1. Introduction
In the context of biomass valorization towards substitution of petroleum-based materials, sorbitans represent bio-sourced alternatives to a large number of petroleum-based molecules; and can be used in a wide range of applications such as additives, pharmaceuticals and monomers for polymer industries [1, 2, 3, 4]. In particular, the hydrogenation of glucose into sorbitol and the dehydration of sorbitol with various catalytic systems have been studied in depth [5, 6]. However, the synthesis of these diols remains, to this day, a technological challenge due to the cost of energy-consuming processes, use of expensive and toxic solvents as well as the intensive purification steps.
To this regard, it is worthwhile to develop “one-pot” protocols for the obtention of sorbitans starting from glucose combining heterogeneous catalytic hydrogenation and an acid-catalyzed dehydration reaction [5, 7]. Conventionally, strong mineral acids are used and they limit the selective obtention of sorbitans to favor its dehydration to isosorbide (produced by double dehydration of sorbitol). Being non-toxic and non-corrosive, gaseous CO2 has attracted attention in replacing environmentally unsafe mineral acid catalysts thanks to the reversible control of reaction mixture’s pH.
In order to study this one-pot reaction (hydrogenation and dehydration), we combined in situ spectroscopic analysis with molecular modeling to better understand the mechanisms and the kinetics in the formation of different reactions’ intermediates [8, 9, 10]. However, the thermodynamics of these multi-component mixtures are not clearly known. We recall that the aqueous dehydration of sorbitol was performed under H2 and CO2 pressure (between 30 and 120 bar); and at high temperature (up to 220 °C). The phase behavior and the mutual solubility of all the components of this multi-component mixture depend strongly upon the pressure/temperature conditions which in turn can significantly influence the selectivity and yield of the sorbitans synthesis during hydrogenation and dehydration occurring in the liquid phase.
The aim of this paper is to determine the thermodynamic behavior of binary, ternary, and quaternary systems composed of sorbitol/water/H2/CO2 at temperatures ranging from 40 °C to 220 °C; at CO2 pressure between 30 bar to 120 bar; H2 pressure at 30 or 60 bar. Additionally, we have focused our investigation on the gas phase using infrared spectroscopy which performs well in situ and in operando investigations. Of particular note, the evolution of the intensity of selected vibrational modes of CO2 and H2O have also been analyzed as a function of temperature and pressure in order to determine the evolution of H2O and CO2 concentrations in binary, ternary, and quaternary mixtures. From the results obtained, it was possible to show that in such multi-component system, a water-rich liquid phase coexists with a CO2-rich gas phase under our experimental conditions (T < 220 °C and P < 120 bar). In addition, it was found that sorbitol remains in the water-rich liquid phase over the thermodynamic range investigated.
2. Experimental setup
2.1. High pressure in situ infrared absorption setup
The in situ analysis of sorbitol–water–CO2–H2 mixture was done with IR spectroscopy in one high-pressure cell (Figure 1). Due to strong absorption of water, the liquid-phase analysis is best performed using ATR-FTIR equipment which can withstand high temperature and pressure. Due to the lack of such equipment, we have focused our study on the gas phase using a home made HP cell which can withstand high temperature of about 250 °C and pressure up to 20 MPa. The home-made stainless-steel cell is composed of three cylindrical windows (one sapphire window for visual observation and two silicon windows for IR absorption with a pathlength of 26 mm). Windows were positioned on the flat surface of an inconel plug with a Kapton foil placed between the window and the plug to compensate for imperfections at the two surfaces (unsupported area principle). Flat Kapton rings were used to ensure sealing between the plug and the cell body. Heating was achieved using four cartridge heaters inside the body of the cell and a thermocouple located close to one cartridge was used to regulate the temperature with an accuracy of ΔT = ±0.5 °C. The cell was connected via a stainless capillary to a pressurizing system which allows the regulation of pressure with an accuracy of ΔP = ±0.1 bar.
Design of the high-pressure experimental device with a high-pressure cell coupled with IR spectrometer for the in situ measurements of the CO2-rich phase of the sorbitol/water/H2/CO2 mixtures.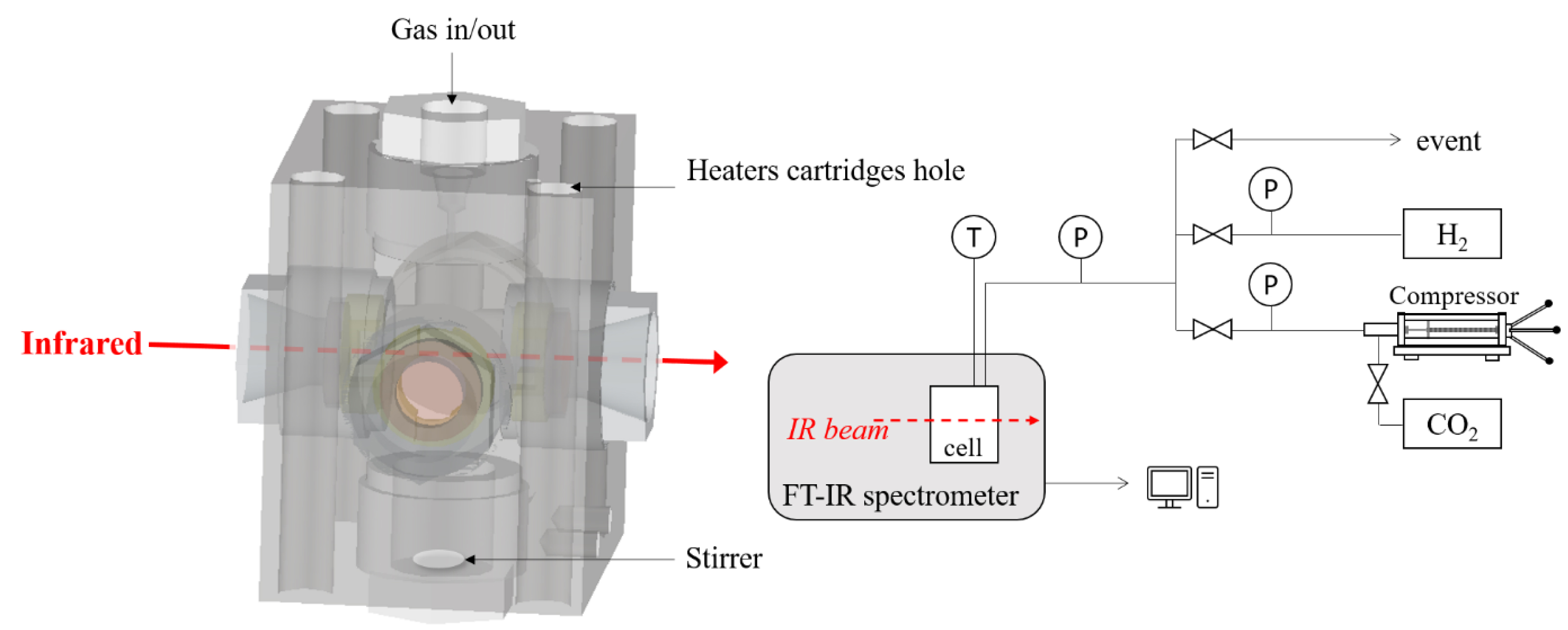
For IR absorption measurements, a Nicolet 6700 FTIR spectrometer equipped with a Globar source, a KBr/Ge beamsplitter and a DLaTGS/KBr thermal detector was used to determine the spectral range, from 400 to 6500 cm−1. Single beam spectra with 4 cm−1 resolution were obtained by Fourier transformation of 100 accumulated interferograms in order to improve the signal to noise ratio.
2.2. Experimental procedure
For the quaternary mixture, the lower part of the cell was filled with 1 ml of an aqueous solution of 30 wt% of sorbitol (purchased from Sigma Aldrich (>98% purity)). The experiments were performed by initially adding a predetermined pressure of H2 (purchased from Air Liquid (99.9999% purity)); CO2 (purchased from Air Liquid (99.95% purity)) was then added to this high-pressure cell using a manual pump (TOP industrie) up to the desired pressure. We mention that the notation 30/30 bar CO2/H2 means that 30 bar of H2 is introduced and then the cell was filled with CO2 until the total pressure reached 60 bar. Making the hypothesis that both CO2 and H2 behave as ideal gases and that their pressures can be added, we assumed that 30 bar of CO2 was added to the cell. The cell volume (8 ml) is fixed and only the pressure can be adjusted.
2.3. Infrared absorption spectra and calibration
Figure 2 illustrates (for the case of a binary H2O/CO2 mixture) the spectral changes of the infrared absorption spectra in the CO2-rich phase that occur with an increase of the temperature from 40 to 220 °C at a constant pressure of 60 bar.
Infrared spectra of the CO2-rich phase of the H2O–CO2 binary system at a constant pressure of CO2 of 60 bar; and temperature from 40 °C to 220 °C.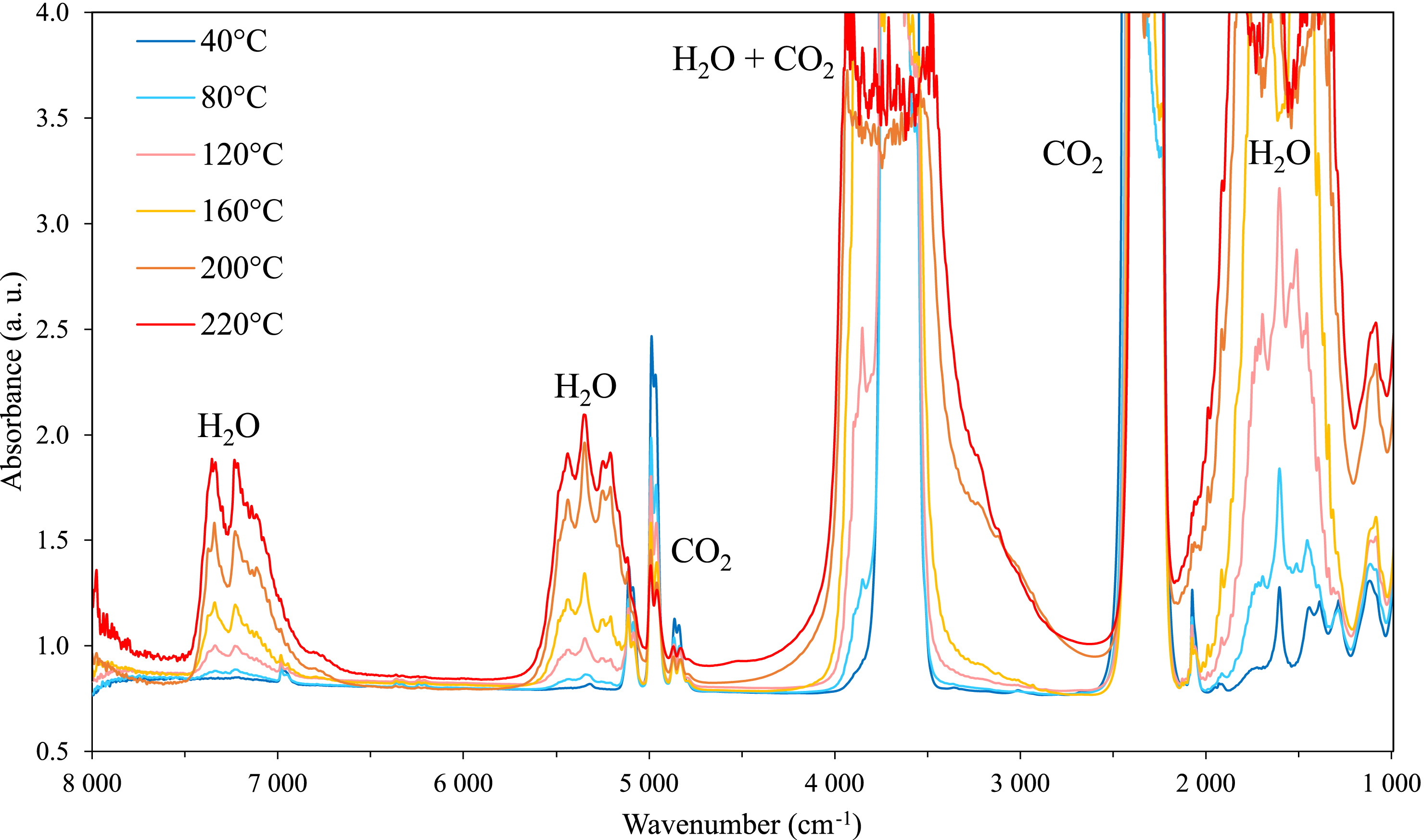
In that region, fundamental and combination bands of CO2 and H2O were observed. However, only the spectral range between 2500 and 3200 cm−1, and between 4500 and 7500 cm−1 are of interest for our purpose under our experimental conditions. Indeed, the strong absorption of CO2 and H2O precludes any quantitative analysis outside this spectral range.
For CO2, the region from 4750 to 5200 cm−1 with overlapping peaks of CO2 at 4800 cm−1, 4950 cm−1 and 5100 cm−1 are of interest and correspond to the combination modes 4𝜈2 + 𝜈3, 𝜈1 + 2𝜈2 + 𝜈3 and 2𝜈1 + 𝜈3, respectively. 𝜈1, 𝜈2 and 𝜈3 are the fundamental vibrational modes of CO2 with symmetric stretch, bending mode and antisymmetric stretch, respectively [11, 12, 13, 14]. The weak peak observed at 6950 cm−1 is assigned to the 3𝜈3 overtone of CO2.
For H2O, the band at 1600 cm−1 which is saturated at temperatures above 120 °C is related to the bending mode 𝜈2 of water [15]. The profile at 5300 cm−1 having an increasing intensity with the temperature, is assigned to the 𝜈2 + 𝜈3 combination mode of water [16, 17]. In 7000–7500 cm−1 spectral range, the profile with a doublet structure observed at 7200 cm−1 with an enhanced intensity with temperature is assigned to the 2𝜈3 overtone of water.
In order to quantify the concentration of CO2, we selected the vibrational mode 2𝜈1 + 𝜈3 of CO2 and determined its epsilon value from its integrated area (from 4900 to 4740 cm−1). This was selected as it negligibly overlaps with any contribution of water. We, then measured the infrared absorption spectra of neat gaseous CO2 at constant pressures of 30, 60 and 120 bar at 40, 80, 120, 160, 200 and 220 °C. Knowing the concentration of CO2 (in mol⋅L−1) from the NIST database (NIST Chemistry Webbook [18]), the pathlength of the cell (l) and measuring the integrated area (A) in 4900 to 4740 cm−1 range; the molar extinction coefficient (epsilon = 𝜀) values are then calculated by applying the Beer–Lambert law: A = 𝜀 ⋅ l ⋅ c (see Table 1). For all temperatures and pressures, it can be seen that there is a significant variation of the epsilon values with increase in pressure. Hence, these values are used as reference for the same given temperature and pressure to determine CO2 concentration in all analyzed mixtures.
Molar extinction coefficients (in L⋅mol−1⋅cm−2) of the 2𝜈1 + 𝜈3 mode of CO2 integrated in 4900 to 4740 cm−1 range
| 40 °C | 80 °C | 120 °C | 160 °C | 200 °C | 220 °C | |
|---|---|---|---|---|---|---|
| 30 bar CO2 | 2.010 | 2.101 | 2.114 | 2.014 | 1.931 | 1.806 |
| 60 bar CO2 | 1.916 | 2.013 | 1.970 | 1.903 | 1.791 | 1.675 |
| 120 bar CO2 | 1.341 | 1.645 | 1.688 | 1.646 | 1.532 | Saturate |
By the same token, to quantify the concentration of water, selecting a vibrational mode of water and determining its epsilon value is required. In water–CO2 mixtures, the molar fraction of CO2 can be calculated from the concentration of CO2 and water. We selected the 𝜈2 + 𝜈3 combination mode of water and used its integrated area from 5800 to 5347 cm−1 to calculate the concentration of water. We emphasize that only the high frequency wing of the mode of water has been considered for integration as the low frequency range is superimposed with a contribution of CO2. Then, the epsilon of this band associated with water is fitted to obtain an experimental molar fraction of water calculated from the experimental water and CO2 concentration, that is consistent with the molar fraction in the literature [19, 20]. As shown in the Figure 3, a good fit is obtained in the whole temperature and pressure range specific to this study when a unique epsilon value of water is used and found to be equal to 140 L⋅mol−1⋅cm−2. Thus, we have evaluated a relative uncertainty of ±5% on the concentration measurements using our setup and data processing methodology.
Comparison of the molar fraction of CO2 in the CO2-rich phase of H2O–CO2 mixtures calculated from our infrared spectra with literature data.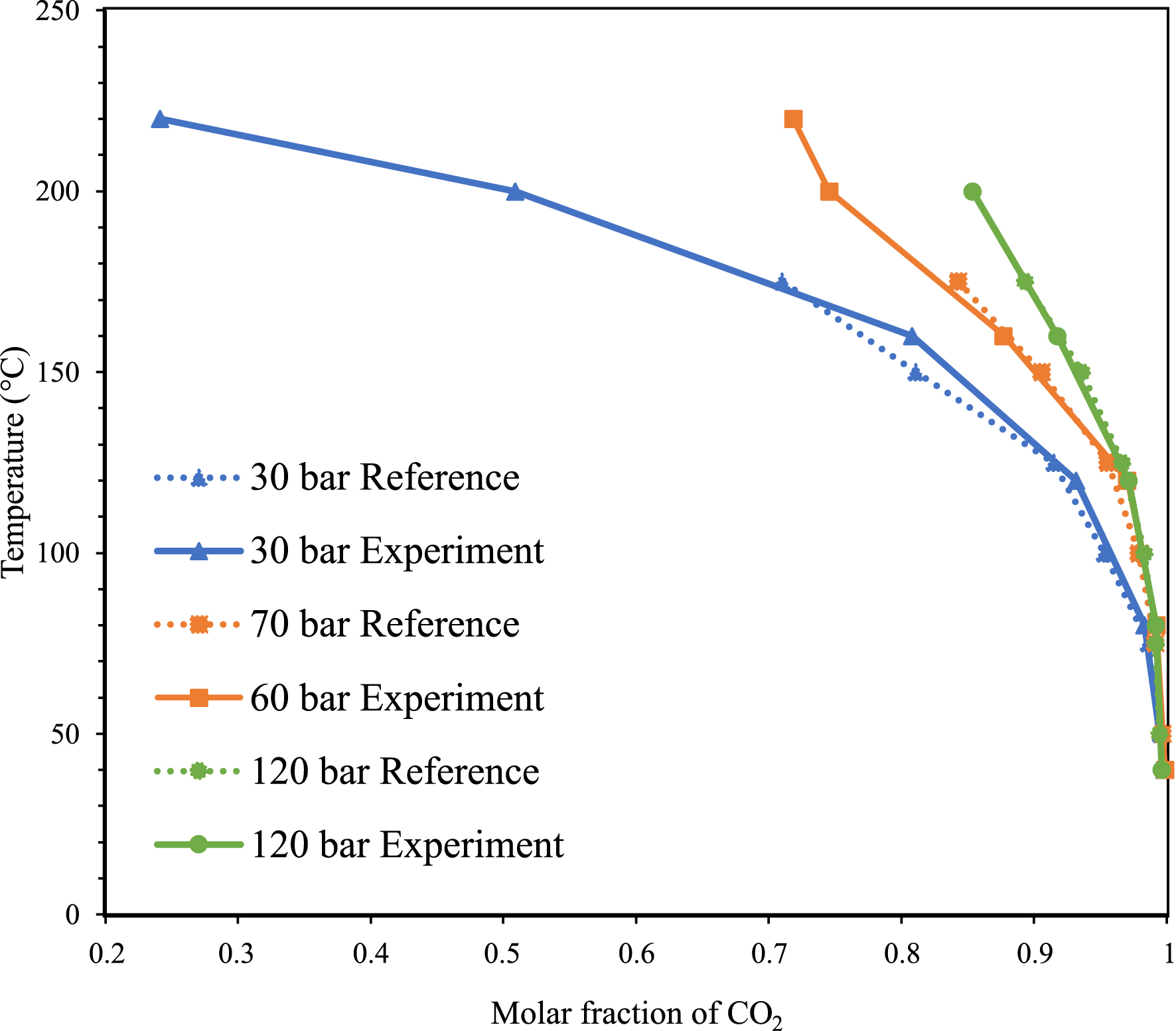
Finally, it is worth noting that if sorbitol is soluble in the CO2-rich phase, one should observe a peak in the alkane region (in the range 2800–3000 cm−1) related to C–H stretching vibrations with a detection limit of about 10−4–10−5 gsorbitol/gCO2. We emphasize that the initial total amount of sorbitol is excessive in comparison to the minimum amount of sorbitol that could be detected.
In a few experiments, the collision-induced IR spectral band of H2 is also observed at about 4150 cm−1 (see Figures 5 and 12 in the results and discussion section). This band has been reported previously for H2 diluted in monoatomic gases under high pressure conditions [21, 22]. Indeed, as the H2 stretching vibration is inactive in IR spectroscopy, the observed H2 spectrum in the CO2-rich phase is due to an induced dipole moment of the H2 molecule that results from its interaction with the surrounding CO2 molecules. Therefore, the induced molar extinction coefficient of the H2 peak strongly depends on the temperature and pressure and precludes the use of this peak for quantitative analyses [23].
3. Results and discussion
3.1. H2O–CO2 binary system
Preliminary studies are done in H2O–CO2 binary system to analyze the influence of water on the CO2 concentration in the CO2-rich phase based on the pressure of CO2 and temperature. Infrared spectra of the gas phase of the binary system are illustrated in Figure 2 and has been described in earlier sections. Beer–Lambert law is applied to determine the evolution of the concentration of CO2 in the CO2-rich phase of the H2O–CO2 binary mixture and compare it with the concentration of neat CO2 in the same thermodynamic range from 40 to 220 °C at constant CO2 pressures (30, 60 and 120 bar as shown in Figure 4).
Temperature versus CO2 concentration at constant pressure in neat CO2 and in the CO2-rich phase of the binary system H2O–CO2.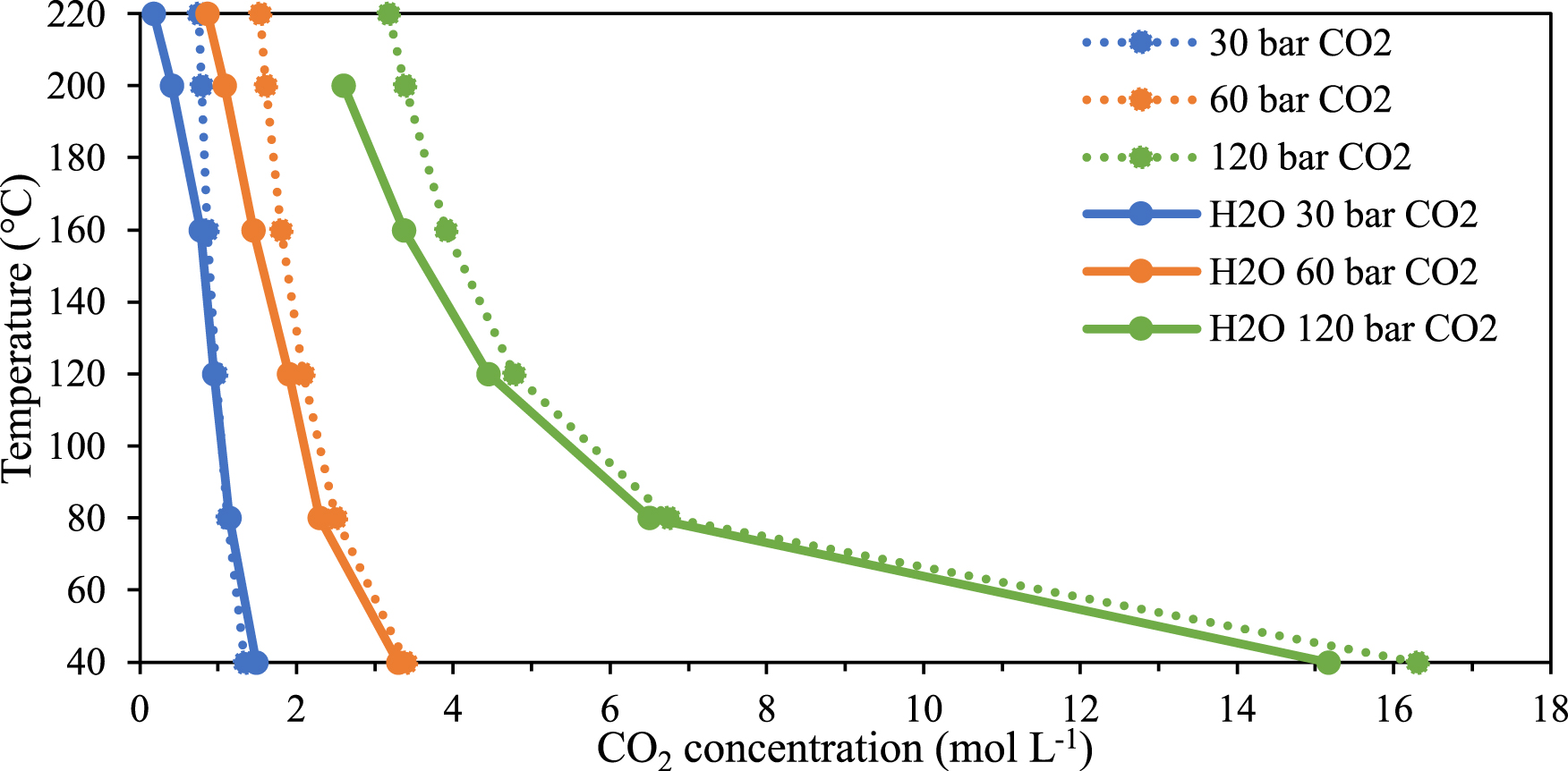
Looking initially at CO2 alone (Figure 4, dotted lines), it can be inferred that the concentration of CO2 gas decreases when the temperature rises. This effect is very pronounced at high pressure of 120 bar and less marked at 60 and 30 bar. In the presence of water (Figure 4, solid line), the same effect is observed but between 160 and 220 °C, the concentration of CO2 decreases further. This effect can be explained by two phenomena: as temperature increases, CO2 dissolves in water and consequently, its concentration in the gas phase decreases and concomitantly, water is “evaporated” in the gas phase leading to a decrease in CO2 concentration. Both phenomena governing the mutual solubility of water and CO2 are consistent with data reported in the literature [19, 20].
Infrared spectra of the binary system CO2–H2 at 90 bar from 40 °C to 220 °C.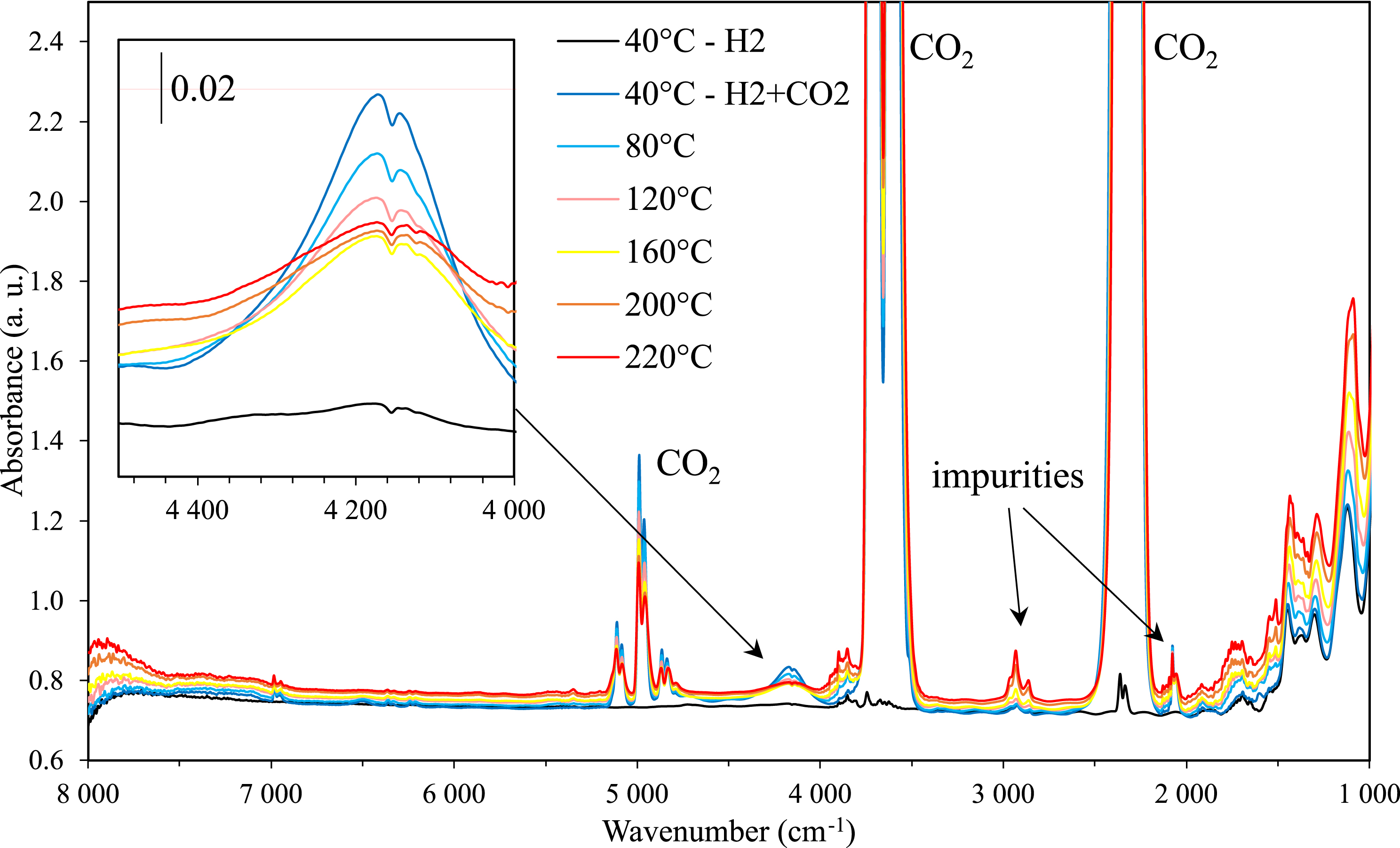
3.2. CO2–H2 binary system
The evolution of the infrared spectra of the binary mixture CO2/H2 (30 bar/60 bar) with temperature is elucidated in Figure 5. We emphasize that above the critical point of CO2, the binary mixture H2/CO2 displays only one gas phase as previously reported in thermodynamic studies on H2/CO2 mixtures [24, 25, 26]. Interestingly, a barely detectable band at 4100 cm−1 in neat H2 is clearly observed after adding CO2 in the cell at 40 °C. Then, its intensity decreases continuously as the temperature rises. This band is assigned to the vibrational stretching mode (vibron) of H2 interacting with surrounding CO2 molecules and the effect of CO2 on this band has been discussed by our group in a recent article [23]. As the intensity of this band is related to “induced” effects, it is not possible to accurately calculate the concentration of H2 in the mixture.
However, in order to determine the effect of H2 on the CO2 concentration, we determined the evolution of the concentration of CO2 in the H2–CO2 binary mixture and compared it with the concentration of neat CO2 in the same thermodynamic range (from 40 to 220 °C) at constant pressures of CO2 (30 and 60 bar) as shown in Figure 6. The concentration of CO2 appears to decrease at 60 bar of H2 (yellow line in Figure 6), while at 30 bar (of H2) negligible effect is observed (grey line in Figure 6). When the pressure of CO2 is doubled from 30 to 60 bar while keeping the pressure of H2 at 30 bar results in a weak decrease of the concentration of CO2 in comparison with neat CO2 at low temperature (below 120 °C). This effect could be attributed to the intermolecular interactions between H2 and CO2 as evidenced by “induced” effects on the vibrational stretching mode of H2.
Temperature versus CO2 concentration at constant pressure in neat CO2 and in the CO2-rich phase of the binary system CO2–H2.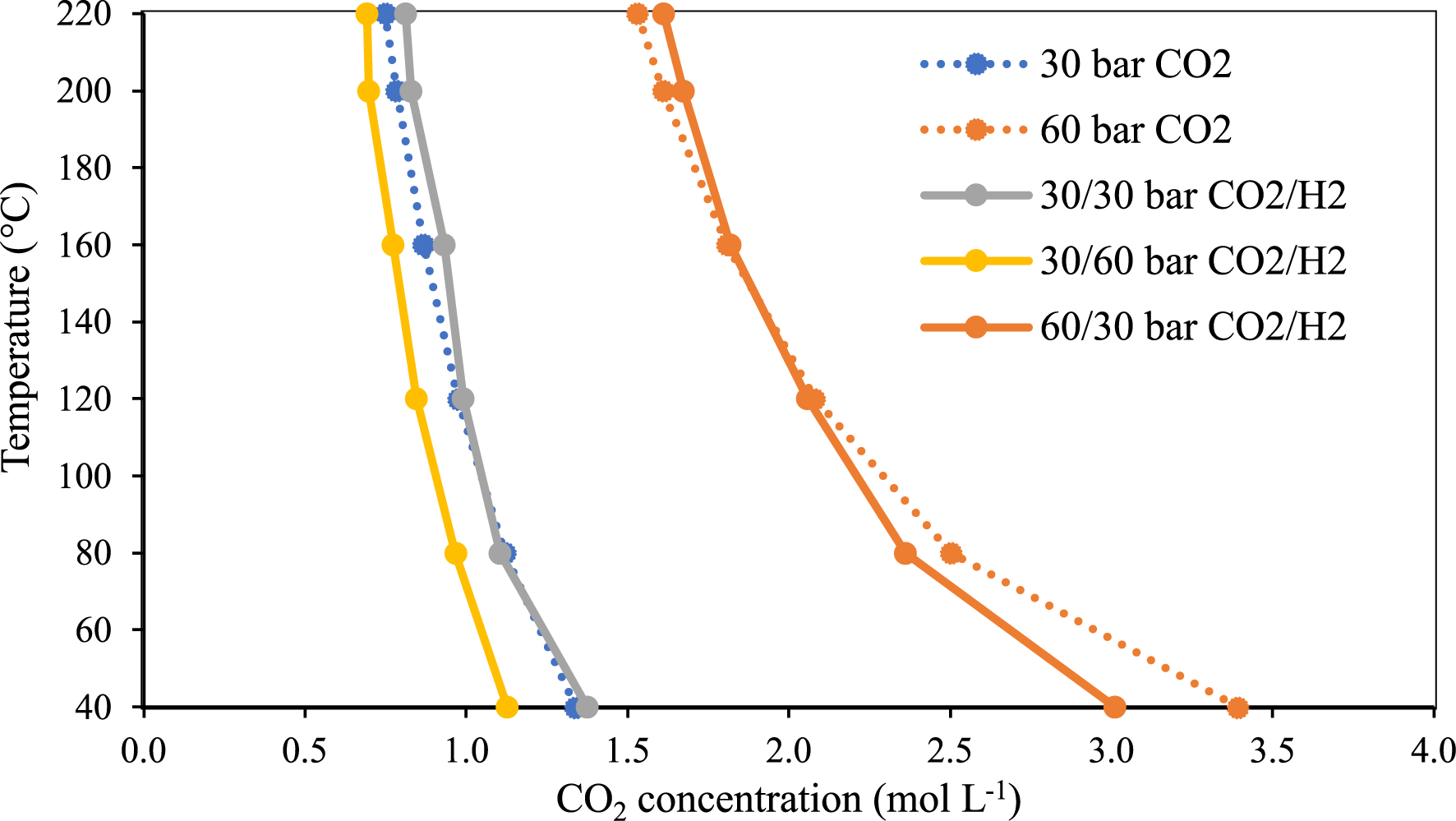
3.3. H2O–CO2–H2 ternary system
The binary system comprising CO2 and H2 is compared to the ternary system where water is added to the high-pressure cell at a given pressure of H2 and CO2 (Figure 7). At temperatures below 150 °C, concentration of CO2 in the H2/CO2 binary mixture is similar to that of the ternary mixture with water. However, at temperatures above 150 °C, a significant decrease in CO2 concentration is observed in the CO2-rich phase of the ternary mixture when compared to CO2-rich phase of the binary system CO2–H2.
Temperature versus CO2 concentration at constant pressure in the CO2-rich phase of the binary (CO2–H2) and ternary (H2O–CO2–H2) systems.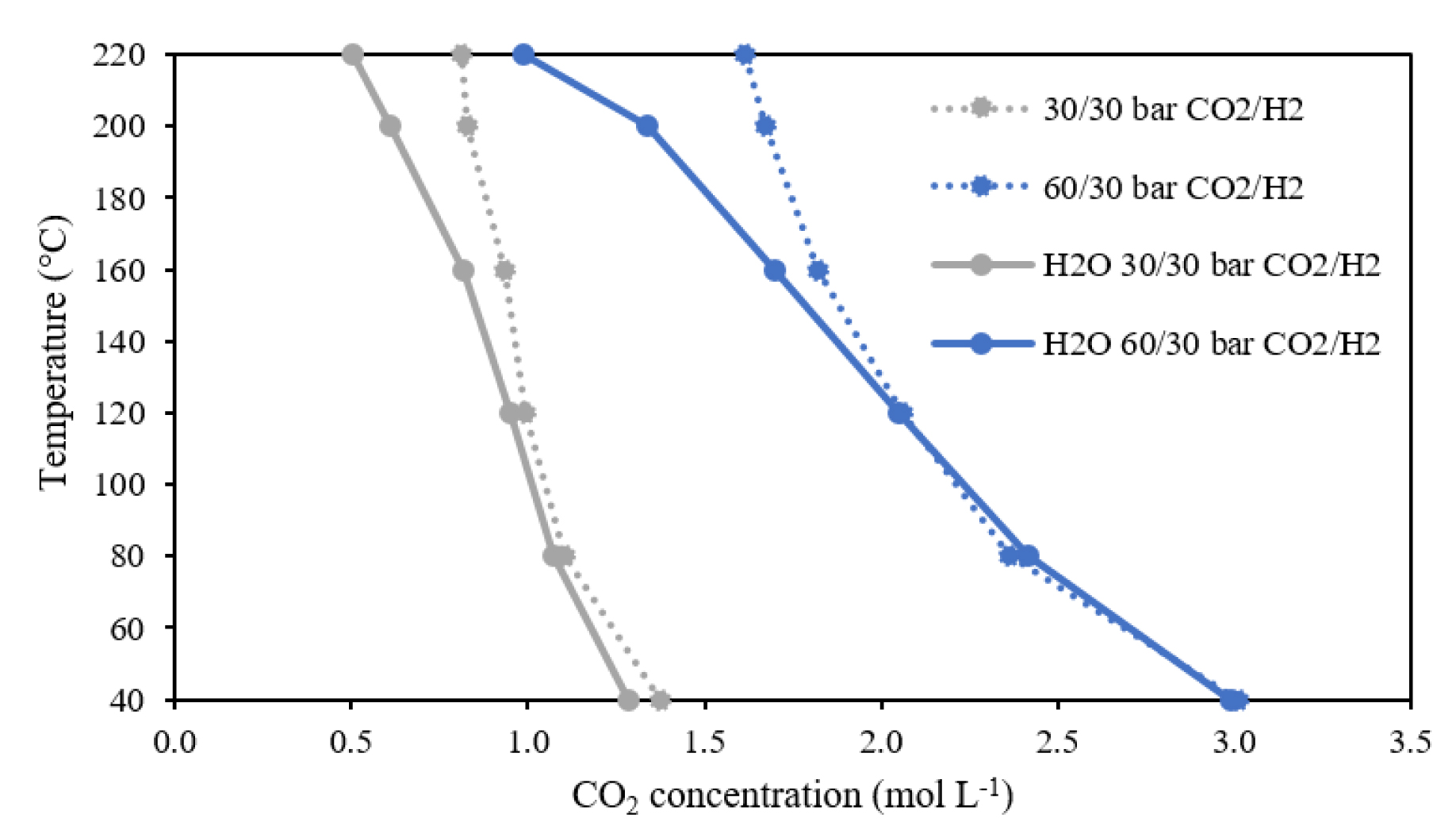
To determine if the presence of H2 has a significant effect on the solvation of water in the gas phase, for a given ratio H2/CO2 (see Figure 8), the evolution of water concentration according to the temperature for the binary (H2O–CO2) system is compared with the ternary system (H2O–CO2–H2). First, the increase of the CO2 pressure from 30 bar to 60 bar slightly increases the concentration of water (dotted lines). For example, at a constant temperature of 200 °C, the water concentration in the vapor phase is 0.36 mol⋅L−1 and 0.41 mol⋅L−1 for 30 bar and 60 bar of CO2, respectively. When H2 is added to the system (full lines), negligible effect is seen when 30 bar of CO2 mixes with 30 bar of H2 (yellow line). However, in the system comprising 60 bar of CO2, the addition of 30 bar of H2 leads to a slight decrease of the solubility water in the CO2 phase (orange lines) and is similar to that measured with 30 bar of CO2. Thus, the lower solubility of water in the CO2 phase in the presence of H2 could be due to the lower CO2 concentration in the CO2 phase as shown for the binary system CO2–H2 (cf. Figure 7) as well as to the fact that H2 is a highly hydrophobic molecule that is expected to limit the solubility of water in the CO2 phase.
Temperature versus H2O concentration at constant pressure in the CO2-rich phase of the binary (H2O–CO2) and ternary (H2O–CO2–H2) systems.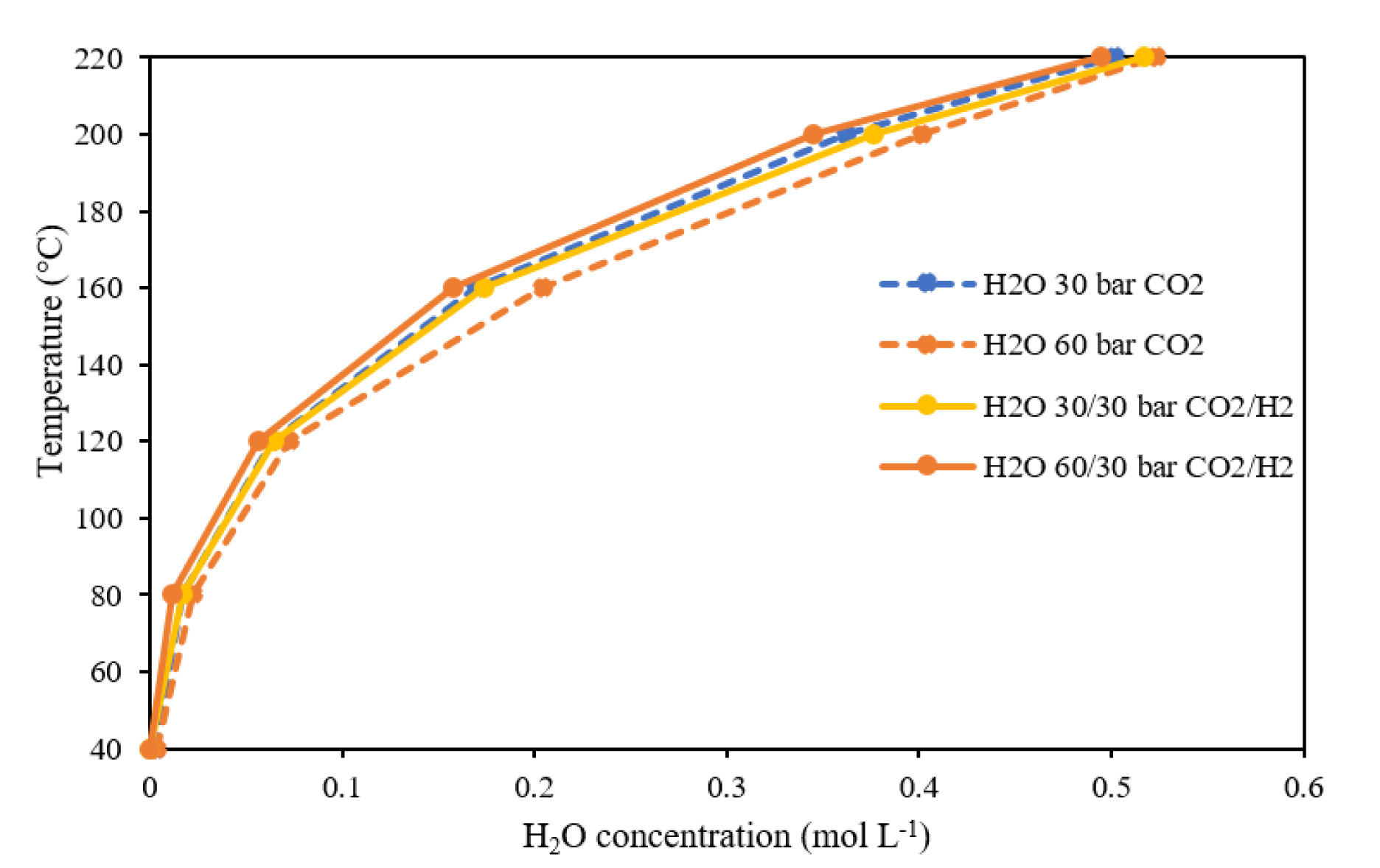
3.4. H2O–sorbitol–CO2 ternary system
Figure 9 displays the infrared spectra of the gas phase of a ternary system, an aqueous solution of 30 wt% of sorbitol under CO2 pressure. It is worth noting that the evolution of the profiles is similar to that observed when the temperature is increased in H2O–CO2 binary system (cf. Figure 2). This suggests that sorbitol is not detected in the CO2-rich phase (under our detection limit of 10−4–10−5 gsorbitol/gCO2) as we have not observed any new peaks related to sorbitol between 2900 cm−1 and 1100 cm−1.
Infrared spectra of the CO2-rich phase of the ternary system H2O–sorbitol–CO2 at 60 bar from 40 °C to 220 °C.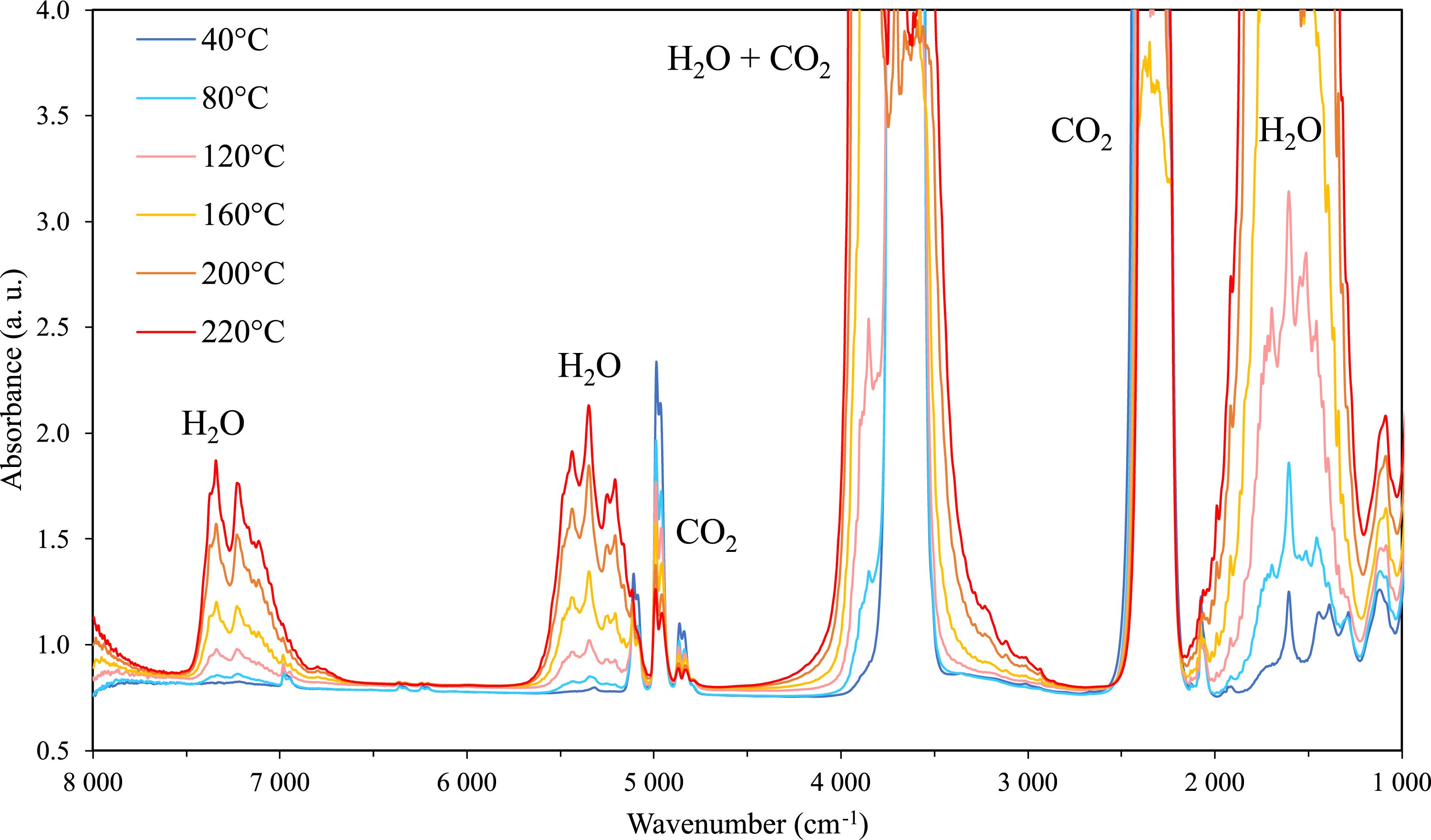
The CO2 concentration in the CO2-rich phase of the H2O–sorbitol–CO2 ternary mixture is compared to that of the H2O–CO2 binary system (Figure 10). No significant difference in the CO2 concentration was seen at 30 or 60 bar of CO2. However, at a higher CO2 pressure of 120 bar, the concentration of CO2 slightly increases (green, full line) when sorbitol is added to water. It can be inferred that the presence of sorbitol in water has no effect on the CO2 concentration in the gas phase at low CO2 pressures and higher pressure of 120 bar, sorbitol modifies the water–CO2 equilibrium.
Temperature versus CO2 concentration at constant pressure in the CO2-rich phase of the binary (H2O–CO2) and ternary (sorbitol–H2O–CO2) systems.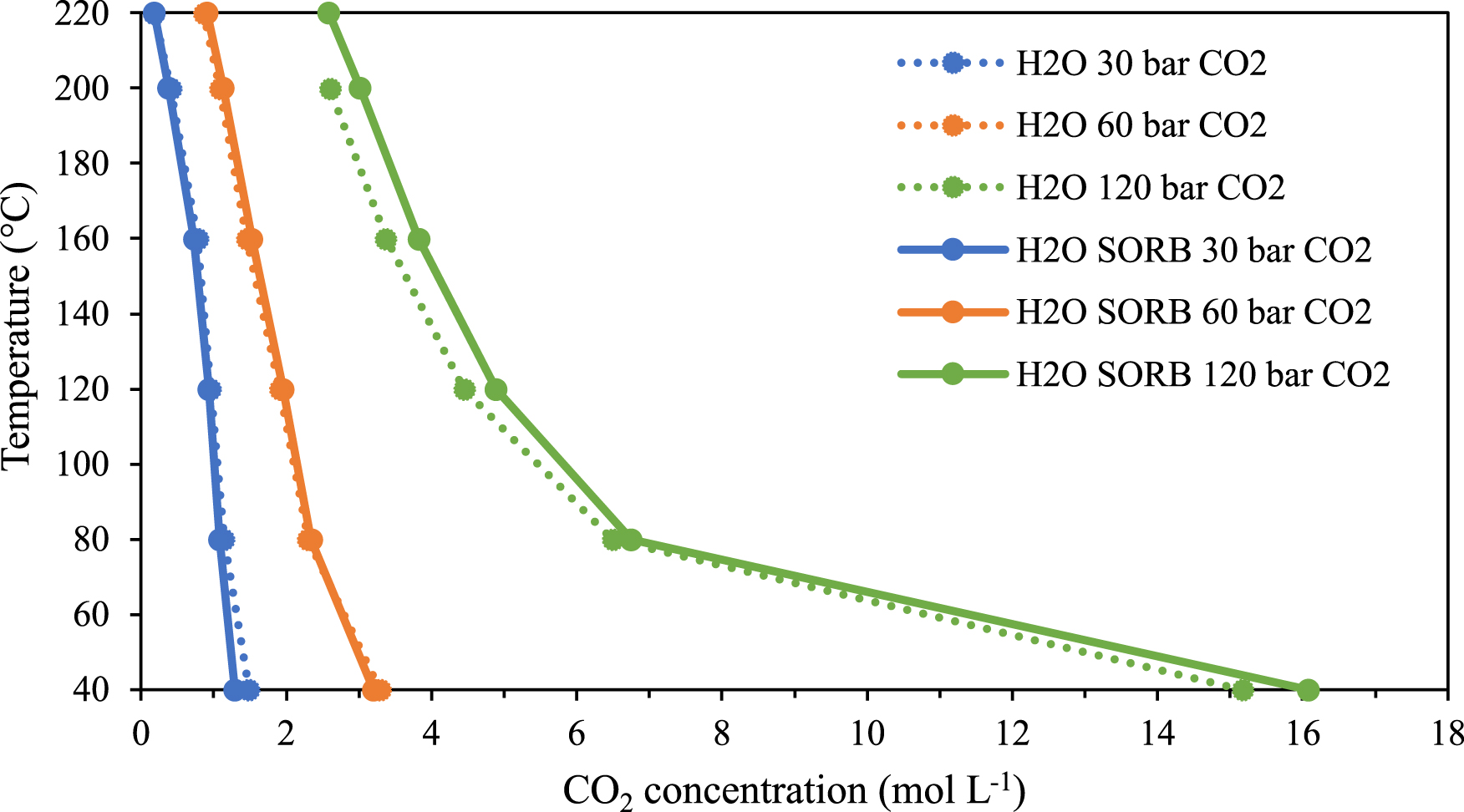
In order to assess the modification of the water–CO2 equilibrium due to the presence of sorbitol in the aqueous phase, the evolution of the concentration of water in the CO2-rich phase for the H2O–sorbitol–CO2 ternary mixture with temperature is observed (see Figure 11) and compared with the results obtained for the H2O–CO2 binary system. As shown in Figure 10, no effect is seen at CO2 pressure < 60 bar, as the concentration of water in the CO2-rich phase is similar for the binary and ternary systems. However, at CO2 pressure of 120 bar, the water concentration decreases from 0.3 mol⋅L−1 to 0.24 mol⋅L−1 at 160 °C. Therefore, it can be inferred that sorbitol prevents the solvation of water in the CO2-rich phase by “keeping” water in the liquid phase and leads to a higher concentration of CO2 in the CO2-rich phase.
Temperature versus H2O concentration at constant pressure in the CO2-rich phase of the binary (H2O–CO2) and the ternary (sorbitol–H2O–CO2) systems.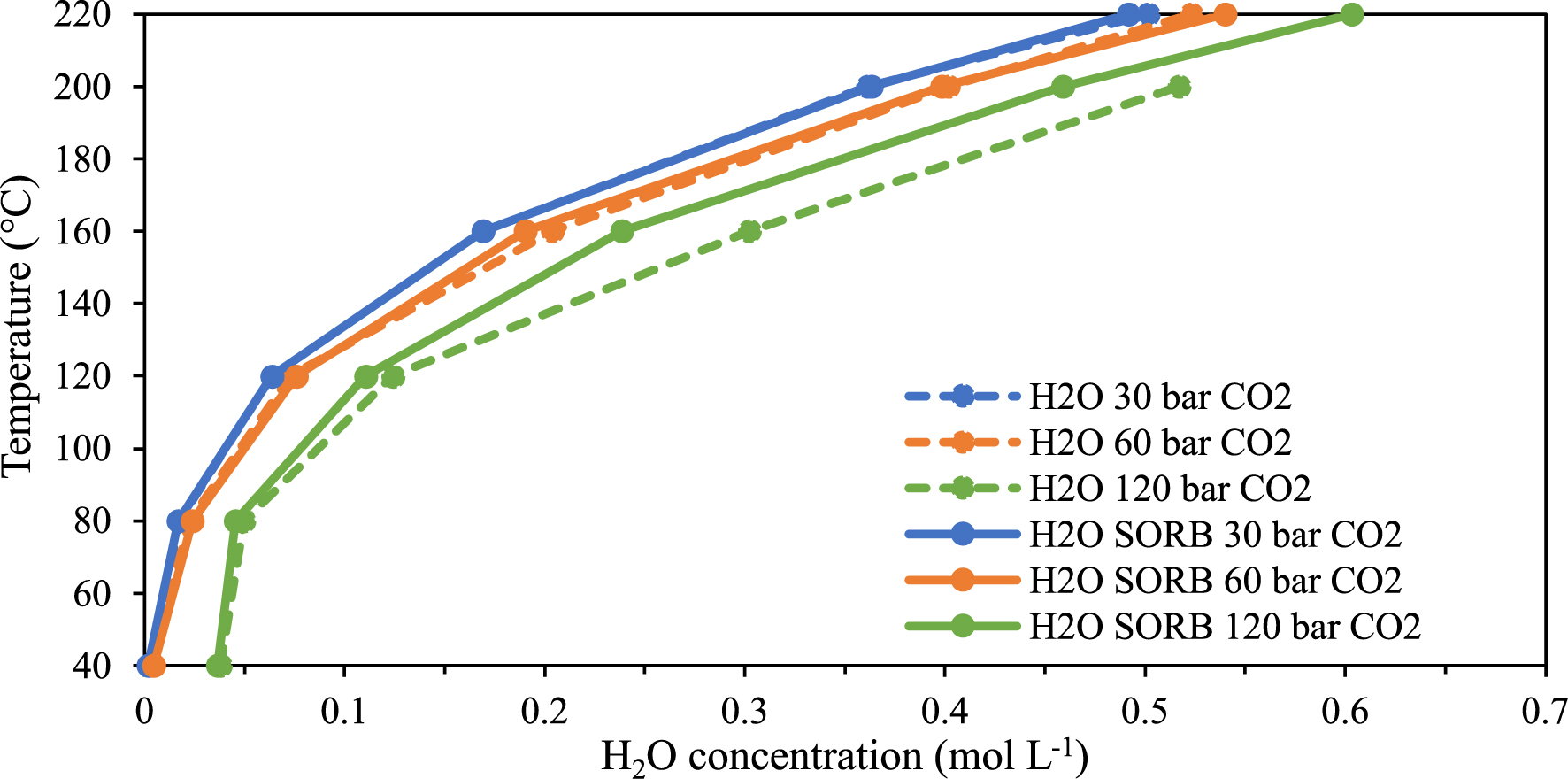
3.5. H2O–sorbitol–CO2–H2 quaternary system
Figure 12 shows the evolution of the infrared spectra in the CO2-rich phase as a function of temperature of the quaternary system comprising an aqueous solution of 30 wt% of sorbitol and the mixture of CO2 and H2 gases. The “induced” peak of H2 at 4150 cm−1 is only observed at low temperatures. With increase in temperature, the band of water increases and overlaps H2 while the bands of CO2 decrease. As seen in the ternary system (Figure 9), sorbitol is not detected in the CO2-rich phase.
Infrared spectra of the CO2-rich phase of the quaternary system H2O–sorbitol–CO2–H2 at 90 bar at temperatures from 40 °C to 220 °C.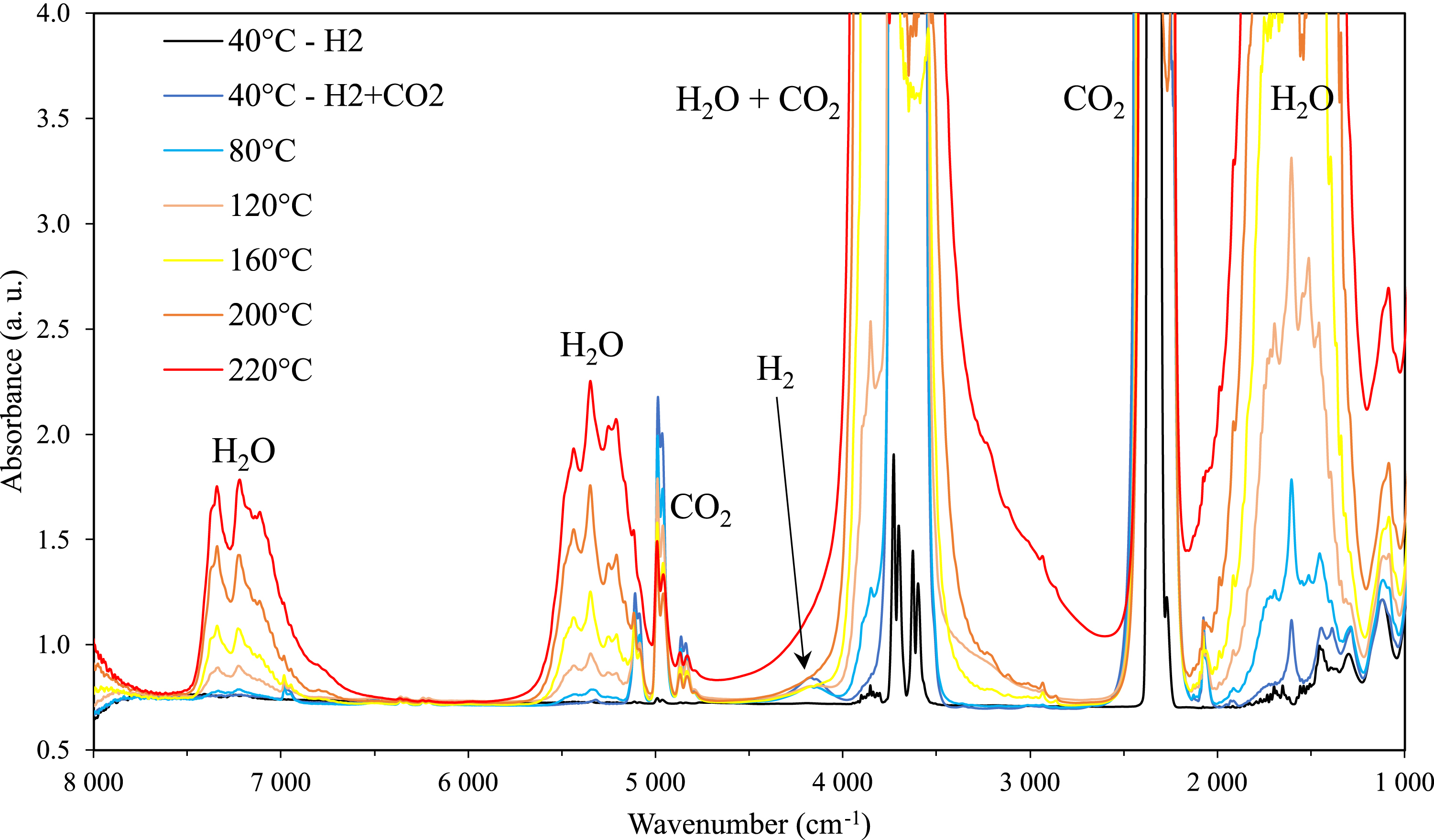
In Figure 13, the concentration of CO2 in the CO2-rich phase for the quaternary mixture is compared with the ternary system H2O–CO2–H2. It appears that at the pressure range investigated (corresponds to conditions used for the catalytic reactions reported previously [8, 10]), the addition of sorbitol does not have a significant effect on the CO2 concentration in the presence of H2.
Temperature versus CO2 concentration at constant pressure in the CO2-rich phase of the ternary (H2O–CO2–H2) and quaternary (H2O–sorbitol–CO2–H2) systems.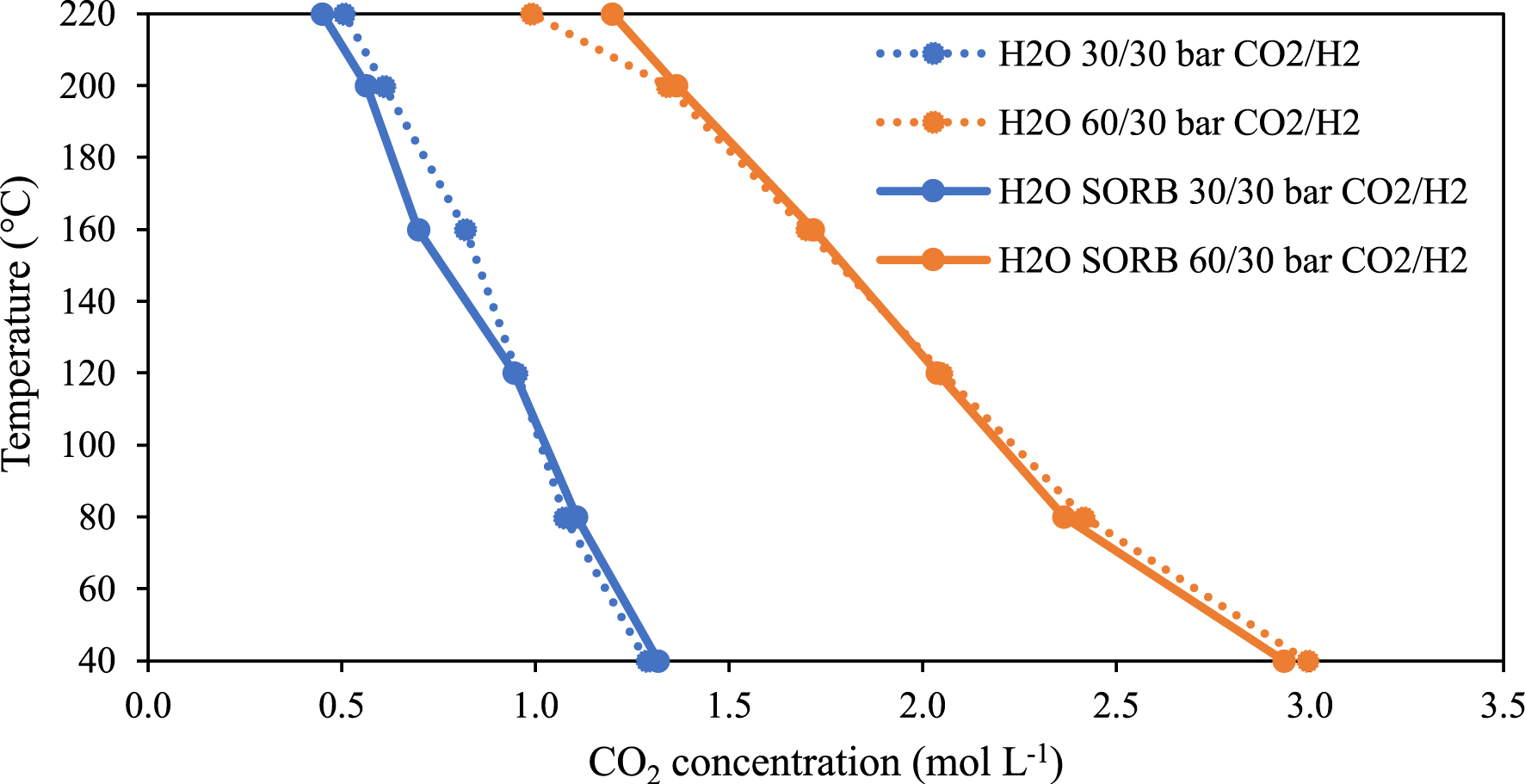
By the same token, the quaternary system is compared to the ternary system H2O–sorbitol–CO2 to study the effect of H2 on water concentration in the CO2-rich phase (cf. Figure 14). Almost no effect is observed, which is consistent with the results reported in Figure 13.
Temperature versus H2O concentration at constant pressure and increasing temperature in the CO2-rich phase of the ternary (H2O–sorbitol–CO2) and quaternary (H2O–sorbitol–CO2–H2) systems.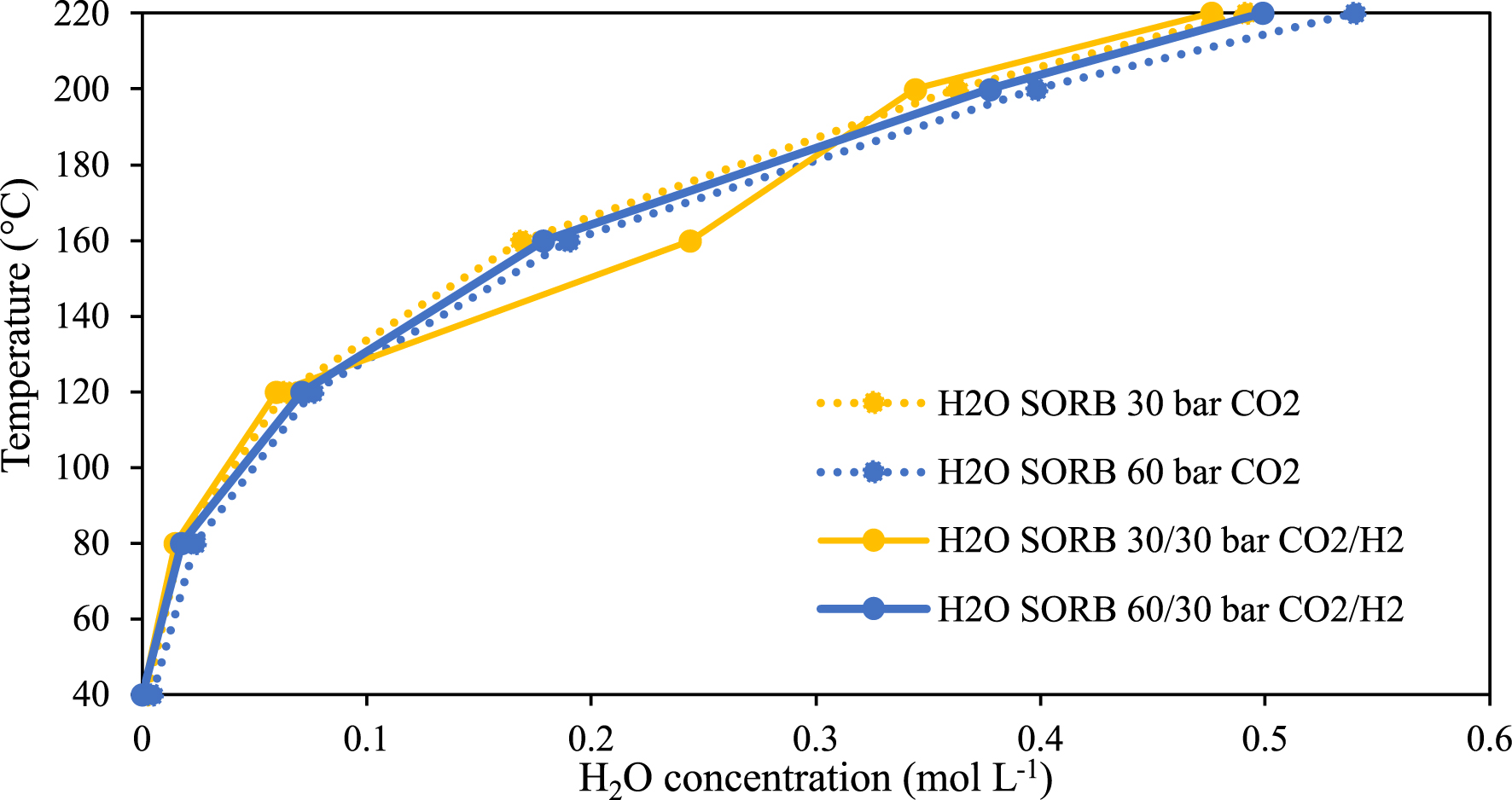
Therefore, at the pressure and temperature range investigated where two distinct water-rich and CO2-rich phase are observed, the presence of sorbitol in the water-rich phase and H2 in the CO2-rich phase have a limited impact on the mutual solubility of water and CO2.
4. Conclusion
The aim of this paper was to investigate, using in situ infrared spectroscopy, the thermodynamic behavior of the H2O–sorbitol–CO2–H2 system which is involved in the one-pot catalytic reaction for hydrogenation of glucose to sorbitol and the acid-catalyzed dehydration of sorbitol by CO2 [8, 10]. Specifically, our study focused on the gas phase of binary, ternary and quaternary mixtures and selected vibrational modes of CO2 and H2O that have been analyzed in order to determine the evolution of the H2O and CO2 concentrations as a function of the temperature and pressure.
At a given pressure of CO2, with increasing temperature, the concentration of water in the gas phase increases with a concomitant decrease of the CO2 concentration when compared with neat CO2. Addition of H2 at a pressure of 30 bar to H2O/CO2 binary system lowered the concentration of both CO2 and water in the gas phase. On the other hand, the addition of sorbitol to water appears to prevent the mutual solubility of CO2 and water, an effect that is significant only at high CO2 pressure (120 bar) investigated in this study.
Finally, the purpose of these thermodynamic measurements was to determine whether sorbitol, present in the liquid water phase at high temperature, could dissolve in the gas phase and reduce the sugar concentration in water, thus affecting the catalytic reactions mentioned earlier. However, an inverse relationship was observed, where both sorbitol and H2 limited the water solubility in the gas phase to some extent. Therefore, we confirm that under our experimental conditions (T < 220 °C and P < 120 bar), the ternary and quaternary mixtures display two distinct phases, sorbitol/water-rich liquid and CO2/H2-rich gas phase, both reactions of hydrogenation and dehydration taking place in the liquid phase.
Declaration of interests
The authors do not work for, advise, own shares in, or receive funds from any organization that could benefit from this article, and have declared no affiliations other than their research organizations.
Funding
The authors acknowledge the Region Nouvelle Aquitaine and the University of Poitiers for their financial support (funding of the PhD of IB). The authors are also grateful to the program “Instrumentation in situ en conditions extrêmes” of the MITI of CNRS for financial support towards infrared and Raman equipment, the INCREASE Federation and the GDR 2035 SolvATE.




 CC-BY 4.0
CC-BY 4.0