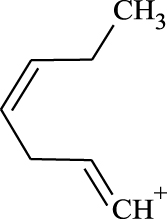
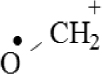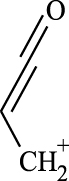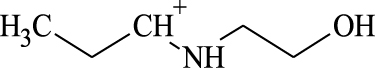1. Introduction
The egg and pupa are critical life stages in holometabolous insects because they are immobile and therefore relatively vulnerable to attack by natural enemies. Eggs generally contain antioxidants or defence molecules and are laid where they are less likely to be found and eaten by predators. However, parental care of hatchings is rare, with a few exceptions (e.g. some Hymenoptera and the burying beetles Silphidae), and care after nutritional independence is not reported other than for vertebrates. Nevertheless, pupae are not defenceless. In Coleoptera, the most diversified group of insects, pupae have evolved several physical, behavioural and/or chemical strategies that enable them to avoid being attacked [1].
The Coccinellidae are a large family in the order Coleoptera with a worldwide distribution, comprising two sub-families, 38 tribes and about 6000 described species [2]. Paradoxically, although there has been a longstanding research interest in this group (e.g. review by [3, 4]), there is no systematic study of the ecology and defence strategies of ladybird pupae. There is only one comparative analysis of the morphology of pupae [5], which includes 32 genera and 57 species of North American ladybirds. However, this seminal work does not refer to any structural or other mechanism of defence. Apart from that study, the information on pupae is scattered, although it could be valuable for phylogenetic studies, similar to those based on larval characters (e.g. [6]). Not surprisingly, the absence of information leads to conflicting views on pupal defences.
The available information on the subject refers to several ladybird pupal enemy avoidance strategies involving physical and behavioural mechanisms (Table 1). However, the Coccinellidae are mainly known for their chemical defences. A wide range of molecules have been identified, among which there are several alkaloids (reviewed in [7]), including those derived from fatty-acids. Many pupae, like the other ladybird stages, are chemically protected by an aposematic system associating alkaloids in the haemolymph with bright and contrasting colours and/or strong odours. In addition, all developmental stages reflex bleed when attacked, instantaneously expelling droplets through the femorotibial articulations or dorsal glands that contain high concentrations of toxic or repulsive alkaloids [3].
Defence strategies of ladybird pupae
| Strategy | Taxa | Reference |
|---|---|---|
| Gin traps | Coccinellini | [8] |
| Coccidulini and Chilocorini | [3] | |
| Rhyzobius chrysomeloides (Herbst) | (∗) | |
| Pupa enclosed in larva exuvium but visible through dorso-longitudinal slit | Chilocorini | [8] |
| Rodolia spp, Anovia circumclusa (Gorham) | [9] | |
| Hyperaspis maindroni Sicard | [10] | |
| Thalassa saginata Mulsant | [11] | |
| Pupa enclosed in waxy [12] larva exuvium | Azya spp | [13] |
| Hyperaspis silvestrii Weise | [14] | |
| Cryptolaemus spp, Nephus spp | (∗) |
(∗) Authors pers. obs.
Furthermore, several species of the tribe Epilachnini [15], as well as Delphastus catalinea (Horn) of the tribe Serangiini [16], Platynaspis luteorubra Goeze of the tribe Platynaspidini [17], and Nephaspis oculatus (Blatchley), Scymnus sinuanodulus Yu and Yao [18, 19], S. ningshanensis Yu and Yao [20] and S. posticalis Sicard [21] belonging to the tribe Scymnini, have a more specific chemical defence. In these species, the larval exuviae folds back and total or partially uncovers the pupa. The pupal cuticle that is visible bears setae on the tips of which droplets can be seen. Behavioural experiments indicate that these droplets are extremely efficient in deterring attacks by ants [16, 21, 22]. For species of Epilachnini, the droplets are reported to contain molecules of the azamacrolides family [7] as well as tocopheryl acetates [23, 24]. The droplets produced by D. catalinea contain polyketides [16]. For the remaining species, the nature of the chemicals in the droplets is unknown.
The present study investigated the chemical defence of the pupae of five species of Scymnus. One of the objectives was to determine whether pupae of all the five species produce droplets. Another was to determine if droplet production by the insect pupae is influenced by larval diet or is of autogenous origin. Finally, based on the annotation of GC-MC analyses, the chemical composition of the droplets produced by Scymnus nubilus Mulsant is examined.
2. Materials and methods
2.1. Rearing methods and comparison of the pupae of different species of Scymnus
Adults of S. interruptus (Goeze), S. rubromaculatus (Goeze), S. apetzi Mulsant, and S. subvillosus (Goeze) were collected near Toulouse (France), and S. nubilus in the Azores (Portugal). These species were chosen because of their availability.
Stock cultures of all five species were maintained in the laboratory for at least 2 generations (around 23 days from egg to adult stage) before the study was initiated. Rearing conditions were LD 16:8 and 22 ± 1 °C. Ladybird adults were kept in 2-L plastic boxes with a piece of corrugated filter paper and were fed three times a week, ad libitum, with either Acyrthosiphon pisum (Harris) or Rhopalosiphum padi L, common prey of Scymnus spp. (e.g. [25]). Two stems of broad bean, Vicia faba L. or Triticum turgidum L. were added to the boxes to improve the survival of A. pisum or R. padi, respectively. Adult ladybirds were transferred to new boxes every 2 weeks and the eggs and larvae that remained in the old boxes were reared in similar conditions to the adults (see above). For each species, all the fourth instar larvae available in the stock cultures were collected and isolated in 3 cm Petri dishes, kept at the same temperature and photoperiod as the stock cultures and fed three times a week an excess of aphids according to the diet treatment. On reaching the pupal stage, they were observed daily to check for the presence of droplets. In addition, pupae were checked for the presence of wax that typically covers the larvae of Scymnus [12] and some of which can be present on the pupae.
2.2. Collection of droplets from pupae of Scymnus nubilus
The mean diameter of the droplets on the tips of setae on the pupae of S. nubilus is about 50 μm and they were collected using Solid Phase Micro-extraction (SPME) fibers (50/30 μm DVB/CAR/PDMS, Stableflex 24Ga, Supelco, Bellefonte, PA, USA). This enabled us to exclusively analyse droplets without collecting other cuticular compounds.
The fibers were conditioned before use by insertion in the GC-MS injector port for 1 h at 270 °C, to remove contaminants that might result in a high background reading in the chromatogram. They were then gently rubbed over the tip of the setae to collect the droplets. The droplets of 45 pupae were collected. For pupae with a dense covering of setae, it is difficult to determine exactly how many droplets were collected per individual; each fiber was loaded with the droplets from 15 pupae and therefore 3 fibers (samples) were used for analyses. Simultaneously, a blank test to check for possible laboratory environment contaminations was performed by leaving another fiber close to the stereomicroscope. Each fiber was then injected directly in a GC-MS.
2.3. GC-MS analysis of droplets collected from pupae of Scymnus nubilus
Analyses were done on a two-step basis. Firstly, two fibers were injected into a low-resolution mass spectrometer quadrupole detector (ISQ QD) coupled to a Trace 1300 gas chromatography (Thermo Fisher Scientific Inc., Illkirch, France) fitted with an apolar capillary column (Restek RTX-5MS 30 m × 0.25 mm, 0.25 μm film thickness, 5% diphenyl and 95% dimethylpolysiloxane) and a splitless injector (280 °C). Then, a third fiber was injected into a high-resolution GCT Premier TOF mass spectrometer (Waters, Manchester, UK) coupled to a gas chromatography 6890 series (Agilent Technologies, Palo Alto, CA, USA) fitted with a similar capillary column to the one described above and a splitless injector. This high-resolution MS enables the determination of the exact mass of the molecular ion and fragments, giving additional information on the elemental composition that is used to improve the annotation of the chromatographic peaks. In both GC-MS, ionization was done by electron ionization (70 eV, source temperature 200 °C). The mass spectra were scanned from m/z 20 to m/z 700. Helium was the carrier gas (1.2 mL⋅min−1). The initial oven temperature was set at 50 °C for 1 min. Then, the temperature was increased by 20 °C⋅min−1 to 140 °C, then by 3 °C⋅min−1 to 300 °C. This final temperature was held for 3 min. Prior to each injection of a fiber loaded with droplets, a control empty fiber, i.e. exposed to the same environmental conditions, was injected.
The purity of the chromatographic peaks was checked with the help of the Mass Spectral Deconvolution and Identification System program (AMDIS 2.70). For each of the fractions the most likely elemental composition was calculated [26]. The limits of each fragment were fixed as follows: C—from 1 to 50 atoms; H—from 0 to 100 atoms; O—from 0 to 7 atoms; N—from 0 to 3 atoms. All data was processed using MassLynx software (Dat version 4.0, Waters SAS, Guyancourt, France). Accurate masses were calculated and used for the determination of the elementary composition of analytes. Moreover, the Kovat’s retention indices were calculated using [27] and by comparing the peaks to an external standard consisting of a solution of n-alkanes (n-C7H16–n-C40H82) of known concentrations. The degree of unsaturation was used to determine how many rings and/or double bonds are present in a compound [28]. The structure of each fragment ion was identified using (i) the exact mass of the fragment in high resolution (ii) the most common fragment ion tables [29], and (iii) the ACD/MS Fragmenter software (2016, 1.1, Advanced Chemical Development, Toronto, ON, Canada).
3. Results
3.1. Observations on the pupae of five species of Scymnus
Observations of the droplets (Figure 1) produced by the pupae of 5 species of Scymnus whose larvae were reared on two different diets are presented in Table 2. These results indicate that all of these species of Scymnus can produce droplets, but their distribution on pupae varies among species with three main patterns: (a) all pupae present droplets and there are droplets all over them, (b) most pupae don’t produce droplets and, when they do, these are present on the pronotum and the rest of the body is partially covered by the exuvium of the last larval instar, and (c) all pupae produce droplets, which are present on the pronotum and the rest of the body is not covered by the exuvium of the last larval instar. The 40 individuals of S. apetzi reared on the different diets did not differ in droplet production, but there are insufficient results for the remaining species to make it a general assertion.
Photograph on the left, dorsal view of a pupa of Symnus nubilus, and on the right, a high magnification photograph of glandular setae on the dorsal integument of a pupa.
Distribution of droplets and presence of wax on pupae of five species of Scymnus when fed on two different aphid diets (Acyrthosiphon pisum or Rhopalosiphum padi)
| Species | Diet | Distribution of droplets and presence of wax | |
|---|---|---|---|
| A. pisum | R. padi | ||
| S. nubilus | 20/20 | – | Large droplets, all over the body |
| S. apetzi | 20/20 | 20/20 | Large droplets, all over the body |
| S. interruptus | 3/22 | 0/10 | Droplets, when present, small and on the pronotum Wax from the last larval exuviae partially covering the body |
| S. rubromaculatus | ∗ | 14/32 | Droplets, when present, small and on the pronotum Wax from the last larval exuviae partially covering the body |
| S. subvillosus | 2/2 | 7/7 | Small droplets present on the pronotum |
Number of individuals with droplets divided by the total number of individuals examined. ∗ Non-accepted prey; – rearing not done.
3.2. Chemical analyses of the droplets produced by pupae of Scymnus nubilus
Figure 2 shows the chromatographic profile of a direct injection of the pupal secretions from a SPME fiber, obtained using the high-resolution GC-MS. This analysis reveals two main peaks at retention times of 24.99 and 28.83 min, which make up 87% of the total area of all the peaks. Analysis of these two major peaks reveals the absence of co-elution, indicating that they are each for a single compound. The small peaks from 0 to 24 min are mainly hydrocarbons and siloxanes eluted from the chromatographic column during the temperature gradient.
A high resolution GC-MS chromatographic profile of the droplets collected from glandular setae on the pupa of S. nubilus.
Analysis of the mass spectra of the two major peaks (Figure 3) indicates a similar fragmentation pattern with ion signals at m/z 55, 69, 81, 83, 95, 102, 123, 210, 240 and 268. In addition, the mass spectra of the two major peaks show a loss of 29. Nonetheless, the peak with a retention time of 24.99 min exhibits a molecular ion at m/z 478.37, while for the peak with a retention time of 28.33 min a molecular ion at m/z 506.40 is detected. Although the two mass spectra show a difference in the intensity of the ion at m/z 268, this can be explained by the difference in the size of the carbon chain [22] and the two peaks can therefore be considered as belonging to the same chemical family.
Mass spectra of the two major chromatographic peaks of the secretion produced by glandular setae on the pupae of S. nubilus. 1 = mass spectra of the peak with retention time 24.99 min; 2 = mass spectra of the peak with retention time 28.83 min.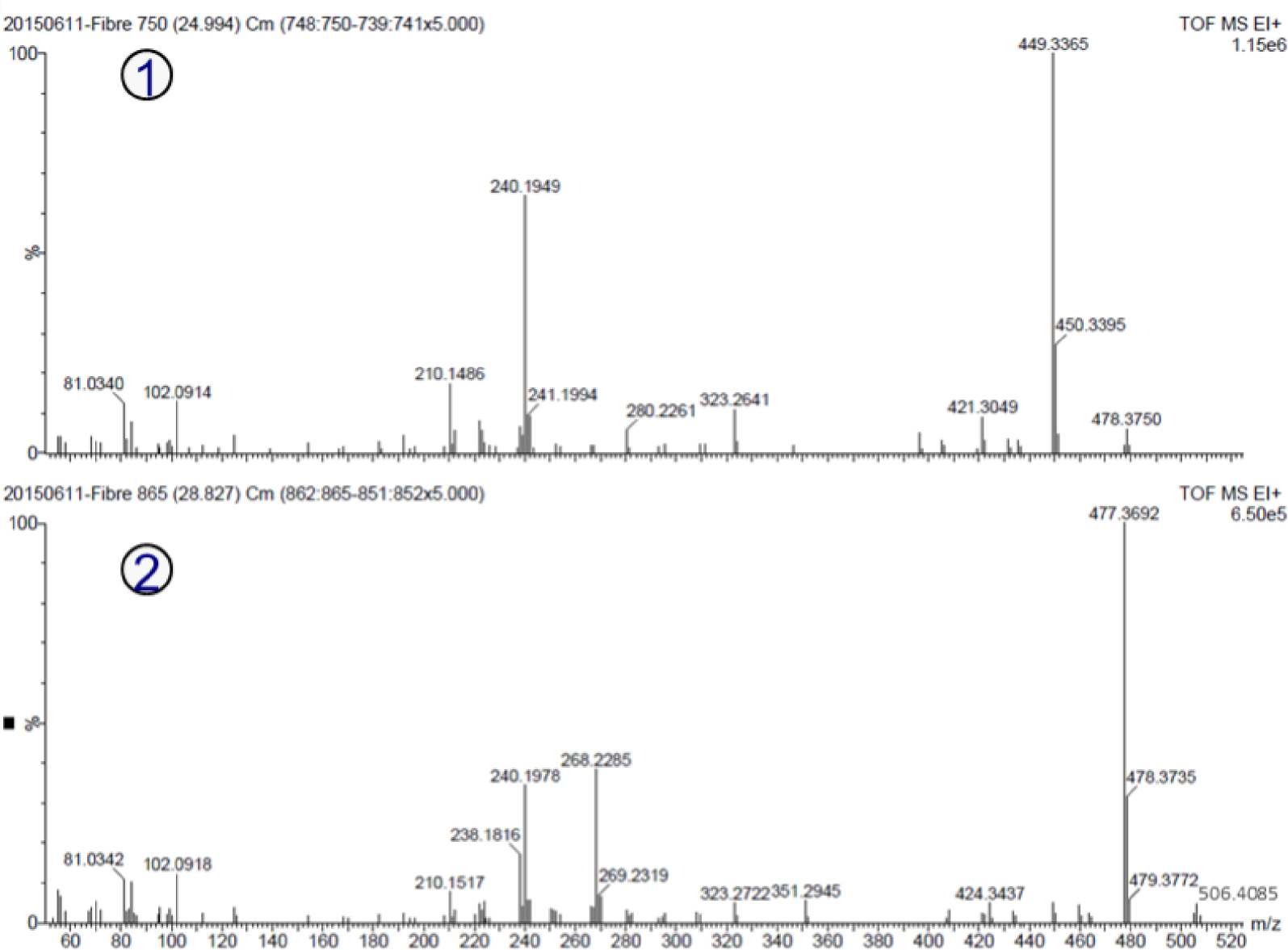
Table 3 shows the m/z values and chemical formula/possible structures of each ion detected by high-resolution GC-MS. According to the accurate mass measurement, all ions are composed only of carbon, hydrogen, oxygen and nitrogen. The proposed chemical formulas for the ion signal at 24.99 min and 28.83 min are C28H50N2O4 and C30H54N2O4, respectively. The two chemical formulas have five degrees of unsaturation, which may indicate the presence of double bonds and/or the presence of a ring. The difference between the two molecules can be attributed to a C2H4 increment typical of an increment in a fatty acid chain.
m∕z values and some structures of major fragments of the mass spectra of the two major compounds in the secretion of the glandular setae on pupae of S. nubilus (cf. Figure 2)
Based on the elementary composition and with the help of the ACD Fragmenter software and the most common fragment ion table, it is reasonable to propose the structure of fragment ions below m/z 110 (Table 3). The following identification of the unsaturation sites is proposed: (i) Fragment at m/z 69.0997, present in both compounds (C28H50N2O4—24.99 min; C30H54N2O4—28.83), can be attributed to C5H indicating the presence of C=C double bond; (ii) The two compounds present a fragment ion at m/z 30.01 related to a carbonyl function C=O; (iii) a neutral loss of 29 amu from both molecular ions is detected. This can be attributed to an ethyl group −CH2–CH3 leading to the fragment ion at m/z 449.33 for the peak at 24.99 min, and at m/z 477.36 for the peak at 28.83 min.
The above results confirm that the two major ion signals on the chromatogram belong to the same chemical family. As it was not possible to exclude the presence of small quantities of other molecules of the same chemical family in the sample, a more thorough analysis of the chromatogram was carried out focused on the three fragment ions at m/z 102, 210, and 240, respectively. The low-resolution GC-MS chromatograms were used for this analysis because the instrument has a higher sensitivity in terms of signal-to-noise ratio (S/N) compared to the high-resolution GC-MS. The limit of detection was set at a S/N of 10:1. This enabled the detection of four smaller chromatographic peaks (Figure 4), whose mass spectra are presented in Figure 5. The spectra are characteristic of ions from the same molecular family according to the intense common fragment ion at m/z 240 but are differenced by the number of insaturations (difference of 2 mass units). The signal-to-noise ratio for these peaks was higher than 25 indicating they cannot be interpreted as noise. They all present similarities in their fragmentation profiles indicating that all 6 peaks belong to the same chemical family. The relative amounts of these peaks (for a total area of 100% under those peaks) were 74.71, 23.7, 0.61, 0.59, 0.25, and 0.14%, respectively, for peaks 1 to 6.
Low resolution GC-MS chromatographic profile (scan filter m/z 240, 210 and 102) showing the six compounds in the droplets produced by the glandular setae on the pupa of the ladybird S. nubilus.
Mass spectra of the four minor chromatographic peaks of the secretion produced by the glandular setae on the pupae of S. nubilus. 3, 4, 5 and 6 are the mass spectra of the chromatographic peaks in Figure 4.
4. Discussion
Species of Scymnus are distributed worldwide and are very common in guilds of aphid and coccid predators (e.g. [30]). However, they are small and dull coloured, which probably explains why there is so little information available on their chemical ecology and life history in general, compared to ladybirds in some other tribes [25].
Pupae of the Scymnus are covered in setae with droplets of defensive compounds at their tips. In this paper, the chemical defences of the pupae of five species of Scymnus were studied. The production and distribution of droplets on pupae was described and the chemical composition of droplets produced by pupae of S. nubilus was determined.
Droplets were produced by the pupae of all the species studied and in addition another 3 Scymnus species are reported producing droplets in the literature [18, 19, 20, 21]. Therefore, it is a widespread phenomenon in this genus. It is also noteworthy that the distribution of droplets on pupae of the 5 species varied, with three different patterns corresponding to the three clades: S. apetzi/S nubilus, S. interruptus/S. rubromaculatus, and S. subvillosus. These clades correspond to the species phylogenetic relationships highlighted by Magro et al. [31] and presented in Figure 6. These patterns do not seem to be related to the distribution of setae, but might be related to their morphology, which was not studied.
Phylogenetic relationships of the 5 species of Scymnus studied, based on Magro et al. [31].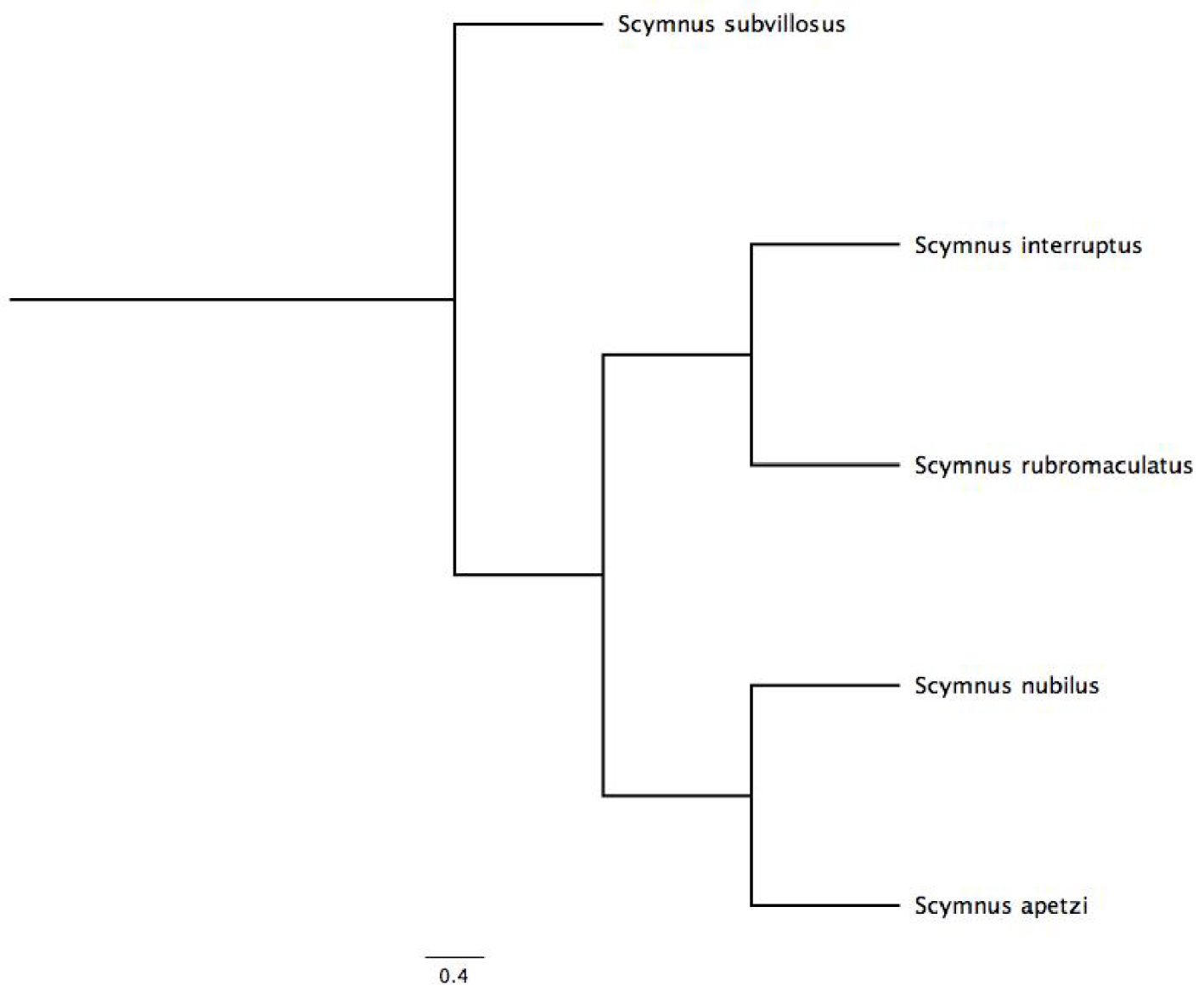
Diet does not seem to influence droplet production, suggesting that Scymnus make a de novo synthesis of the compounds in the droplets, which is generally the case for the defensive compounds in the Coccinellidae [32], but this needs to be verified. Furthermore, the chemical composition of droplets produced by pupae given different diets was not analysed, which could be interesting as there is a putative case of a ladybird sequestering defensive chemicals, and an additive effect of autogenous defences and chemicals sequestered via certain foods cannot be excluded [33].
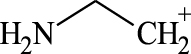
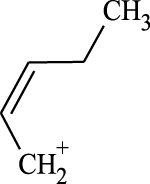
The GC-MS analyses of the chemical composition of S. nubilus droplets indicated the presence of 6 compounds, the two major ones corresponding to C28H50N2O4 and C30H54N2O4 elemental compositions. Four characteristics of these molecules are important: (i) presence of oxygen, (ii) for every two molecules of nitrogen there are 4 molecules of oxygen, (iii) there are five degrees of unsaturation related to C=C and C=O double bonds and (iv) the compounds are volatile (low polarity) in the GC-MS without the need of derivatization, indicating that the nitrogen atom probably lies within a ring. As the volume of droplet liquid available is very small it is not possible at this stage to carry out further analyses, such as purification and complete structural identification by NMR and circular dichroism. Searches in the Pherobase database [34], however, indicate the azamacrolides family, i.e. fatty acid-derived alkaloids (Figure 7). Moreover, the chemical analyses of this study may have missed some compounds because GC-MS cannot detect chemicals with high boiling points and there are some reports of such high-boiling chemicals in the droplets produced by pupae of ladybirds (e.g. [35]). Thus, an analysis of droplets using HPLC-MS is needed, which will require much larger volumes of droplet liquid than available in the present study.
(A, B) Mono-Azamacrolides produced by Epilachna varivensis [22]; (C) di-Azamacrolide produced by Epilachna borealis and Subcoccinella vigintiquatuorpunctata [35]; (D) structure suggested in this study for those produced by pupae of Scymnus nubilus, where R1 and R2 are two CH3–CH2-ethyl chains, R3 and R4 are two mono-unsatured chains, and R5 and R6 a methyl or ethyl chain.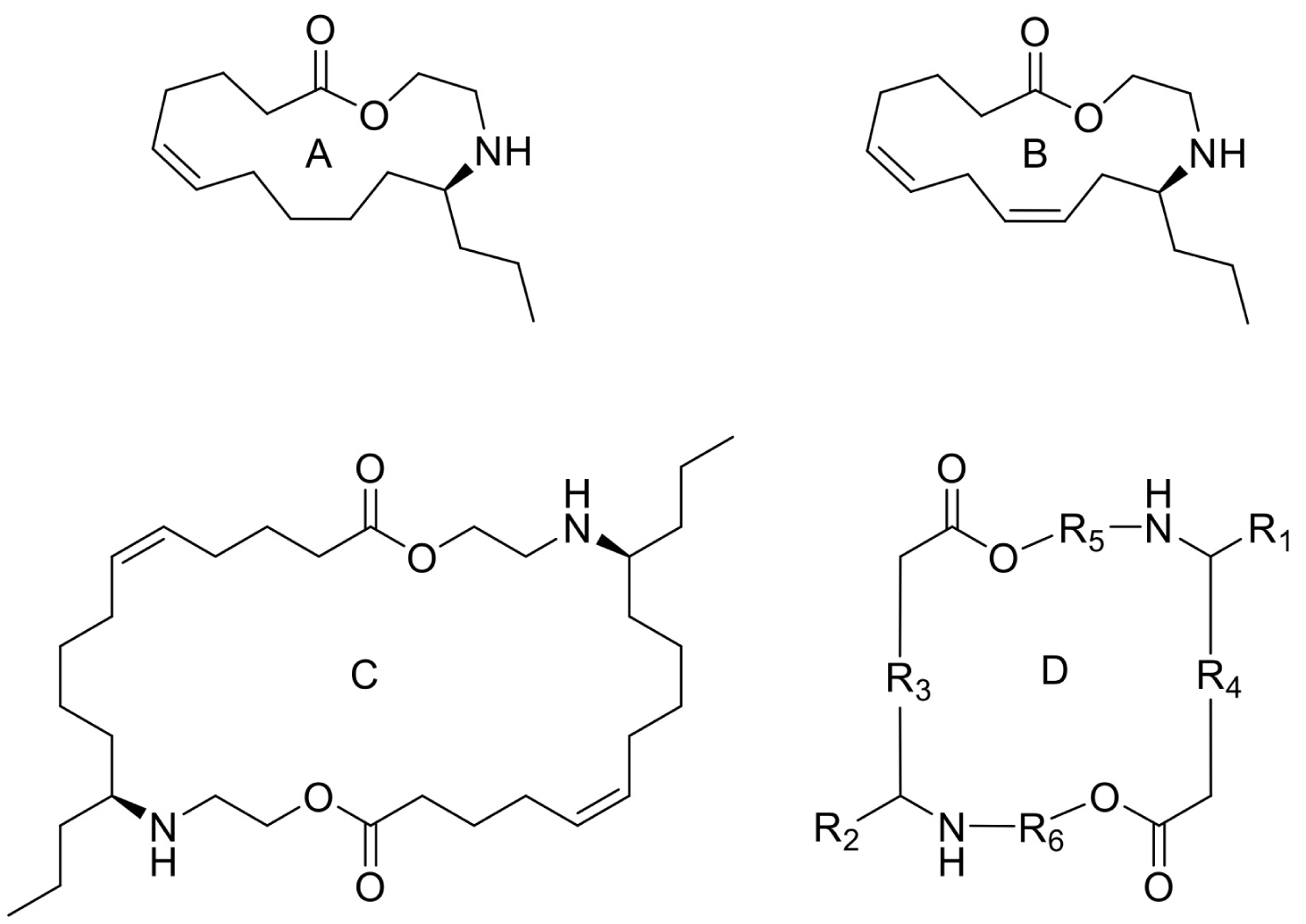
It is noteworthy that azamacrolides are present in the liquid on the tips of setae on pupae of the ladybird tribe Epilachnini [15, 22]. Comparison of the mass spectra of the liquid collected from S. nubilus with that of the mono-azamacrolide epilachnene reported by Attygalle et al. [22] for Epilachna varivensis Mulsant (Figure 7A, B) revealed they both have six fragment ions at m/z 44.093, 55.0176, 55.0540, 69.0997, 84.0816 and 95.0858. The mono-azamacrolides, however, only have three degrees of unsaturation whereas in the case of S. nubilus it is five degrees of unsaturation. Moreover, the ions at m/z 268 and 240 are complementary and the sum of the two fragments leads to the molecular ions at m/z 478 ( = 240 + 240 − 2) and m/z 506 ( = 268 + 240 − 2) indicating the presence of a macrocycle resulting from the condensation of two subsequent molecules. Thus, it is likely that S. nubilus produces di-azamacrolides as it is the case for the Epilachnini Subcoccinella vigintiquatuorpunctata (L) (Figure 7C, D). A full comparison is not possible as there are no GC-MS mass spectra of di-azamacrolides. The lack of available reference compounds remains a problem for studies on natural product for which the size of the sample available is very small.
The study by Seago et al. [6] based on the simultaneous analysis of molecular data and morphological characters of both ladybird adults and larvae shows that an integrative approach can be important to resolve evolutionary relationships within the Coccinellidae. Nevertheless, the evolutionary history of the Coccinellidae is blurred by rapid radiation and inferring the major relationships of the ladybird tribes remains a challenge [2, 36]. As specialized metabolites have been used in studies on many taxa as complementary tools to traditional morphological and gene-based phylogenetic exploration [37], we recommend additional studies on the defence traits of ladybirds to help elucidate the internal phylogenetic relationships within the Coccinellidae.
Conflicts of interest
Authors have no conflict of interest to declare.
Acknowledgements
We thank G. Espinasse and J.-F. Garrigues for technical support in collecting droplets and rearing the insects. We are also grateful to J. Bouajila for helpful comments on an early version of the manuscript. Finally, we would like to thank T. Dixon who assisted with manuscript editing. The laboratory “Evolution et Diversité Biologique” is part of the “Laboratoire d’Excellence” LABEX TULIP (ANR-10-LABX-41) and LABEX CEBA (ANR-10-LABX- 25-01).




 CC-BY 4.0
CC-BY 4.0