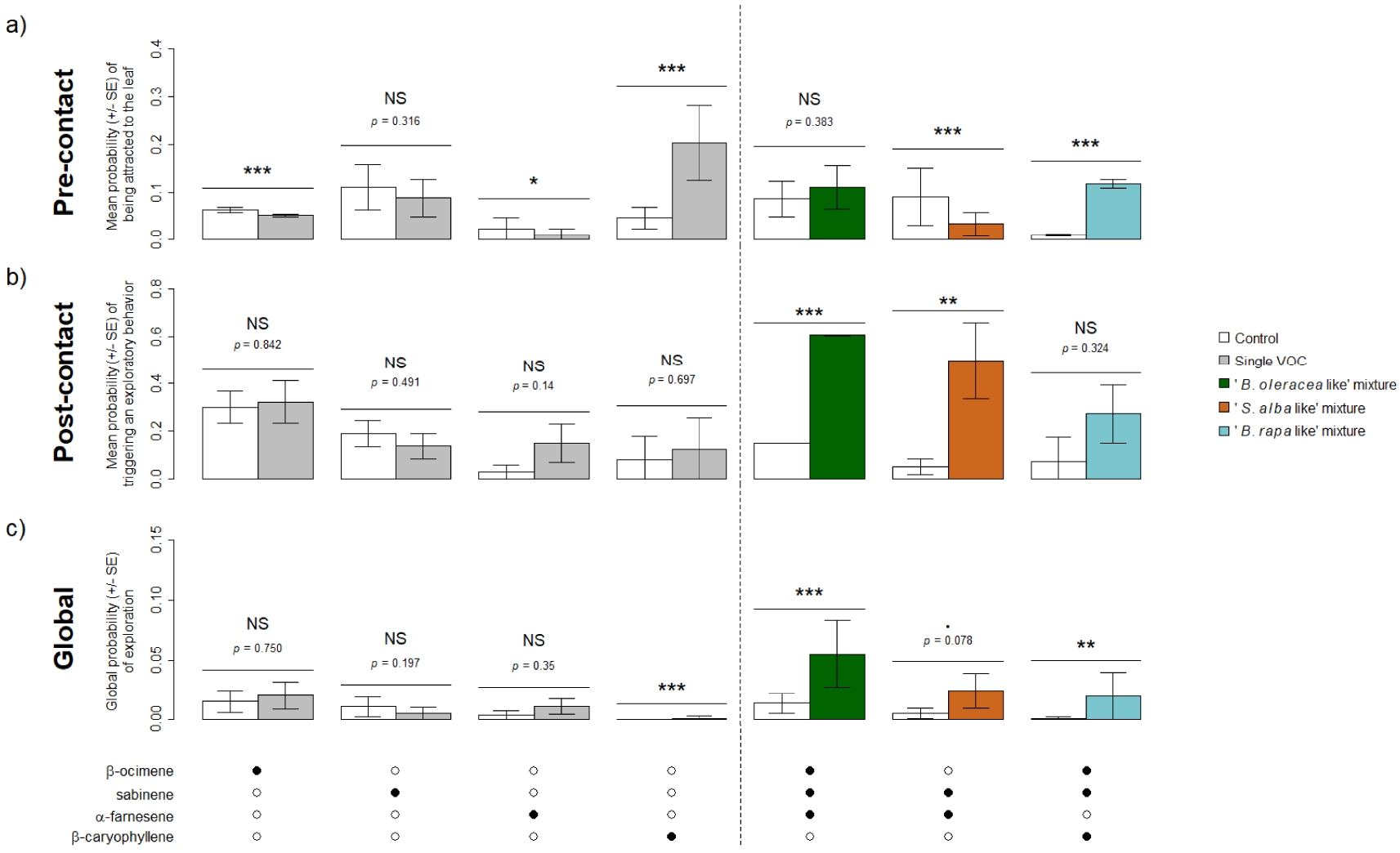
4. Discussion
Plant-emitted VOCs are well known to mediate distance attraction of phytophagous insects [14]. However, much less studied is the fact that VOCs can also act on insect behavior once in contact with the plant (but see [28, 29]). Moreover, when the effect of VOCs on the host selection process is investigated, the distinction between distance and contact effects is only rarely made. The present study highlights the importance of making this distinction by showing that pre- and post-contact effects of VOCs can be independent.
Olfactometer experiments had already shown that D. radicum females are attracted differently to the three plant species tested here [47, 48]. Using GC-MS identification and subsequent multivariate analyses, we show that these species could be distinguished based on their VOC blends, and each characterized by a specific emission profile. These results are in agreement with the literature since species-specific blends are usually observed (e.g. [65, 66, 67]). The specificity of the blends characterized in our study has already been reported [47], and although species-specific mixtures identified here are not exactly similar to those described previously, all VOCs identified here, but the aromatic ethylacetophenone, have already been shown to be emitted by these species [47, 68]. Among the compounds identified in this study, α-farnesene and β-caryophyllene may be involved in distance attraction of females [47]. Indeed, our behavioural observations showed that β-caryophyllene (alone or in combination) induced a significant pre-contact effect, i.e. attraction. Eucalyptol and cis-3-hexenyl acetate, also present in the different volatile profiles but not detected by female antennae in our experiments (but see [64]), reduce and stimulate oviposition, respectively, in field situations [43]. Other volatile sulphur compounds also produced and emitted by Brassicaceae but not present in our blends are also known to affect the female behavior. For example, allyl isothiocyanate is attractive to gravid females and stimulates their oviposition [41, 69], whereas DMDS has a deterrent effect on oviposition [45]. In any case, the specificity of the mixtures emitted by each plant species appears logically as a starting point for phytophagous insects to evolve interspecific discrimination abilities.
Electrophysiological recordings revealed that not the whole blends are detected by females’ antennae, but only four ubiquitous terpenes which combinations are species-specific. While a blend of plant VOCs may contain more than a hundred compounds, electrophysiological antennal recordings have shown that only a subset of these VOCs is usually detected by insects [14], which is confirmed by our results. Among the olfactively active compounds, behavioral experiments showed that β-caryophyllene had an attractive pre-contact effect on D. radicum females, which supports an important role of this compound in plant-insect interactions as it has been shown attractive to a diversity of generalist and specialist phytophagous insects (e.g. [70, 71, 72]). However, it is the only VOC which induced pre- or post-contact effect when applied alone, while all three VOC mixtures had such effect. Indeed, β-ocimene, α-farnesene and sabinene appeared neutral or even repellent alone, whereas their combinations increased the probability to observe an exploration behavior. Consistently, although the behavioral pattern was quite similar between β-caryophyllene alone and the only mixture that included it, the global probability of exploration appeared higher when β-caryophyllene was combined with β-ocimene and sabinene. These results are in line with the many experiments showing that stronger behavioral responses are obtained with specific blends or combinations of VOCs rather than with individual compounds [6]. Furthermore, the striking example of Webster et al. [21, 22, 23] clearly demonstrates that the signaling value of a mixture of VOCs is determined by the whole mixture rather than by the sum of the signaling values of its individual components. Although not all possible combinations were tested in our study, our results clearly support this idea. Nonetheless, further behavioral studies with more realistic concentrations and ratios of VOCs would be necessary to conclude about natural host selection processes in D. radicum, as these two factors are known to be of importance [6, 20, 21, 70].
VOCs have long been recognized as key cues mediating distance attraction in phytophagous insects (e.g. [73, 74, 75]). Their involvement in oviposition stimulation once in contact with the plant has also been shown in several species, although this aspect was much less studied [28, 29, 76, 77]. Our behavioral experiments confirm that in D. radicum, VOCs induce pre-ovipositional exploration behaviors at contact. Moreover, although the global probability of D. radicum to explore artificial leaves, which results from both the distance attractiveness and the ability to stimulate exploration behaviors once in contact, was (nearly) significantly increased by all three VOC mixtures, the effect of these mixtures was strikingly different: for the “B. rapa like” mixture this effect is due to an increased attractiveness, while for the “S. alba like” and “B. oleracea like” mixtures it is due to a higher stimulation of exploration behaviors once in contact with the leaf. This demonstrates the relevance of distinguishing between pre- and post-contact phases when studying the effect of VOCs on host selection processes. Indeed, it shows that a given behavior can be initiated by different and independent mechanisms, although these are mediated by the same kind of plant cues.
In conclusion, this study provides a better understanding of the action of plant-emitted VOCs on the behavior of a specialist phytophagous insect, and confirms the importance of VOC combinations in mediating plant-insect interactions. More importantly, it demonstrates that the effects of these chemical cues may be independent when considered at distance and once the insect makes contact with the plant, and that a given type of cues (here, VOCs) can mediate several aspects of host recognition processes of phytophagous insects.
Conflicts of interest
Authors have no conflict of interest to declare.
Funding
This work was supported by a doctoral fellowship from Université Rennes 1 and a Research grant from the GDR MediatEC, France.
Acknowledgements
We are grateful to Magali Proffit and Florence Nicolè for their precious help in the development of the volatile capture device, and to Nicolas Barthès for his help in GC-MS data analysis. We also acknowledge the GDR MediatEC, which grant partly funded this work.




 CC-BY 4.0
CC-BY 4.0