1. Introduction
Aqueous foams, which are composed of air bubbles separated by thin liquid films stabilized by surfactant molecules, have been widely used in hydrometallurgy for the concentration of various species: ions, colloids, ore particles, microorganisms [1, 2, 3, 4, 5] …At industrial scale, foams are often employed to separate mineral particles in froth flotation processes [6]. Their use has been extended to various extraction processes such as deinking of aqueous solutions via flotation [7, 8], and in the nuclear industry for extraction and concentration of uranium, in aggressive and strongly acidic environments [9, 10]. All these examples take benefit of the peculiar features of liquid foams: These foams are mostly composed of gas, so that less than 10% of their volume fraction is water, even less than 5% in the case of dry foams. The resulting volume of aqueous phase to be treated is considerably reduced. However, to be effective in terms of extraction, the extraction must be carried out through the liquid gas interfaces developed in the foam, and not by the transport of the liquid in the aqueous films, as this fraction must be minimized. The gas can be inert (N2), simply air, containing O2, or also more reactive such as an O3/O2 mixture. The use of an oxidizing gas brings additional reactivity to the solutes in the aqueous phase. Foams also contain surface active agents, which have detergency properties and can remove impurities from a surface and/or activate it. The choice of the surfactant is key for the stability of the foams, as the lifetime of the foams strongly depends on coalescence of bubbles, facilitated by the drainage of liquid out of the films [11]. Coalescence events lead e.g. to the detachment of mineral particles from the interface and loss of the particles into the initial slurry during flotation processes. Leaching of ores using foams was proposed in the 70’s [12], however with scarce applications so far to our knowledge. Recently, the possibility to use foam based on diluted aqueous hydrochloric or sulfuric acid solutions for the leaching of metallic copper or silver has been demonstrated [13]. The perspectives opened by these model studies drove our attention to the application of foams for the leaching of copper from electronic waste (e-waste).
The recovery of metals from e-waste, known as Urban mining [14, 15, 16], has drawn considerable attention during last decade. The high content in copper, much higher than in natural ores, has led to the set-up of pyrometallurgical and hydrometallurgical processes dedicated to copper recovery [17]. Latter processes always rely on a leaching stage, generally based on the use of a large amount of a concentrated aqueous solution. In the case of copper, it is well known that the sole action of H+ in the acid is not sufficient to leach metallic copper, and that an oxidant is needed [18]. Metallic copper is thus generally leached using nitric acid, ferric sulphate in sulfuric acid, or a mixture of hydrochloric acid with hydrogen peroxide [19]. Electroleaching can also be implemented, either directly on adequate waste which are connected to the anode, or indirectly through the electrogeneration of a reactive intermediate such as chlorine in chloride media [20]. Latter two approaches require specific equipment. The use of O2 as an oxidant suffers from slow gas–liquid transfer, so that the use of a hydrochloric acid solution stirred under air is not sufficient for copper leaching. Acceleration of gas–liquid transfer when employing a foam proved to be sufficient to observe significant leaching of copper in the above mentioned study [13], which lets suggest that further process development is possible using air as oxidant. It is thus the purpose of this article to report our progress on the leaching of copper from e-waste with hydrochloric acid-based foams. Brij®S10 (polyoxyethylene(10) stearyl ether, C18H37(OCH2CH2)nOH, n ∼ 10) was employed to generate the foam, as it is a standard neutral surfactant readily available at industrial scale, which is known for its good foaming ability and stability in acidic media [21], and has been previously studied in our laboratory [22]. The present study resulted in a leaching strategy based on two stages, which enables efficient recovery of base metals, with most copper almost quantitatively leached during the second stage employing an acidic foam.
2. Materials and methods
Approximately 500 kg of waste printed circuits boards (WPCB) were provided by a waste recovery company operating in France. Concentrated nitric acid and concentrated hydrochloric acid were purchased from Carlo Erba reagents. Surfactant Brij®S10 (polyoxyethylene(10) stearyl ether, C18H37(OCH2CH2)nOH, n ∼ 10) was purchased from Sigma-Aldrich and used without further purification. Gas used was filtered air provided through a compressor.
2.1. Grinding of e-waste
Batteries and aluminum heat sinks were first manually disassembled from WPCB before the entire sample (485 kg) was shredded with an industrial cutting mill to a particle size of less than 30 mm. The sample was subsequently quartered with a rotary divider; one quarter (122 kg) of the sample was then shredded anew, with an industrial cutting mill equipped with a bottom sieve with perforation size of 10 mm. The −10 mm sample was further divided with a riffle splitter to obtain sub-sample masses of approximately 4 kg. Two of these fractions were gathered to form a sample of 7.4 kg, which was then shredded in its entirety in a lab knife mill (Retsch SM2000 with tungsten carbide grinding tools), fitted with a bottom sieve with perforation size of 2 mm. The weights of the disruptive materials and of the lost material amounted to less than 9 and 1% of the initial materials respectively. The former were added to the final ground sample to produce the same composition as the original WPCB materials. This sample was then dry-sieved up to 500 μm, and wet-sieved from 500 μm down to −40 μm.
2.2. Elemental analysis of WPCB samples
Samples of weights varying between 4 and 6 g were taken from each of the previously formed size fraction, and subjected to a hot, atmospheric leaching in aqua regia, at 200 °C during 2 h. After cooling, each leachate was filtered (filter porosity of 43 μm), and the resulting filtrate was diluted with weakly concentrated nitric acid (0.5 mol⋅L−1). The concentrations of Fe, Cu, Ni, Zn, Pb and Co in the filtrates were measured by flame AAS (VARIAN 220 FS).
2.3. Leaching using foam
In a 250 mL two-neck round bottom flask, was introduced the solid (either Cu 40 μm powder, Cu wire, Cu 1 mm beads, or samples of ground WPCB detailed in Table 1) to be leached (ca. 500 mg, weighed with precision), followed by 10 mL of a 2 mM BrijS10 aqueous HCl solution of fixed concentration (see text for details). Air was bubbled during ca. 5 min using a fritted glass tube, at 5 mL/min flow, to grow the foam to approximatively 100 mL (a mark was put on the outside of the flask). The flask was then connected to a Buchi rotavapor, and rotated at 100 rpm. At fixed time intervals, rotation was stopped and the system was left to settle over few min, an aliquot of the leaching aqueous solution was taken (ca. 200 μL using a glass Pasteur pipette) and centrifuged during 1 min at 6000 rpm, and 100 μL of resulting solution were taken and diluted into a 1.0% (w/w) HCl/HNO3 with HNO3/HCl = 9 (v/v) aqueous solution. Air was also regularly reinjected, approximatively once per h, to regenerate the foam as it progressively collapses. Concentrations of metals in the aliquots were determined by inductively coupled plasma atomic emission spectroscopy (ICP/AES, SPECTRO ARCOS ICP Spectrometer, AMETEK Materials Analysis). Given concentrations are calculated as the means of three replicates on three different wavelengths for each metal; relative standard deviations were determined and lie between 1 and 4%.
Average composition of ground WPCB particles according to their size
| Particles size | Weight contenta | |||||
|---|---|---|---|---|---|---|
| Cu (%) | Fe (%) | Ni (%) | Pb (%) | Zn (%) | Co (mg/kg) | |
| >2 mm | 13.0 | 57.1 | 0.99 | 0.14 | 2.28 | 485 |
| 1–2 mm | 15.2 | 11.2 | 0.43 | 1.06 | 1.89 | 78 |
| 500 μm–1 mm | 25.2 | 8.3 | 0.50 | 1.74 | 1.95 | 551 |
| 250–500 μm | 24.0 | 7.7 | 0.50 | 1.58 | 1.40 | 845 |
| 100–250 μm | 18.8 | 9.8 | 0.40 | 1.50 | 1.40 | 818 |
| 63–100 μm | 5.5 | 19.3 | 0.46 | 1.55 | 1.53 | 557 |
| 40–63 μm | 2.7 | 15.2 | 0.32 | 1.00 | 1.07 | 240 |
| <40 μm | 2.5 | 9.3 | 0.35 | 1.53 | 1.33 | 162 |
| Total | 17.8 | 12.4 | 0.47 | 1.25 | 1.83 | 314 |
| 0–750 μm | 15.9 (1.8) | 14.8 (1.2) | 0.38 (0.07) | 1.3 (0.2) | 1.8 (0.3) | 392 (107) |
Note: a The sum of contents in metals does not reach 100% as WPCB also contain organic polymers and inorganic (glass) fibers.
2.4. Pre-treatment of PCB particles
About 5 g of WPCB weighed with precision were put in contact with 60 mL (L/S ratio: 12 mL/g of solid) of an aqueous 3 M HCl solution in a glass vial. The resulting mixture was heated to 60 °C and stirred for 3 h at room temperature (20 °C ± 2 °C). The resulting mixture was then filtered on a Büchner set-up equipped with a paper filter under vacuum. The resulting solid was used without further treatment for subsequent leaching using foam, or stored in glass vials under N2 atmosphere.
3. Results and discussion
3.1. Grinding of e-waste
The collected WPCB were first of all shredded and ground. In order to evaluate the optimal size for leaching stage, various fractions have been sieved and collected (Table 1).
The copper content is strongly dependent on the size of the particles. The large particles contain much more copper, which can be related to the fact that (i) copper is employed in coils of wires and printed circuits, and (ii) copper is a ductile metal, difficult to shred into tiny pieces. Thus, the leaching efficiency according to mean copper particle size rapidly appeared as a key point to study [23].
3.2. Set up of experimental device for foam leaching
The design of solid–liquid–gas reactors has been the subject of numerous chemical engineering studies, which will not be discussed here. In the frame of this study, several approaches have been envisioned to design a suitable tool for leaching experiments at the laboratory scale. First of all, a classical column was tested, but it rapidly appeared that it is complicated to have an homogeneous suspension of particles in the foam. Dense particles, and especially large copper particles, tend to fall to the bottom of the column and thus in the liquid employed to generate the foam. Light particles are rapidly expelled from the column at the front-end of the foam, and hydrophobic particles accumulate at the periphery of the column, with very poor contact with foam (see Figure SI1). As it is important to have a homogeneous dispersion of the solid in the foam we turned to a rotating drum, which can be easily simulated with classical laboratory apparatus and glassware (Figure 1).
Schematic description of the experimental procedure employed for ground WPCB leaching using foams.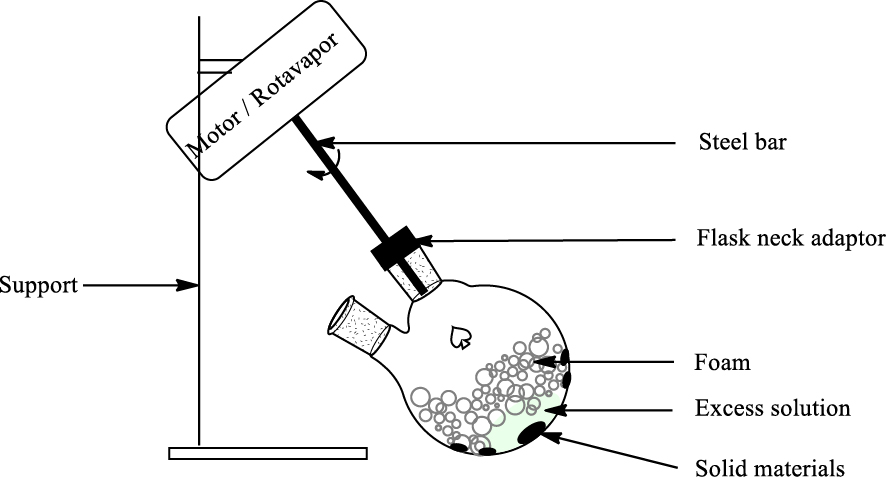
First set-up was based on a simple round bottom flask attached to a mechanical stirrer inclined with an about 30° angle, and placed on a wooden support that would limit friction during rotation. The set up was upgraded using a Buchi rotavapor, which limits friction and enables to heat the flask at a controlled temperature using a water bath if desired. A two-neck flask was employed to enable regular sampling of the reaction mixture, and if necessary gas injection to regenerate the collapsed foam. Leaching was performed at room temperature: in these conditions, foam remains stable over more than 1 h without further gas injection. Experiments at 40 °C were attempted in order to increase leaching rate, however, gas injection was necessary after 15 min as the foam collapsed more quickly, so that the effect of temperature has not been investigated. Such a laboratory set-up is seen as a rough model for rotating drum devices, which have been developed at large scale for leaching of various solids including e-waste, as well as other classical operations such as filtration. In our study we did not investigate the optimal volume of foam to employ, as a small volume of liquid may lead to very large volumes of foams, which could be an important drawback for further scaling-up or industrial application. Each leaching experiment was repeated at least three times, and the error bars displayed result from the mean results over these repetitions.
3.3. Leaching of pure metallic copper
The first studies on pure metallic copper led to mitigated results (Figure 2). First of all, as expected, small particles (copper powder, 40 μm average particle size) led to rapid dissolution. But larger particles (copper beads, 1 mm diameter) led to sluggish Cu leaching. Latter result could be explained by the fact that the beads sink and stay at the bottom of the rotating flask. On the other hand, the small metallic particles were well dispersed in the foam, facilitating the contact to liquid (HCl) and gas (O2 in air) necessary for the reaction. However, results on Cu wire were quite disappointing, even if the wires did not sink in the solution and were well dispersed in the foam. These results will be discussed later in the paper. Similar experiment conducted in solution without surfactant and with continuous air bubbling (5 mL/min) led to 2 to 3 times lower Cu dissolution yield, even after 5 h. In parallel, experiments performed with the surfactant but without addition of air led to negligible dissolution. It is reasonable to assume that the use of foams enables a better transfer of gas into the liquid. As described before [13], two physical transfer reactions are important: (i) gas–liquid transfer of O2, and (ii) diffusion of dissolved O2 and HCl in the liquid phase towards the surface of the solid. These issues are easily answered using liquid foams. The liquid fraction of foams was not properly controlled in our system, in comparison with previous work [13], only adjusted through regular bubbling in the foam to reach a ca. 10 fold volume of liquid initially employed. The concentration in surfactant (2 mM) was fixed to ensure sufficient stability of the foam: lower concentration led to progressive collapse of the foam, whereas higher amount did not increase foam stability.
Evolution of Cu leaching using foams according to the Cu source (foam prepared using 3 M HCl and 2 mM Brij S10, 10 mL of liquid for 500 mg solid).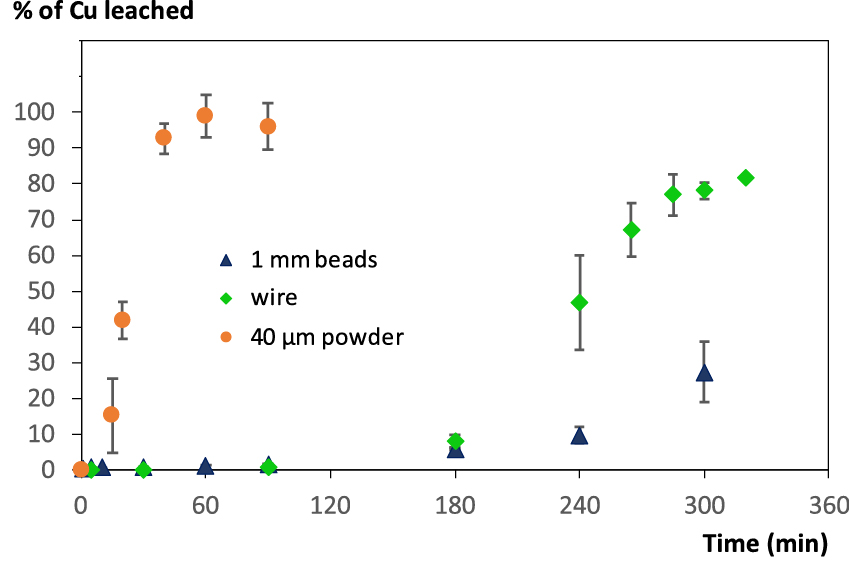
The kinetic profile also rose our attention. The initial Cu dissolution rate is close to zero in the case of beads and wire, and appears to be low in the case of powder, as if an initiation step was necessary in the Cu dissolution mechanism. Furthermore, the sigmoidal profile observed suggests an autocatalytic mechanism. Such an autocatalytic mechanism has been previously reported in ammonia solutions [24]. Depending on the oxygen (and ammonia) concentration, the autocatalytic term was found to be predominent. To our knowledge, no similar mechanism study on metallic copper has been performed in chloride media, although the oxidizing features of cupric chloride are well known, and have been employed for copper minerals [25] or even gold [26] leaching. In the present study, the occurrence of an autocatalytic mechanism and the impact of cupric ions were clearly proven by two key experiments (Figure 3): First of all, addition of a small quantity of Cu2+ ions (10% respective to totally Cu content) led to a decrease by a factor of 2 of the duration needed for complete Cu dissolution. More impressive was the gain in Cu dissolution observed when starting from a 1/1 mixture of wire and powder: The kinetics profile was very close to that observed with Cu powder alone (see Figure SI2). The remarkable increase in reaction kinetics can be interpreted from a chemical point of view by dissolution of Cu metal by Cu2+ ions present in solution rather than by dissolved O2 and H+, as latter reaction involves 3 reagents (Cu, O2 and H+ ions). However, there is still a visible induction period, suggesting that the surface state of the Cu metal is also important. Physical activation of the Cu via e.g. Friction with the Cu particles can be envisioned. Latter parameter was not further investigated, as available reports in the literature suggest very complex mechanisms [27].
Evolution of Cu wire leaching using foams upon addition of 40 μm Cu powder or 10 wt% CuSO4 (foam prepared using 3 M HCl and 2 mM Brij S10, 10 mL of liquid for 500 mg solid).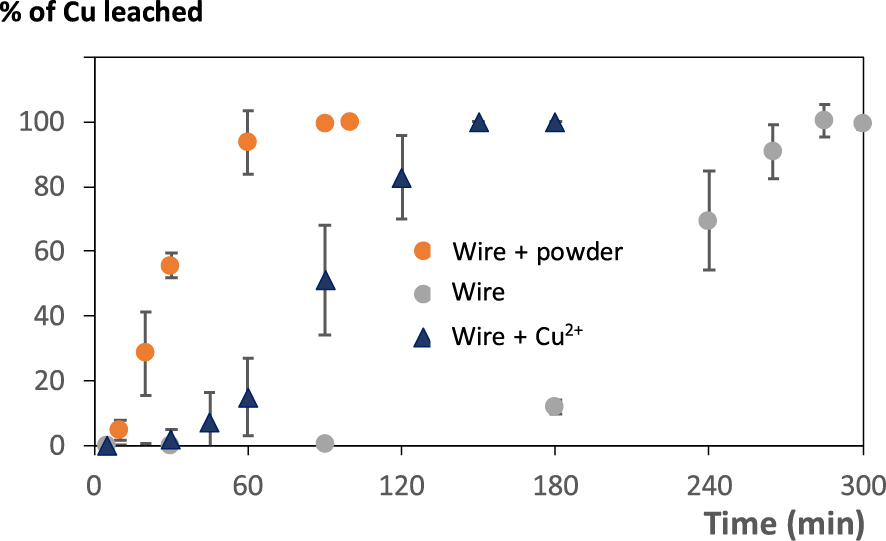
Altogether, these results suggest that the presence of copper particles of various dimensions in the ground e-waste is a favorable factor for efficient copper leaching, and we turned to leaching experiments on a real sample of ground WPCB, of size lower than 750 μm, obtained after classical shredding and grinding operations of composition given in Table 1.
3.4. Leaching of copper from ground e-waste
Copper leaching from ground WPCB with foam using the experimental conditions described above led to rather poor results in comparison with previous results obtained on pure metallic copper (Figure 4). The dissolution of Cu is (i) incomplete and (ii) much slower than in the case of the mixture of Cu powder and Cu wire described in previous section (and even slower than in the case of Cu wire alone).
Evolution of Cu leaching from WPCB particles using foams, with comparison with a mixture of Cu wire and Cu 40 μm powder (foam prepared using 3 M HCl and 2 mM Brij S10, 10 mL of liquid for 500 mg solid).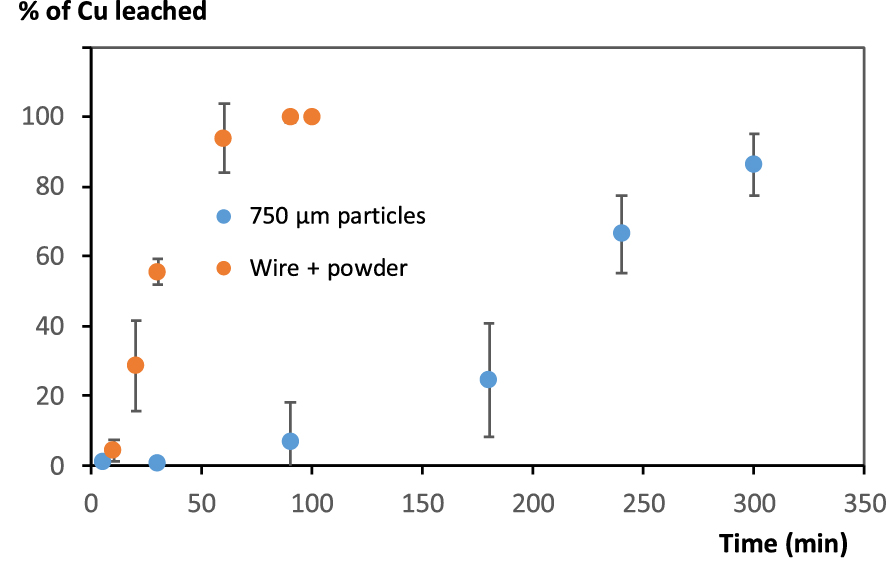
E-waste are composed of numerous metals, with most of them expected to be oxidized more easily than copper (Table 1). The leaching of metallic copper is based on an acidic oxidizing process, and in these conditions, other metals such as Zn, Fe, Ni, Al, etc … which are more reducible than Cu are expected to be also leached [28]. Consequently, it is reasonable to suppose that experiments led on ground WPCB did not give satisfactory results regarding Cu leaching, as these other metals were preferentially attacked, and Cu preserved. After 5 h reaction time, the solution was still very acidic (control using pH paper) and simple calculation showed that protons were still in large excess: the limit in Cu leaching probably arises from kinetic limitations, but we did not continue the experiment over a longer period for practical reasons. Rather, a first treatment of ground WPCB was set in place. Attack of the solid with aqueous HCl in solution led to significant leaching of Al, and no leaching of Cu (Table 2). Leaching of other metals such as Fe, was moderate (Table 2). Al leaching kinetics were rapid (Figure SI3), but Fe leaching was much slower, and difficult to push to completion (Table SI1).
Leaching yield of Al, Fe and Cu upon treatment of ground WPCB with aqueous HCl of variable concentration during 5 h at room temperature (5 g of ground WPCB, size <750 μm, for 60 mL aqueous HCl solution)
| Metal | HCl concentration (mol⋅L−1) | |||
|---|---|---|---|---|
| 0.1 | 1 | 3 | 5 | |
| Cu | <1% | <1% | 1% | 3% |
| Fe | 8% | 15% | 24% | 22% |
| Al | 5% | 56% | 92% | 95% |
Such a pre-treatment of ground WPCB should thus improve the efficiency of Cu leaching with the foam as less acid should be consumed by metals more reducible than Cu such as Al. Also, the possibility to leach sequentially different metals using the same acid is appealing: It can be envisioned to leach reducible metals with HCl alone, then Cu with HCl and O2 in the foam, leaving noble metals in the residue for further treatment (not discussed in this article). Thus, ground WPCB (size <750 μm) was processed in two stages: In a first stage, Al and some Fe were leached using a 3 M HCl solution (L/S ratio: 12 mL/g of solid). This operation does not leach significant Cu quantity (Table 2). In a second stage, the residue was further treated with an HCl based foam, and the Cu leaching is almost complete (Figure 5).
Influence of preliminary treatment of ground WPCB with aqueous HCl (60 mL of 3 M aqueous HCl solution for 5 g of ground PCB during 3 h at room temperature) on the outcome of Cu leaching using foam (foam prepared using 3 M HCl and 2 mM Brij S10, 10 mL of liquid for 500 mg solid).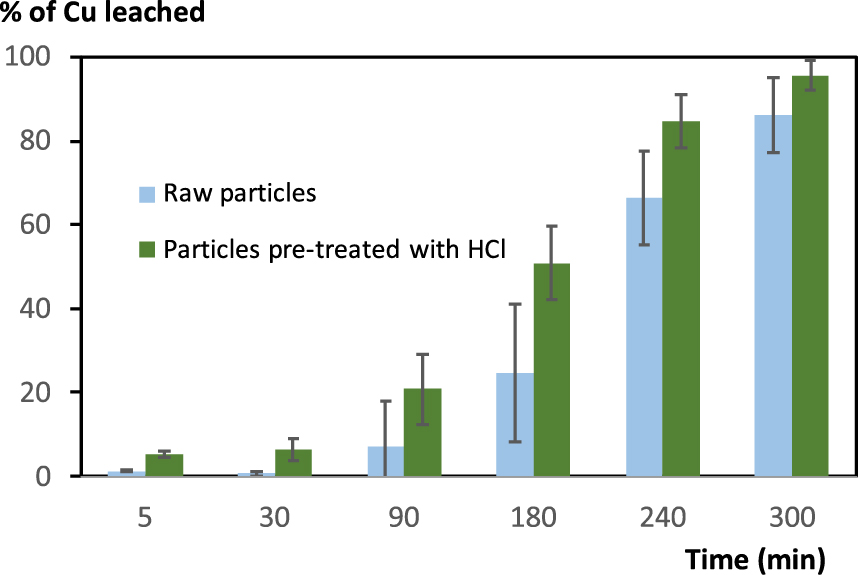
Although the Cu leaching yield is almost quantitative (97 ± 3%), the leaching rate remains low, and the improvement regarding the untreated WPCB sample appears marginal. Therefore, we decided to have a closer look at the impact of the fineness of the grinding. Two particle fractions obtained after sieving and rich in Cu were studied (Figure 6).
Rate of Cu leaching from WPCB particles using foams according to the particles size (foam prepared using 3 M HCl and 2 mM Brij S10, 10 mL of liquid for 500 mg solid).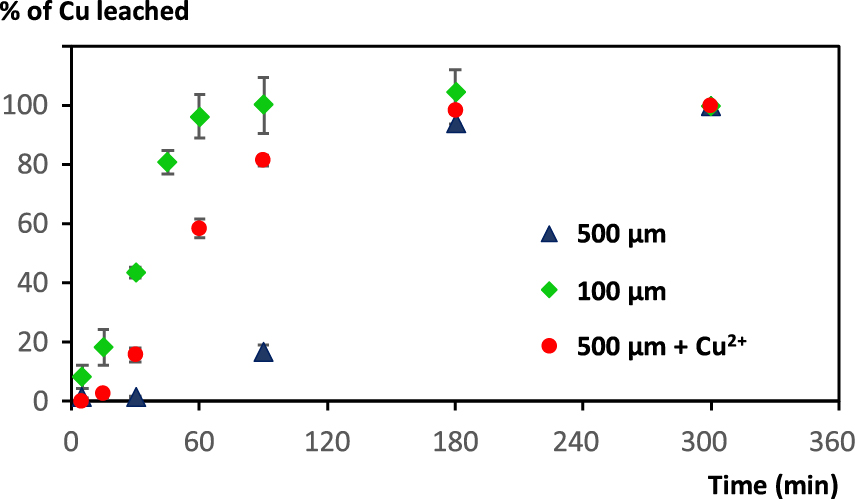
As observed previously on metallic Cu alone, leaching is more rapid when mean particle size is smaller. Quantitative Cu leaching was observed on the 100–250 μm fraction after 2 h, whereas the reaction profile on the 500–1000 μm particles fraction is sluggish, and quite similar to the non-sieved (<750 μm) batch previously employed. Also, addition of 10% Cu2+ ions enabled to increase the leaching rate (Figure 6). There seems to be still a short induction time, shorter than that observed on Cu wire (Figure 3). This point was attributed to the fact that during the pre-treatment, the very fine Cu particles should be preferentially dissolved, as they are more reactive. Altogether these results demonstrate that fine grinding of WPCB is not compulsory for efficient Cu recovery using foams. A minimum of grinding is necessary to achieve liberation of all Cu contained inside components, but excessive grinding is useless as leaching efficiency can be attained by initial addition of a small quantity of Cu2+ ions, which will be recovered afterwards. Also, in a continuous process, there should be always some Cu present in the leaching solution as Cu2+ ions. Furthermore, as stated beforehand, Cu is difficult to grind into very fine particles, and most Cu is contained in large particles: The 500–1000 μm fraction is the richest in Cu compared to other fractions. To our knowledge, the balance between cost in energy (not evaluated here) to lower the mean particle size, and Cu leaching efficiency has not been evaluated yet, whatever the leaching strategy.
3.5. Leaching of major metals from WPCB during the entire process
Based on these results, we examined the outcome of five major metals (Cu, Fe, Ni, Zn and Al) during the two-stage leaching sequence: first stage is a leaching in solution using a 3 M aqueous HCl solution (S/L ratio 1 L/83 g) during 5 h at room temperature, and second stage is a leaching in foam using again a 3 M aqueous HCl solution containing 2 mM Brij S10 (S/L ratio 1 L/50 g) during 5 h at room temperature. Repartition of these 5 metals between first leachate, second leachate and solid residue is given in Figure 7.
Repartition of major metals contained in ground WPCB according to the leaching stage and remaining solid residue (1st stage: 3 M HCl solution, S/L ratio 1 L/83 g, 5 h; 2nd stage: foam prepared using 3 M HCl and 2 mM Brij S10, S/L ratio 1 L/50 g).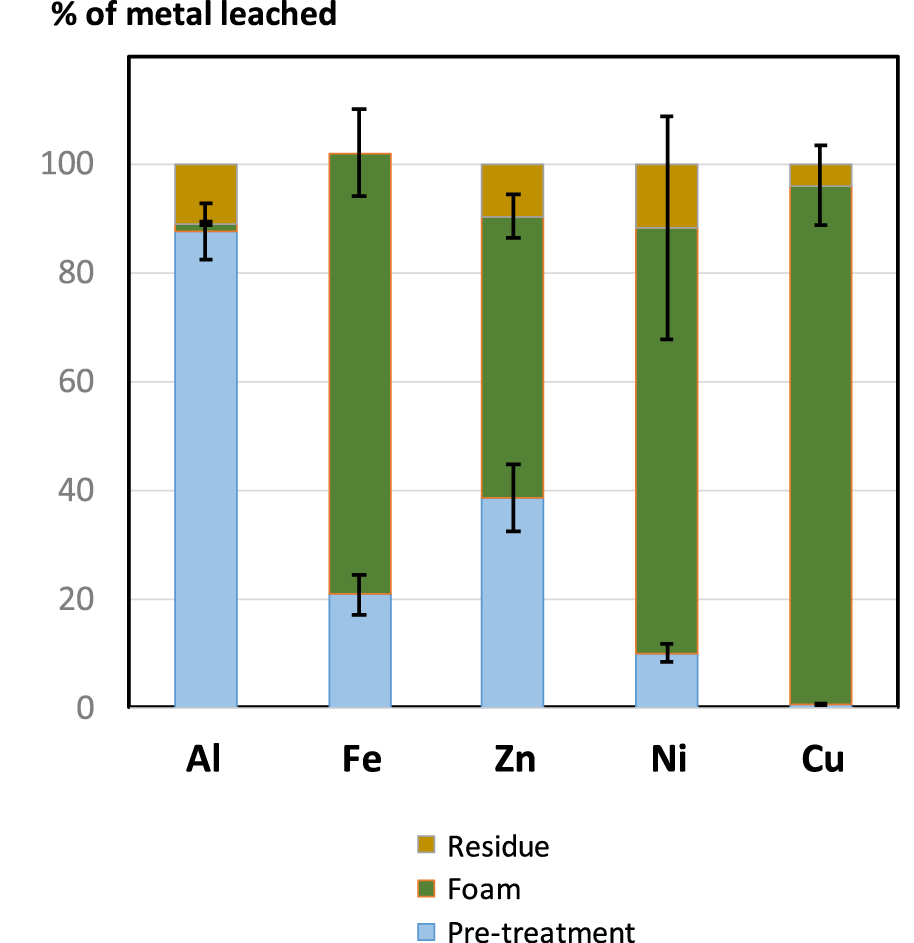
As expected, most Al is leached during the first stage. Remaining Al is found in the residue, probably as oxide incorporated in the glass fibers that reinforces the epoxy plastic support. Then, almost all the Cu is leached during the second stage, with a non-significant loss in the solid residue. The outcome of Fe, Zn and Ni is more intriguing as these metals are mostly leached during the second stage, although the acid quantity obtained in the first stage largely exceeds the amount required for complete dissolution. Passivation of metallic Zn with a resistant oxide surface can be invoked, but this argument may not stand for Fe, which passivation by oxide is inefficient. The subsequent isolation of copper from the foam leachate after foam collapse has not been investigated. A neutral surfactant employed, with a very low amount (2 mM, i.e. 1.4 g⋅L−1). Therefore, interferences with classical precipitation or electrodeposition processes are not anticipated. However, it is not clear whether these processes will be affected by easy foam formation, or if the final purity of copper will be altered. Finally, the reuse of the surfactant will require further dedicated studies.
4. Conclusion
Foams appear as an interesting alternative to classical aqueous solutions for the leaching of Cu from ground WPCB. By simply adding a small quantity of readily available surfactant Brij S10 (2 mM, i.e. ca. 1.4 g/L) into a dilute aqueous HCl solution, efficient Cu leaching was observed after injection of air into the solution and formation of the foam. The foam is stable over extended period and increases gas–liquid transfer, so that O2 contained in air can act as Cu oxidant. This approach is different from classical use of foams in hydrometallurgy (particular and ionic flotation, foam fractionation). Foams are used as a biphasic fluid medium to replace aqueous solutions employed for leaching, minimizing the amount of liquid employed. In continuation to our simple laboratory setup, our intention is to develop a pilot reactor (ca. 1 L scale), fully characterized in terms of gas–liquid transfer, with adequate gas injection and samplings systems. The efficiency of Cu leaching by the foam was improved by performing a first leaching in HCl solution (without surfactant), and a 2-stage strategy that enables complete Al removing, as well as part of Fe, Zn and Ni, was devised. The final leachate contains Cu in a concentration close to 10 g/L, and its processing using existing techniques such as precipitation can be easily envisioned. Furthermore, during this study we evidenced that Cu in e-waste accumulates in fraction of larger size during grinding, and that the average size of the fraction with the highest Cu content (500–1000 μm) is well suited to leaching with foams. Leaching is complete after 5 h, and the kinetics can be improved through addition of 10% Cu2+ ions to the leaching medium. Thus, extensive milling of the waste, which can be quite energy consuming, is not necessary. This study also revealed a particular behavior of Fe: although this metal was expected to be easily removed during first stage of leaching using dilute HCl aqueous solution without foam (i.e. without O2 as an oxidant), it proved to be reluctant to dissolution. On the contrary, during the leaching of Cu with the foam, Fe was easily dissolved along with Cu. The mutual interactions between both metals during leaching with HCl/O2 has not been studied to our knowledge. Detailed studies dedicated to the understanding of the mechanism of both Cu dissolution by HCl/O2 in foam and relationship with Fe are underway and will be reported in due course.
Declaration of interests
The authors do not work for, advise, own shares in, or receive funds from any organization that could benefit from this article, and have declared no affiliations other than their research organizations.
Funding
We would like to acknowledge ANR-17-CE08-0016 FOAMEX.
Acknowledgements
We would also like to acknowledge C. Monteux, N. Pantoustier, P. Bauduin and P. Perrin for fruitful discussions, and B. Baus-Lagarde for assistance during the ICP-OES analyses.




 CC-BY 4.0
CC-BY 4.0