1. Introduction
Layered (or lamellar) double hydroxides (LDHs)-type materials are also known as anionic clays or hydrotalcite-like compounds and have attracted a lot of attention in recent years. The mineral hydrotalcite [Mg6Al2(OH)16](CO3)⋅4(H2O) was the first LDH to be identified in Sweden in 1842 [1]. Because of its water content (hydro), and similarity to talc (talcite), the substance was named hydrotalcite [1], which typically exists in nature under the form of sheets or as a fibrous mass and can also be easily manufactured. The general formula for LDHs is:
Figure 1 shows the schematic of the LDH structure. The metallic active site can either be confined at the intermediate levels of the double-layer structure or disseminated uniformly within the layered structure by cation substitution. Bivalent and trivalent cations M(II) and M(III) create a positively charged layer when they are octahedrally coordinated into six hydroxyl groups. Divalent cations (M2+ = Zn2+, Ni2+, Cu2+, Co2+, etc.) and trivalent cations (M3+ = Fe3+, Cr3+, etc.) can either partially or completely replace Mg2+ and Al3+ respectively in the hydrotalcite structure [2]. Anions (An−) and water molecules form interstitial layers, which neutralize the metal hydroxide layers’ positive charge. There are no strict restrictions on the nature of the interlayer anions. For example, simple inorganic anions, such as carbonate, and nitrate [3] can be used as interlayer anion, as well as organic anions, such as terephthalate, acrylate, and lactate [4]. Furthermore, coordination compounds, polyoxometalates, and biomolecules like nucleoside monophosphates have been successfully intercalated [5].
Schematic structure and different preparation methods of LDHs.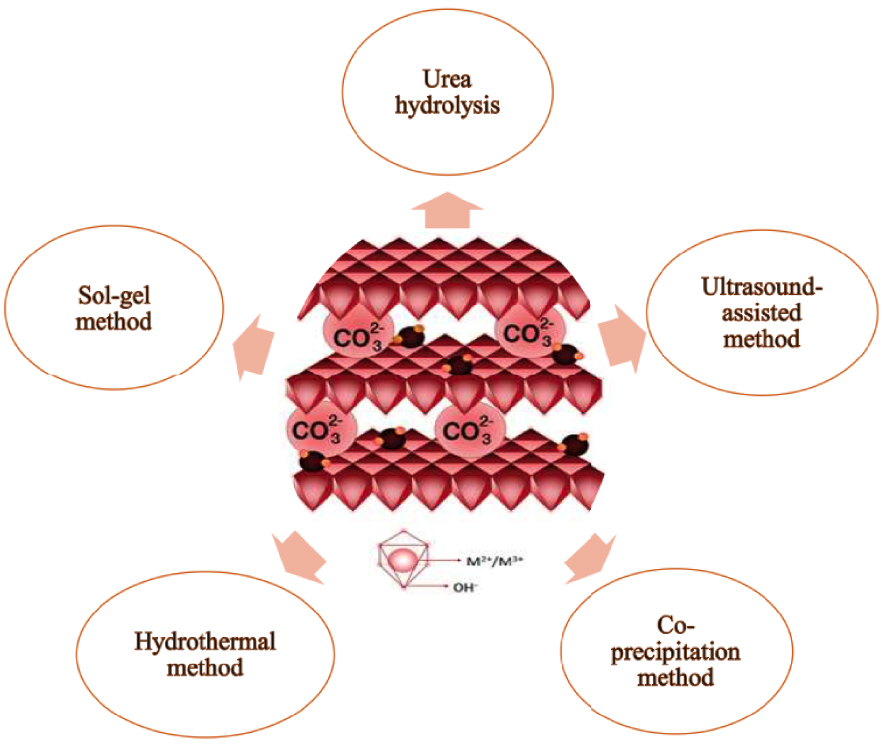
Due to their adaptability, compositional flexibility, fundamental makeup, enormous surface area, redox characteristics, and biocompatibility [6], LDHs received extensive academic research for use in practically all scientific domains such as catalysis [7], wastewater treatment [8], drug delivery [9], biomedicine [10]. LDHs can be used as synthesized maintaining their lamellar structure [11], as catalyst support [12] and as catalyst precursor [13]. The LDH precursors allow to design a variety of catalysts including (1) homogenized mixed oxides when the LDH structure is calcined, and (2) reconstituted LDH obtained from the rehydration of mixed oxides with anionic solutions [14].
Oxides produced by calcining LDHs exhibit a wide range of characteristics, uniform elemental dispersion, and persistence even after thermal treatments or reduction. They have been used as catalyst in different reactions, such as methanation, dry reforming of methane, total oxidation of volatile organic compounds (VOCs), hydrodesulfurization of fluid catalytic gasoline cracking, NO reduction, and removal of SO2 and NOx [15]. There are several traditional methods to prepare LDHs including urea hydrolysis, sol–gel method, hydrothermal synthesis, and co-precipitation [16]. Nowadays, research is still being conducted to develop materials with increased surface areas, smaller particle sizes, and tunable pore diameters.
Among the existing synthesis routes, ultrasound (US) technology has gained a lot of attention due to some advantages including the ability to create high-temperature and high-pressure regions through the formation and collapse of microbubbles (cavitation), increase mass transfer processes as a result of system pressure variation, and apply large amounts of vibrational energy to small volumes with little heating [17]. The combined consequences of those three processes induce a reduction of synthesis time, enhanced solid textural characteristics, and greater and stronger active basic sites [17]. In order to highlight the interest of ultrasound-assisted synthesis (UAS) of LDHs, we summarize the most recent advances in the synthesis of LDHs with ultrasound-assisted methods (including co-precipitation, urea hydrolysis, hydrothermal and US-assisted methods) and their parameters. Finally, we will consider the effect of ultrasounds in the application of these materials in different fields.
2. Layered double hydroxides preparation methods
2.1. Urea hydrolysis
Urea (CO(NH2)2) is a weak Bronsted base that hydrolyzes slowly and is easily soluble in water. In the production of LDHs, urea can be utilized as a substitute precipitating agent. In comparison to Na2CO3, urea has the advantage of hydrolyzing slowly, resulting in a gradual degree of supersaturation during precipitation. It is very soluble in water and can produce ammonium cyanate or its ionic form (1) (, NCO−) through controlled hydrolysis in aqueous solutions. Prolonged hydrolysis produces in basic medium (2) or carbonic acid H2CO3 in acidic medium, eventually evolving to CO2 (3) according to the following reactions [18]:
| (1) |
| (2) |
| (3) |
The simplicity of washing is another benefit of employing urea. Due to the predominance of charge-balancing as a by-product of urea decomposition, the biggest disadvantage of urea hydrolysis is the inability to select the major counter ion intercalated in the LDH structure [19]. Urea hydrolysis becomes a simpler process than co-precipitation if is the desired intercalated anion as neither extra NaCO3 nor strict atmospheric control are needed [20].
2.2. Sol–gel method
The sol–gel method allows for the synthesis of very pure LDHs [21]. In this process, room-temperature water or organic solvents are used to hydrolyze the metal precursor salts or their organic constituents. These materials are sometimes made soluble using a different solvent. It is necessary to add either an acid or a base to the hydrolysis solution to speed up the precipitation process to produce highly distributed metals in the solution. Detailed work on the synthesis of magnesium and aluminum LDH by the sol–gel method was performed by Prinetto et al. [22]. The composition of the precursors, temperature, and aging time have direct effects on the structural properties of LDHs [23]. For instance, it is possible to enhance the surface area or particle size of synthetic LDHs by lowering the reaction temperature [21]. This preparation method has a long processing time [24].
2.3. Hydrothermal method
Typically, this synthesis is carried out in autoclaves by starting with a basic solution containing the metals that would make up the LDH and using the autogenous pressure produced by heating at temperatures between 60 and 200 °C. Aging is the same process but when the temperature is lower than 60 °C. The combination is held in hydrothermal conditions for a period of time that could range from hours to many days. Using hydrothermal techniques, it is possible to create bigger particles with more crystallinity [2]. For instance, the conditions to obtain the largest crystals of magnesium and aluminum LDH (1653 Å) are 200 °C for 24 h [25].
2.4. Co-precipitation method
The technique with the highest usage rate for producing LDHs is co-precipitation. By evaporation or by altering the solution’s pH, inorganic salt solutions are supersaturated with an alkaline solution in this approach. The degree of solution supersaturation affects the structural morphology and particle size [26]. Not all ions will precipitate out of the solution at low pH levels, and metal ions may dissolve at very high pH levels. It is important to choose the saturation pH carefully, which is determined by the cation types employed [21]. This approach involves progressively adding an aqueous solution of bivalent and trivalent metal salts into an aqueous solution of the desired interlayer anion (usually carbonates), along with an alkaline solution with a set pH to promote precipitation. By altering the ratio of metal salts, it is simple to change the proportion of divalent and trivalent ions in the hydroxyl layers when using this method [27]. The type of anions and cations, pH, the ratio of different metals, temperature, and aging time all have a significant impact on how the metal-LDH material precipitates. One of the major drawbacks of this method is the difficulty to control the size of precipitating particles and their agglomeration [28].
2.5. Ultrasound-assisted synthesis (UAS)
US irradiations have been recently used during the co-precipitation method either in the maturation phase [29] or from the beginning of the co-precipitation, instead of magnetic stirring [30, 31]. When synthesizing materials like LDHs, the medium is heterogeneous, constituted from a liquid–solid phase, causing a violent jet of liquid to emerge towards the surface. Hence the name of asymmetric cavitation whose effects cause surface cleaning, destruction of limit layers and enhancement of mass and heating transfer [32]. This last effect is due to turbulent mixing and acoustic streaming. The collision of microjets on the surface creates erosion and defective sites and consequently leads to particle fragmentation. In addition, US causes dispersion of small groups of LDH layers and reduce agglomeration during the crystal growth step, giving rise to small crystal sizes. Consequently, the enhancement of chemical reactions in a liquid–solid system is due to interparticle collisions generation and to increase in specific surface area by fragmentation of friable solids [33].
Data published on LDH materials prepared by US-assisted methods are summarized in Table 1. In most papers, the technology used is an ultrasonic bath, which is mainly employed for cleaning laboratory instruments and in analytical grade solvent degassing. Transducers are located at the bottom of the tank, which limits cavitation activity. Consequently, in this device type, the acoustic field generated is not homogeneously distributed in the total volume [34, 35]. In comparison to other US devices, the power density generated by a bath is very low [36]. In order to increase the acoustic density in the medium, some authors have worked LDH synthesis using an ultrasonic horn [37]. This device is a direct irradiation method because the horn is partly immersed in the reaction medium and can produce a high quantity of power in a small volume. Regarding the operational parameters, it is interesting to note that the authors of different articles describe them in an incomplete way which suggests that they have little knowledge of US technology. The US frequencies used are between 20 to 80 kHz, which means that mechanical effects are preferentially sought for the synthesis of LDH materials. However, we need a study on the synthesis of LDH materials at high frequency (>100 kHz) in order to analyze the impact of chemical effects of US on the physicochemical properties of these materials. This parameter may play an important role in the synthesis of LDHs, since as shown in Table 1 there are no studies on the effect of this factor on the characteristics of the resulting material. The irradiation time varies from 10 to 600 min but the delivered powers are often omitted or described without indication of volume which does not allow access to power density. Another important factor mentioned in most papers is temperature. We observe that temperatures vary between room temperature (25 °C) and 65 °C. Only three papers have applied a temperature higher than 65 °C. This temperature range is considered low compared to the temperatures used in several conventional methods. This factor is one of the advantages of using US in the synthesis of inorganic materials.
Published articles on LDHs prepared by UAS
| LDH | Device | Frequency (kHz) | Power (W) | Time (min) | Temperature (°C) | Application | Reference |
|---|---|---|---|---|---|---|---|
| Co–Al | Horn | 20 | — | — | 25 | Toluene oxidation | [31] |
| Mg–Co–Al | Bath | — | 100 | — | 80 | Water treatment | [38] |
| Mn–Ni | Horn | 20 | 100 | 150 | — | Supercapacitor | [39] |
| Ni–Al | Bath | — | — | 60 | 65 | Removal of the reactive azo dye (Remazol Brilliant Violet RBV-5R) | [40] |
| Mg–Al diacid | — | — | — | 60 | — | Intercalation of diacid in LDH | [41] |
| Zn–Mg–Al | Bath | 50 | — | 30, 120, 600 | 25 | LDH activated by ultrasounds induced reconstruction | [42] |
| Fe–Al | Bath | 80 | 160 | 15, 30, 60, 90 | 65 | Reduction of bromate | [43] |
| Ca–Al | Bath | — | — | 40 | 55 | Adsorption of U(VI) and Cr(VI) | [44] |
| Mg–Al | Horn | — | — | — | 0, 10, 25, 50 | Knoevenagel and aldol condensations | [30] |
| Mg–Co–Al | Bath | 42 | — | 90 | 65 | Hydrogen production by oxidative steam reforming of ethanol (OSRE) | [45] |
| Ni–Mg–Al–Ce | Bath | — | — | 260 | 25, 60, 90 | Incorporation of cerium in NiMgAl LDH | [46] |
| Ni–Co–Mg–Al | Bath | 50 | — | 60 | — | Oxidative steam reforming of ethanol (OSRE) | [29] |
| Co–Mg–Al | Bath | 50 | — | 10 | 25 | VOCs oxidation | [47] |
| Ni–Al | Bath | 45 | 200 | — | 50 | Carbonyl sulfide removal | [48] |
| Mg–Al | Bath | 25 | 140 | 60 | 65 | Fluoride removal | [49] |
| Fe–Al | Bath | 80 | 160 | 30 | 65 | U(VI) removal | [50] |
| Mg–Al | Horn | 20 | — | — | 25 | Toluene and CO total oxidation | [51] |
| Zn–Al | — | 40, 59 | 250, 175, 88 | — | — | Synthesis of small particle size of Zn–Al LDH | [52] |
| Mg–Al | Bath | 40 | — | 480 | 95 | Fire suppression agent | [53] |
There are many advantages to this preparation method like time saving, reduction of crystallite size, increase in specific surface area, better active phase dispersion in the catalysts [54]. Those advantages are mentioned in the synthesis of several types of inorganic materials but few articles have studied the preparation of LDHs by US irradiation.
As mentioned above, LDHs can be synthesized by several preparation methods. Each of those has its advantages and limitations. The advantages vary according to the principle of applied method while limitations range from long preparation times to difficulty in controlling particle size. US assistance appears to be an alternative to those methods, combining several advantages and limiting their drawbacks. In the following section, we focus on the effect of US on the properties and performance of LDHs in different applications.
3. Applications of LDHs obtained from UAS
As mentioned above, LDHs are used in many fields (catalysis, adsorption, pharmaceutics, electrochemistry, etc.) in their initial form or as oxides after thermal treatment. This is due to their characteristics like simplicity in synthesis, distinctive structure, uniform distribution of various metal cations in the brucite layer, surface hydroxyl groups, flexibility, and high chemical and thermal stability. Moreover, the capacity to intercalate a variety of anions (inorganic, organic, biomolecules, and even genes), sustained delivery of intercalated anions, intercalated anions with interlayer spaces, swelling properties, oxo-bridged linkage, and high biocompatibility are all desirable characteristics. Here, we will discuss the effect of US in the synthesis of LDHs on the physicochemical properties and performances of those materials in different areas.
3.1. Remediation processes
In the field of water treatment, the removal of pollutants such as heavy metals, bromate, reactive dyes, and fluoride from wastewater is a topic of great interest. When released into the environment, they seriously harm both ecological security and human health.
3.1.1. Elimination of heavy metals
Due to the low cost of LDHs, tailored properties, and their affinity for anions like dichromate and uranyl, they can be used as anions exchangers and adsorbents for dangerous anions detected in wastewater [55]. Many studies have shown that LDHs can have more advantages if obtained by UAS. Li et al. [44] prepared Ca–Al LDH by co-precipitation method under US irradiation. They obtained the expected material at 55 °C for 30 min with no aging. In those conditions, higher specific surface area and narrower particle size distribution were obtained. As a consequence, this material demonstrated its efficiency in removing uranium and chromium. In addition, removal efficiency was not affected by the presence of competing ions such as nitrate radical and chloride. Likewise, Xie et al. [50] synthesized Fe–Al LDH for removing U(VI). They also prepared the adsorbent material with the US assistance (50 kHz, 160 W, during 30 min in an ultrasonic bath at 65 °C). Scanning electron microscopy (SEM) evidenced that the structure of US-prepared Fe–Al LDH (Fe–Al (30 min)) was characterized by better particle dispersion and narrower particle size distribution than that prepared classically (Fe–Al (0 min)). Figure 2 shows that U(VI) residual concentration was lower after using Fe–Al (30 min) rather than Fe–Al (0 min). This indicates that the US-prepared material has a removal capacity higher than that obtained by the conventional method.
Effect of solid-to-solution ratio on U(VI) removal by Fe–Al LDHs. Experimental conditions: initial concentration of U(VI) 10.0 mg/L, contact time 120 min, pH 6.0, 298 K [50].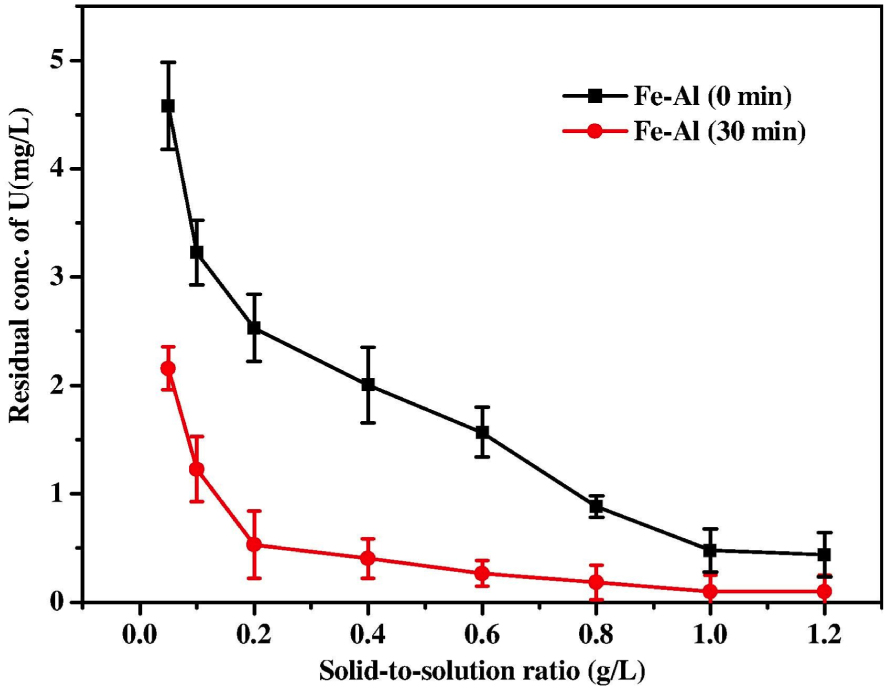
However, other materials have shown higher adsorption capacity such as mesoporous silica prototype, nano-flake Fe-sludge chars, and Ca–Al LDH. The partial oxidation of Fe2+ into Fe3+ leads to a decrease in the number of reactive sites and contributes to losing the removal efficiency after three experiments.
3.1.2. Elimination of bromate
The catalyst Fe–Al prepared using LDH precursors by the US-assisted co-precipitation method was used as an adsorbent to remove bromate from the solution [43]. Bromate () is an oxyhalide disinfection byproduct (DBP) during chlorination or ozonation in bromide-containing water treatment [56]. Due to high solubility, stability, and non-biodegradability in water, bromate is difficult to eliminate once formed [57]. Adsorption by LDH materials is one of the methods used to remove bromate by ion exchange [58]. According to Figure 3, anion exchange adsorption and reduction appeared to be two distinct processes involved in the potential mechanism of bromate removal by Fe–Al LDHs. First, bromate in solution was rapidly adsorbed onto Fe–Al LDHs by ion exchange between sulfate and bromate. Then, adsorbed bromate was reduced by Fe2+ and the harmless reduced products, bromide, entered the solution. Thus, Zhong et al. [43] confirmed ultrasound irradiation to be a simple and fast method to assist in the preparation of Fe–Al LDHs. That study showed that when irradiation time increased from 0 to 30 min, bromate removal efficiency of Fe–Al LDHs was increased.
Schematic representation of bromate removal by Fe–Al LDHs [43].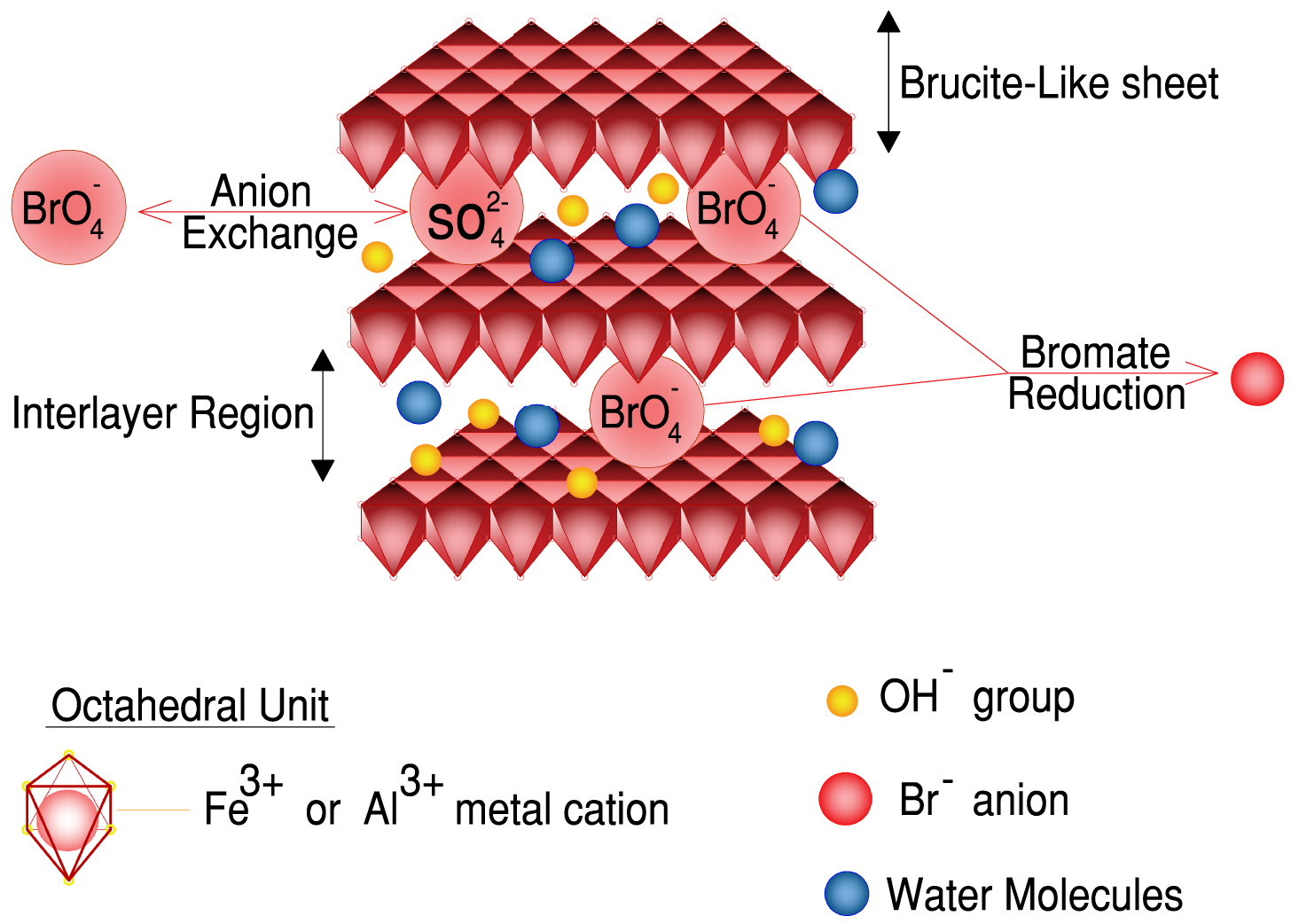
3.1.3. Removal of reactive dyes from wastewater
LDHs are also employed in removal of reactive dyes present in wastewater generated by different industries (textile, printing, plastic, cosmetics, etc.) [59]. Pahalagedara et al. [40] worked on the synthesis of Ni–Al LDH by sonochemical method for removal of a reactive azo dye. The results show that the US-prepared material exhibited 100% dye removal efficiency in 6 min, while the same material prepared by the conventional co-precipitation method required 60 min. Bharali et al. [60] prepared Ni–Al LDH using US and evaluated it in the removal of congo red. Uniform and well crystalline materials were obtained. In terms of activity, US irradiation led to a 12.7% improvement in the removal of congo red. In addition, the as-prepared material could be used in three successful adsorption cycles. Likewise, Kostic et al. [38] have prepared Mg–Co–Al LDH by US-assisted co-precipitation method (100 W, in an ultrasonic bath at 80 °C) and used it for removal of another dye (RB19). UAS led to an increase in specific surface area from 8.1 to 47.7 m2⋅g−1 that results in significant improvement of dye sorption capacity, from 57.5 to 367.9 mg⋅g−1.
3.1.4. Elimination of fluoride
Fluoride excess in drinking water can cause dental fluorosis, skeletal fluorosis, etc. [61]. Among different methods used for fluoride removal from water, adsorption technology is the most employed because of several advantages like high efficiency, simplicity, and profitability [62]. Chang et al. [49] studied the synthesis of magnetic magnesium–aluminum LDHs by a combination of magnetic Fe3O4 nanoparticles (MNPs) and Mg–Al LDH. In that work, US technology was applied in two steps. Firstly, the Fe3O4 MNPs were synthesized by US-assisted reverse-co-precipitation and then redispersed in water. Secondly, two different solutions were prepared: one containing aluminum and magnesium nitrates and the other containing NaOH and Na2CO3. Both solutions were added dropwise to the dispersion of Fe3O4 MNPs under US irradiation, in order to obtain the composite Fe3O4–Mg–Al-LDH. The average particle size of the US-prepared calcined composite was 80 nm, while that of the sample obtained by conventional co-precipitation was around 120 nm. In addition, there was an increase of approximately 14% in specific surface area. The material prepared with US irradiation showed an adsorption capacity 60% higher than that of the composite prepared without US assistance. Moreover, the same authors studied the effect of different US parameters on the particle size of the composite before calcination. They concluded that an optimal time of 30 to 60 min is required. Indeed, if the time was prolonged to 120 min, the average particle size increased. In addition, higher power density leads to more cavitation bubbles which generate more shock waves and microjets and consequently decrease the level of agglomeration.
3.2. Preparation of fire suppressants
LDH materials were also used as fire suppression agents [63]. The researchers have demonstrated the benefits of particles with spherical shapes and large surface areas because they are easily spotted and remain suspended for a long time in the flame zone. Several methods have been used to prepare LDHs with various shapes and good dispersion such as reverse microemulsions or using a polar solvent/surfactant system [64]. However, those methods are complicated, because in some cases it is necessary to use extra templates. Ni et al. [53] synthesized microspheres of Mg–Al LDH by simple sonication in a mix of ethylene glycol and water using an ultrasonic bath operated at 40 kHz during 480 min at 95 °C. X-ray diffraction (XRD) patterns showed formation of pure LDH structure without any other diffraction peak. Moreover, the authors observed a significant increase in specific surface area for the sample submitted to US irradiation (164.7 m2⋅g−1) compared to that prepared in silent mode (43.8 m2⋅g−1). In this work, Ni et al. studied the effect of frequency and sonication time on particle shape. They remarked that the optimal frequency and sonication duration were 40–50 kHz and 8–10 h, respectively. The fire suppression experiments showed that microspheres obtained with US assistance enable a decrease in extinguishing time.
3.3. LDHs as inorganic fillers in nanocomposites
LDHs can be used as nanofillers in a polymer matrix. However, it is difficult to delaminate LDHs into a polymer matrix [65] because of their high charge density and high anion content which lead to strong electrostatic interaction between sheets. One of the strategies used is surface modification [66]. Dinari et al. [41] prepared a chiral diacid-modified LDH which was employed in the preparation of polyvinylpyrrolidone (PVP)/modified LDH nanocomposites using US in three steps. SEM and TEM (transmission electron microscopy) photographs showed no aggregation in the different synthesized materials. Thermal analyses revealed that the introduction of small amounts of the modified LDH improves thermal stability of the nanocomposites at higher temperatures.
3.4. LDHs as catalyst precursors
The main challenge in catalyst development is then to improve active phase dispersion in order to enhance activity and stability [67]. Catalysts elaborated by the LDH route may be an alternative solution because they show high thermal resistance and high dispersion of active phase as well as high specific surface area [68]. Indeed, transition metal catalysts prepared by the LDH route have demonstrated their efficiency in many applications. In literature, the elaboration of catalysts by the LDH route with US assistance is carried out for a variety of applications.
3.4.1. Total oxidation of volatile organic compounds
VOCs are classified as dangerous for human health and environment because of their properties like volatility, toxicity, and diffusivity [69]. One of the techniques used for VOCs removal is catalytic oxidation [70]. LDHs can be used as precursors to synthesize catalysts for total oxidation of volatile organic compounds (VOCs).
Pérez et al. [47] prepared Co–Mg–Al LDH by conventional co-precipitation and by sonication method during aging step for only 10 min at room temperature using an ultrasonic bath at 50 kHz. US use led to a 22.5% increase in specific surface area as well as to higher catalyst reducibility (36.6% increase in H2 consumption). In addition, pore volume increased from 0.2209 cm3⋅g−1 to 0.4552 cm3⋅g−1 upon US irradiation. Furthermore, US-prepared samples exhibited narrowest pore size distribution in comparison to the conventionally prepared ones. Those characteristics led to better catalytic activity in terms of butanol conversion as shown in Figure 4 as well as to higher selectivity towards CO2 and H2O, the desired products. It is then inferred that US use during the aging step leads to catalysts with better characteristics and performances, with a shorter preparation time.
Temperature at 100% butanol conversion of MOCo CT and MOCo US [47].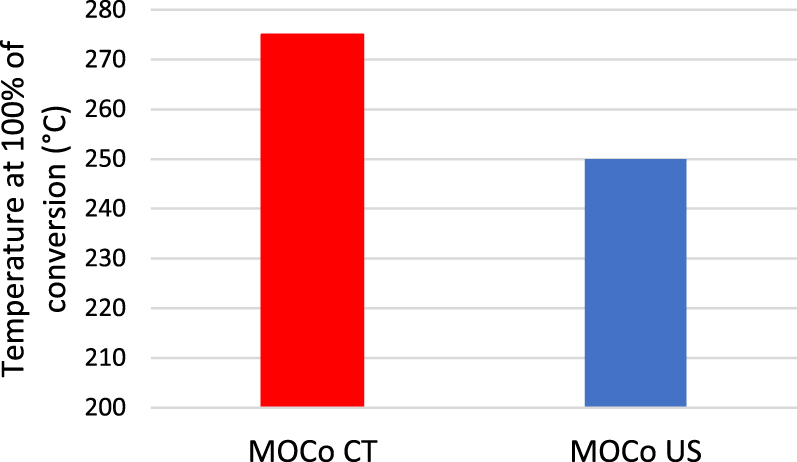
In addition, Genty et al. prepared the catalyst Co–Al using the LDH route by conventional co-precipitation and US assistance (ultrasonic horn, 20 kHz at room temperature) for applications in toluene oxidation [31]. The results showed an increase in specific surface area of 26.75%. TEM analysis indicated larger and less regular size of oxide particles for the solid resulting from conventional synthesis. From XRD patterns, the crystallite size of oxides was reduced between 25.53 and 61.21% under US irradiation according to the material composition. The H2-TPR (Hydrogen temperature-programmed reduction) analysis showed Co3O4 species reduction at lower temperatures for the US-prepared sample. Finally, the catalytic test of the US-prepared sample showed a 50% toluene conversion at a lower temperature than the sample prepared by co-precipitation (Figure 5), due to a higher amount of Co2+ on the surface of the US-prepared sample.
Temperature at 50% toluene conversion of Co6Al2HT500 and Co6Al2HTUS500 [31].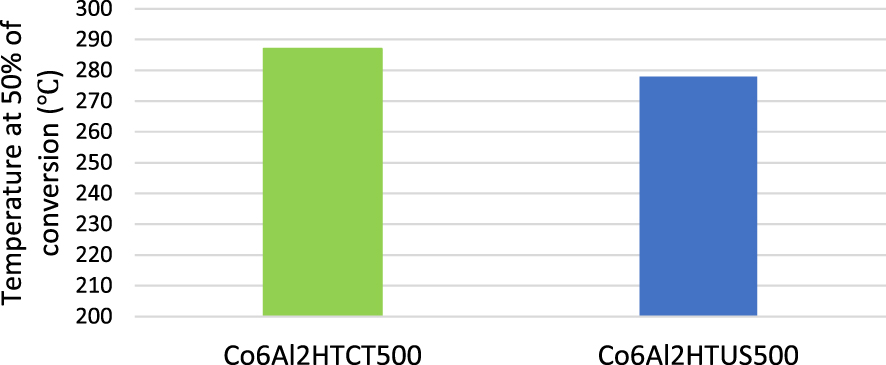
Likewise, the same authors worked on the UAS of another LDH composition (Mg–Al) under the same conditions [51]. Those materials were used as catalysts for total oxidation of toluene and CO. An increment of 51% in specific surface area of hydrotalcite precursors was observed. XRD results showed an increase in the crystallite size of MgO from 4.5 nm for the US-prepared sample to 11.6 nm for the sample prepared by co-precipitation. The activities of the calcined samples were compared by T50 (temperature at which 50% of initial reactant is oxidized) of CO and toluene. T50 decreased from 479 to 423 °C with the US-prepared catalyst. Likewise, T50 of CO oxidation decreased from 285 to 273 °C.
3.4.2. Knoevenagel and aldol condensations
Furthermore, LDHs can be used as precursors to synthesize catalysts for Knoevenagel and aldol condensations. Climent et al. [30] prepared Mg–Al LDH with a Mg/Al ratio of 3 by three different methods. One of those was US irradiation during the precipitation step without the aging step. SEM showed that US-prepared samples present homogeneous morphology with highly dispersed particles characterized by an average size of 80 nm, while average particle size varied between 100 and 540 nm for the samples prepared by the two other methods (classical co-precipitation and microwave-assisted method). N2 adsorption at 77 K showed that the sample obtained by calcination of US-prepared LDHs had the largest specific surface area (20% increase in comparison with the two other methods). The results show that the US-prepared mixed oxides lead to the best catalytic activity. In the Knoevenagel condensation between benzaldehyde and malononitrile, yield increased from 23 to 50% in 15 min. In addition, in the Knoevenagel condensation between benzaldehyde and ethyl cyanoacetate, an increase from 31 to 60% was detected in 5 min using the US-prepared material. In the aldol condensation of citral with acetone, yield increased from 68 to 93% and selectivity to pseudo-ionones increased from 82 to 95%.
3.4.3. Oxidative steam reforming of ethanol
Moreover, oxidative steam reforming of ethanol (OSRE) is another application of catalysts prepared using LDHs as precursors. OSRE is the combination of two reactions, steam reforming of ethanol and partial oxidation of ethanol [71]. OSRE enables hydrogen production [72]. Espitia-Sibaja et al. [45] prepared CoMgAl LDH by US-assisted co-precipitation method (ultrasonic bath, 42 kHz, 90 min at 65 °C) with different percentages of cobalt and tested the materials in OSRE. This study revealed that Co–Mg–Al LDH can be synthesized by the US-assisted co-precipitation method and the resulting oxides can be used as catalysts in the OSRE reaction. The average aggregate size determined by the Scherrer equation from XRD patterns of the mixed oxides was 4 nm. US irradiation facilitates diffusion of Co particles into the brucite lattice. This diffusion maintains the crystallite size after the calcination process. Furthermore, Muñoz et al. [29] prepared Ni–Co–Mg–Al LDH by co-precipitation and US-assisted co-precipitation. Sonochemically synthesized solids had higher basicity (CO2 adsorbed = 268 μmol⋅g−1) than the conventional catalyst (CO2 adsorbed = 199 μmol⋅g−1). This fact may be connected to the development of surface defects, such as exfoliation or erosion of the material’s outer layers, disintegration of agglomerates into smaller particles, or formation of grain boundaries where the oxygen atoms in metal–oxygen (M–O) bonds are not perfectly coordinated [17]. Indeed, some researchers link the development of isolated surface defects to increased basicity of the M–O bonds after sonication of LDHs precursors [30]. Another work involving US in LDH synthesis was led by Macedo Neto [46]. US irradiations were used to incorporate large cations like cerium in Ni–Mg–Al LDHs. Cerium in LDHs offers several advantages such as easy and high reducibility as well as high oxygen storage capacity. The as-obtained materials showed better textural properties and an increase of 38% in specific surface area. In addition, calculation of lattice parameters revealed a better incorporation of cerium ions within the LDH structure when US is applied.
3.4.4. Removal of carbonyl sulfide
Another catalytic application of LDH-derived oxides is the removal of carbonyl sulfide (COS) by desulfurization. COS in petroleum gas and natural gas leads to catalyst deactivation as well as corrosion of equipment used in different treatments of petroleum fractions. Moreover, sulfur compounds emissions in atmosphere produce acid rain [73]. Zhao et al. [48] prepared Ni–Al LDH using US irradiation during the aging step in an ultrasonic bath (45 kHz) with a power of 200 W at 50 °C. SEM images (Figure 6) showed that the US-prepared material (NiAl-UHTLCs) was characterized by homogeneous morphology and highly dispersed particles, whereas the sample prepared without US (NiAl-HTLCs) showed a dense appearance.
SEM image of NiAl-HTLCs (a) and NiAl-UHTLCs (b) [48].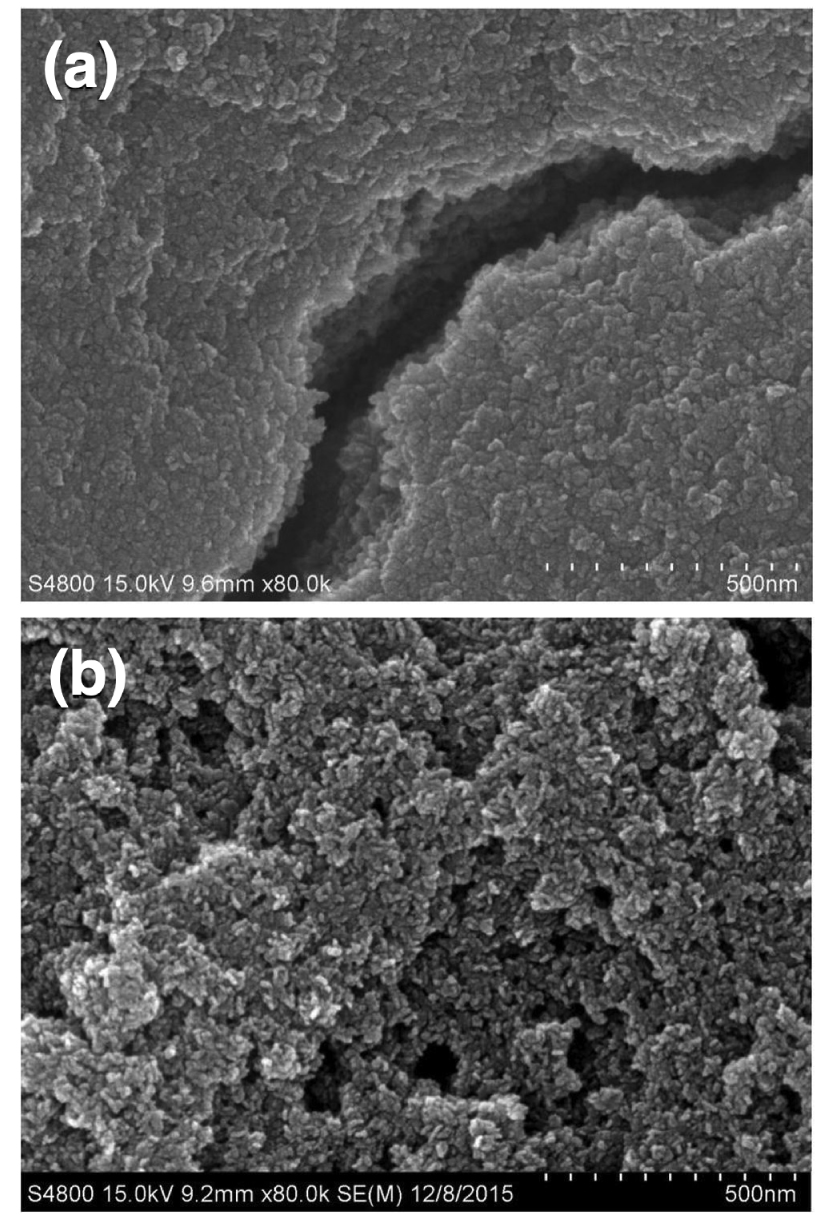
This is because bubbles coming from the acoustic cavitation phenomenon cause erosion when collapsing. The temperature-programmed desorption (TPD) of CO2 showed that US irradiation led to a higher number of weak and moderate basic sites than the catalyst prepared by the conventional method. US-prepared Ni–Al mixed oxides led to better catalytic activity (100% of COS removal in 90 min) compared to that obtained by the conventional method (100% of COS removal in only 90 min). This difference is due to better particle dispersion and formation of well-developed pore structures. In addition, in this type of application, the removal rate increased with the number of basic sites [74]. The results showed that US irradiation leads to a sample with more weak and moderate basic sites. As shown in Figure 7, moderate basic sites (M–O) lead to the formation of H2S by hydrolysis: COS + H2O → H2S + CO2. Then, H2S is oxidized into elemental sulfur by oxygen-containing functional groups (M–O pairs). This is the possible mechanism of COS removal when using US in the preparation method.
Possible enhancing mechanism of ultrasound assistance in the synthesis of Ni–Al hydrotalcite for carbonyl sulfide removal (where M is a metal) [48].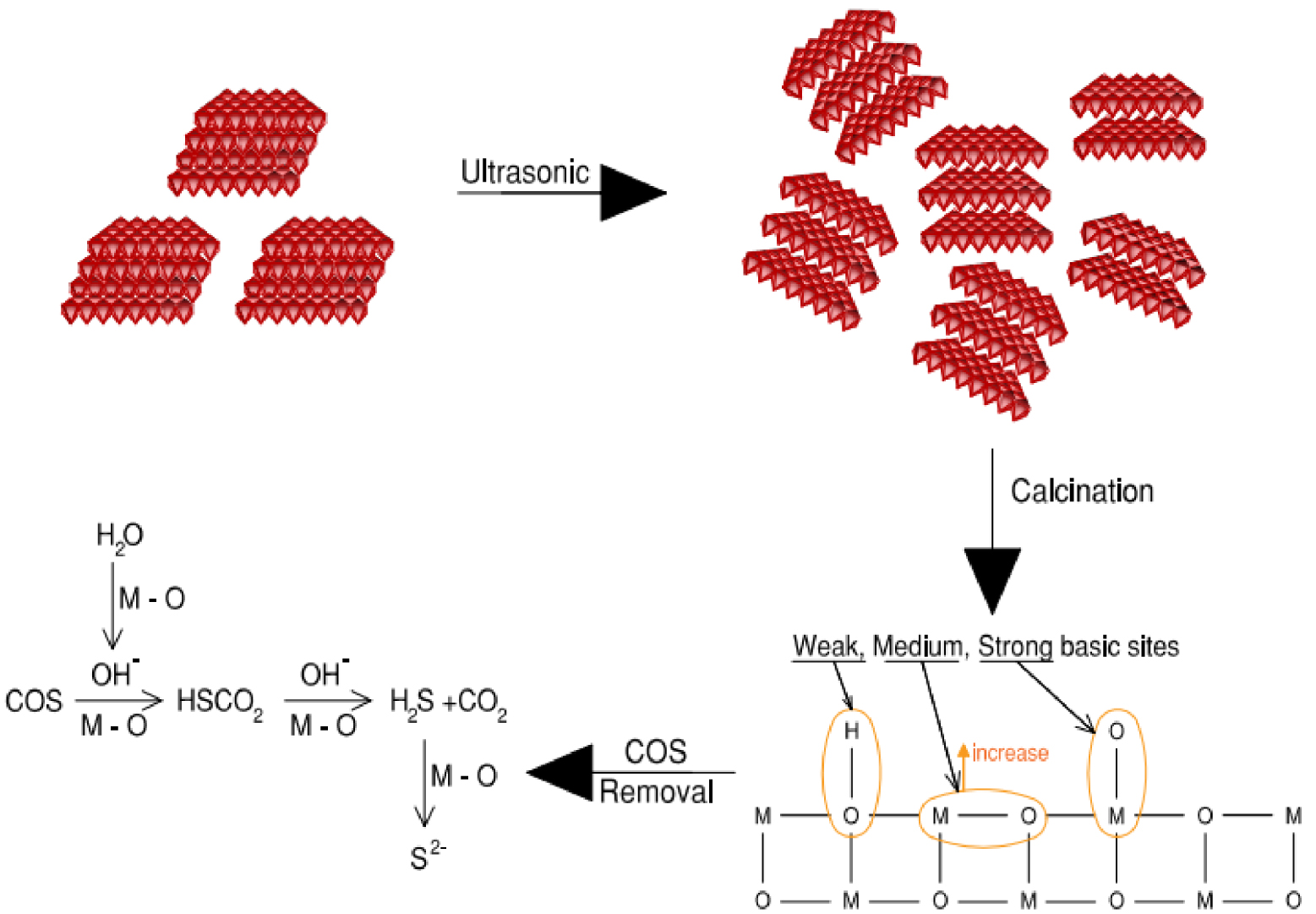
3.4.5. Oxygen evolution reaction
Munonde and Zheng [75] prepared Ni–Fe LDH for oxygen evolution reaction (OER) which is applied in electrochemical water splitting. The objective was the generation of clean energy in the form of oxygen and hydrogen fuels. The electrochemical performance of LDHs was enhanced by exfoliation into single or thinner nanosheets. This process increases mass transfer, exposing more active sites and accelerating electron transfer [76]. US irradiation decreases the size of stacked nanosheets from 70–800 to 30–300 nm and the thickness from 25–40 nm to 12–0 nm in an optimal sonication duration of 15 min. Moreover, XRD patterns show that diffraction peaks became sharper with increased intensities after US exfoliation. This indicated the increase in periodicity of crystallographic directions. The US-exfoliated Ni–Fe LDH catalyst showed advanced performance with 250 mV overpotential at a current density of 10 mA⋅cm−1, that is 100 mV less than the initial Ni–Fe LDH catalyst.
3.4.6. Carbonylation of glycerol
LDHs can also be used as catalysts using the “memory effect”. This effect is defined as the reconstruction of a mixed oxide obtained by thermal decomposition of LDHs immersed in an aqueous solution [77]. Generally, the reconstruction of LDHs is performed by hydrothermal treatment [78]. Álvarez et al. [42] studied the reconstruction of Zn–Al and Zn–Al–Mg LDH by mechanical stirring and ultrasonication during rehydration. Those materials have been applied in the carbonylation of glycerol with urea. Firstly, LDH structure is not completely reconstructed because ZnO is detected in the samples treated by the two methods. Moreover, there are no significant differences in LDH specific surface area. But a 30% decrease in surface area by comparison to the parent hydrotalcite was observed for both treatments. Higher total basicity was obtained in the reconstructed material prepared by ultrasonication for 10 h. Likewise, after adding magnesium, the number of basic sites achieved a maximum in samples US-treated for 2 h. In this study, the turnover frequencies (TOF) were used for comparing the activity of reconstructed LDHs in the carbonylation of glycerol with urea. The results showed that US irradiation increases TOF values.
As shown above, US irradiation offers many advantages over non-US methods. Those characteristics are very important in many fields such as catalytic applications. For example, high basicity, well-dispersed active sites, and small particles are very important features in a catalyst for methanation reaction (CO2 + 4H2→CH4 + 2H2O, 𝛥H298 = −165 kJ⋅mol−1) [79]. High basicity allows more CO2 to be captured, and small, well-dispersed active site particles allow higher contact area between reactants and catalyst, leading to better activities.
4. Conclusion
Layered double hydroxides (LDHs) are promising materials for a wide range of applications. LDHs offer significant benefits over the use of homogeneous alkali hydroxides or alkoxides as well as in heterogeneous catalysis. In each of those applications, the form of the material (original form or as mixed oxides obtained after thermal treatment), the composition and required properties vary. In this review, the impact of ultrasound (US) use in the synthesis of LDHs has been discussed. First of all, the review of the literature devoted to the US-assisted synthesis of LDHs shows that in the majority of cases the experimental conditions are poorly or insufficiently described to permit comparison of the results obtained. As a result, experimental designs are not well described and this does not allow for optimization of the syntheses and of the performance induced by US irradiation. US has been used for a variety of applications and very satisfying results were obtained compared to silent methods. US irradiation can be done in one or more steps of the synthesis process and enables reduction of the overall synthesis time compared to conventional preparation methods from minimum 19 h to only 30 min. This is very important from an industrial point of view because it leads to producing materials with less energy. Furthermore, acoustic cavitation leads to better dispersion of particles characterized by a small size and a narrower particle size distribution since US leads to a 25–33% particle size reduction in comparison to the same materials prepared by classical method. Moreover, US irradiation leads to an increase in specific surface area from 22% to more than 100% in comparison to the conventional methods. This is due to the brutal collapse of cavitation bubbles resulting in shockwaves and microjets toward the surface of solid particles causing local defects, surface erosion and particle fragmentation. Eventually, there is no doubt that future research on these US-prepared materials will broaden their range of application and therefore increase the potential of LDHs. Moreover, using high US frequency is an important choice for studying the impact of one of the parameters related to US irradiations on the properties of the prepared materials. In addition, companies working on the development of efficient new US devices have a role to play in scaling up LDH synthesis from small-scale (laboratory scale) to large-scale. This transition requires cooperation between laboratory researchers and companies to improve the production of LDH materials using US.
Declaration of interests
The authors do not work for, advise, own shares in, or receive funds from any organization that could benefit from this article, and have declared no affiliations other than their research organizations.





 CC-BY 4.0
CC-BY 4.0