1. Introduction
Metal complexes have long been used as therapeutic agents. In the modern era, arsphenamine, toxic to syphilis-causing Treponema pallidum, or antimony compounds, toxic to leishmania, have been successfully developed as metal-based drugs in the early 20th century. However, most modern medicines are organic molecules. There has been a revival of inorganic medicinal chemistry with the discovery that cis-Pt can be toxic to cells with high division rate, with application as anticancer agent. Most of these compounds are meant to be used in living organisms and exert their activity at a cellular level. Because cells are compartmented, bioactivity can vary depending on the subcellular distribution and on the organelle reached. Cells are a very particular environment, with high viscosity, molecular overcrowding impacting diffusion [1], and abounding with metal cations and Lewis bases, which are strong competitors for metal cation coordination. This is why the use of metal complexes raises new specific challenges associated with possible decoordination, metal exchange, etc., that participate to their metabolization, with the possibility of precipitation when the metal cation is released. Predicting these exchanges is not easy due to the complexity of biological media. Exploring the integrity of a complex in cells is key to understanding its activity. Thus, it is important to integrate the design of metal complexes with their study in a cellular context, an approach for which the terms cellular inorganic chemistry or inorganic chemical biology can be coined [2].
Most of the metal complexes studied in the literature for their bioactivity are used as anticancer, antibacterial or antifungal agents, with requirements of selectivity—for cancerous cells, bacteria or fungi—versus normal healthy cells of the host. Hitherto, most bioanalyses have focused on evaluating the toxicity towards cancer cells, microorganisms, parasites, or fungi, which should be selective relative to vertebrate noncancerous cells. There is another class of metal complexes meant to restore the normal cellular life or rescue cells from a pathological or intoxication situation. This is the case for instance of functional mimics of rhodanese, an enzyme that detoxifies cyanide by catalyzing its conversion to thiocyanate [3, 4]. Other catalytic metal-based drugs [5] can be developed to protect the cell from oxidative damages, as catalytic antioxidants mimicking protective enzymes such as superoxide dismutase (SOD) or catalase (CAT), called SOD mimics and CAT mimics [6, 7, 8, 9]. Like the corresponding enzymes, these complexes are meant to catalytically react with reactive oxygen species (ROS), and do not act as inhibitors of ROS production. They must be nontoxic redox catalysts and they are meant to restore the basal activity in cells under oxidative distress. Their characterization therefore requires the development of specific non-routine strategies to evaluate their bioactivity, which is to restore normal cell life from a situation where it has been compromised [2, 10, 11, 12]. Note also that, as activation of paramagnetic dioxygen (S = 1) can be performed by paramagnetic species, there is a strong intertwining between biochemistry of metal ions and metal complexes and that of dioxygen and ROS. This is why, in parallel with the development of SOD and CAT mimics, we have developed a particular interest in cellular models related to pathologies of oxidative stress that will be described in the following.
2. Oxidative stress, distress and eustress
Dioxygen (O2) is necessary for aerobic life. Its ground state is S = 1 with two unpaired electrons in the highest occupied molecular orbital (HOMO). This diradical ground state nature (triplet state 3O2) explains its slow reaction with organic molecules (mostly S = 0) [13]. O2 sequential monoelectronic reductions lead to superoxide (O2∙−), hydrogen peroxide (H2O2) or hydroxyl radical (HO∙), and then H2O (see Figure 1). The partially reduced species—O2∙−, H2O2 and HO∙—are much more reactive than dioxygen itself, with no spin-state kinetic barrier for the reaction with singlet molecules, and hence they are called reactive oxygen species (ROS). They are continuously produced in biological systems by endogenous mechanisms, and their concentration is maintained to low enough levels by protective pathways such as superoxide dismutase or catalase, proteins carved by evolution that catalyze respectively (7) and (8)1 .
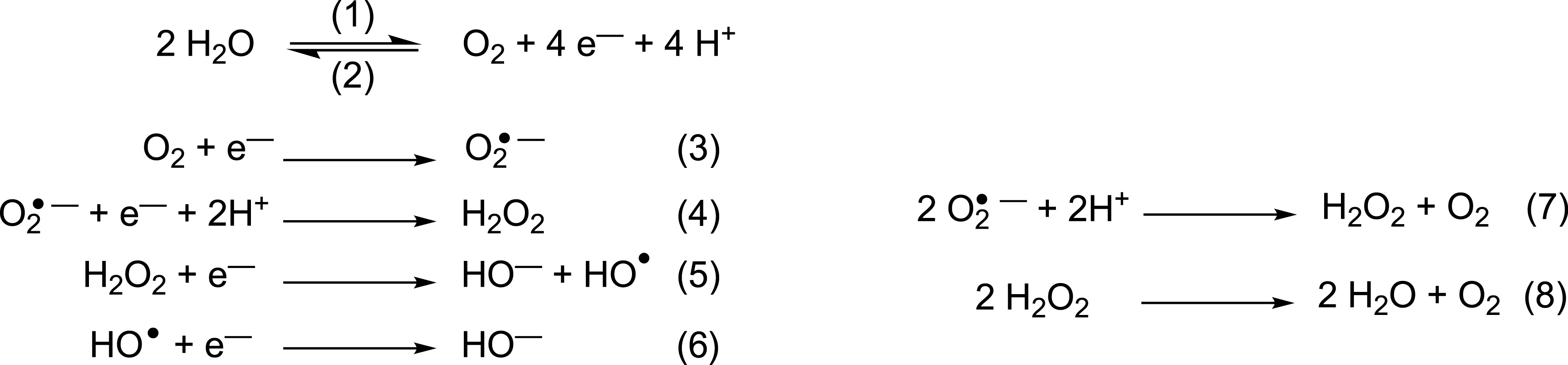
Oxidation of water (1) and reduction of dioxygen (2). Reduction cascade of O2 leading to reactive oxygen species ROS (3) to (6). Dismutation of O2∙− (7) and H2O2 (8). Note that the superoxide radical anion can be noted O2∙− or
In the last two decades, it has become clear that cellular responses to redox imbalance are more complex than previously assumed. On the one hand, cells facing redox imbalance often react with an overshoot effect, as first evidenced in the case of reoxygenation after hypoxia [14]. On the other hand, it turned out that moderate oxidative imbalance (essentially due to spatially and temporally controlled H2O2 production) is used at different scales by cells and tissues as a signaling device to perform physiological tasks. Such signaling by H2O2 was, for instance, observed to be necessary for cytoskeleton rearrangement needed during migration [15, 16], leukocyte navigation towards a wound [17], neural stem cell maintenance and neurogenesis [18, 19], or tissue regeneration in fish or amphibian [20, 21]. Among ROS, H2O2 is clearly suited to this signaling function due to its comparatively long half-life and thus diffusion properties, moderate reactivity, and membrane crossing ability via a subfamily of aquaporins. That is why H2O2 has been particularly studied as signaling molecule. To study its spatial distribution in real time, new genetically-encoded sensors have been developed (reviewed in [22], see also part 2.3 for the HyPer probe) [23, 24]. These improved sensors allow analyzing the dynamics of redox balance in live organisms (reviewed in [25]). More recently they were used to revisit H2O2 dynamics and roles in neuronal differentiation [26], brain patterning [27], neuroinflammation [28], Shh signaling in the developing floor plate [29], appendage regeneration [30], and in hypoxia/reoxygenation [31]. Linking these findings with the observation that activation of several growth factors was accompanied by H2O2 production, H. Sies proposed the new concept of “oxidative eustress” [32, 33, 34]. According to this concept, H2O2 is a central redox signaling molecule in physiological contexts, transient and localized bursts of low levels of H2O2 (in the 1–10 nM range) being required for fundamental developmental and homeostatic processes from intracellular decisions to intercellular communication and organism patterning. In contrast, “oxidative distress” corresponding to supraphysiological levels of H2O2 (above 100 nM) definitely leads to various damages, with an intermediate range of concentration (from 10 to 100 nM) leading to adaptive stress responses.
3. A range of biological models to evaluate antioxidant activity
Metal complexes are of paramount importance for redox reactions, and they occupy a prominent position in the growing research field to fight oxidative stress. Metal complexes can be designed as antioxidants bioinspired by natural cellular antioxidants, such as SOD or CAT, leading to SOD mimics or CAT mimics able to catalyze the dismutation of O2∙− and H2O2, respectively. Their chemical design has been discussed in the literature by us and others [6, 7, 8, 9, 35, 36, 37, 38] and we will not focus on that aspect in the present article.
The evaluation of these mimics goes first through the characterization of their reactivity with either O2∙− or H2O2 out of any cellular context, which we call intrinsic activities; this term refers therein to the reaction kinetics with O2∙− or H2O2, possibly the catalytic constant in case of a true catalytic SOD/CAT mimic. But what will be mainly discussed in this section is the evaluation of the bioactivities in cells.
Many biological assays have been developed to characterize antioxidant activity of SOD and CAT mimics, ranging from fluorescent probes to SOD-deficient organisms [12, 39, 40]. We have been interested in the study of metal-based antioxidants in the context of inflammatory bowel diseases (IBDs). In that context, we have been developing three cellular models to evaluate the bioactivities of metal-based catalytic antioxidants, SOD or CAT mimics:
(a) activated macrophages [11, 41].
(b) intestinal epithelial cells made highly sensitive to bacterial lipopolysaccharide (LPS, HT29-MD2 cells); the strong inflammation induced by LPS, presumably mediated by oxidative stress, is mitigated in the presence of SOD or CAT mimics [2, 10, 11, 42].
(c) HyPer-HeLa cells genetically engineered to express a H2O2-sensing protein [11]; in these models we have characterized the bioactivity of a range of Mn-complexes described as SOD mimics in the literature [11].
3.1. Evaluation of the effect of antioxidants on the superoxide (O2∙−) flow generated by macrophages
Macrophages are immune cells meant to produce ROS against invading bacteria. High ROS flow can be induced upon stimulation with bacterial lipopolysaccharide (LPS), interferon γ (IFN-γ) and phorbol 12-myristate 13-acetate (PMA) (typically 24h, slow activation of the inducible NO synthase (iNOS) by LPS and IFN-γ followed by PMA, for the fast activation of the NADPH oxidase (NOX) (1h)) [41, 43, 44]. Quantification of O2∙− concentration is made possible by using ferricytochrome c as a selective O2∙− UV–vis marker. As cytochrome c is not able to penetrate cells, only the O2∙− released extracellularly is measured [41, 45]. Murine macrophages RAW 264.7 are first activated by LPS and IFN-γ for 24 hours. Cells are then incubated with PMA, ferricytochrome c and a chosen concentration of antioxidant that is nontoxic for the cells (typically estimated with a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay) in an homemade noncolored medium [11]. The absorbance of the supernatant at 550 nm, where there is a band for ferrocytochrome c, gives an estimate of the amount of O2∙− in the extracellular medium (typically after 1h of incubation with ferricytochrome c) (see Figure 2). This assay was shown to provide a positive result with antioxidants not able to enter cells [11, 41]. This is the case of the commercially available CuZnSOD, an efficient catalyst of the dismutation of O2∙− into dioxygen and H2O2 with a kinetics close to diffusion limit. The CuZnSOD overall negative charge at physiological pH and its large size prevent its cell penetration when incubated in the extracellular medium. For that reason, we have labeled this experiment “extracellular activity assay” [11, 41].
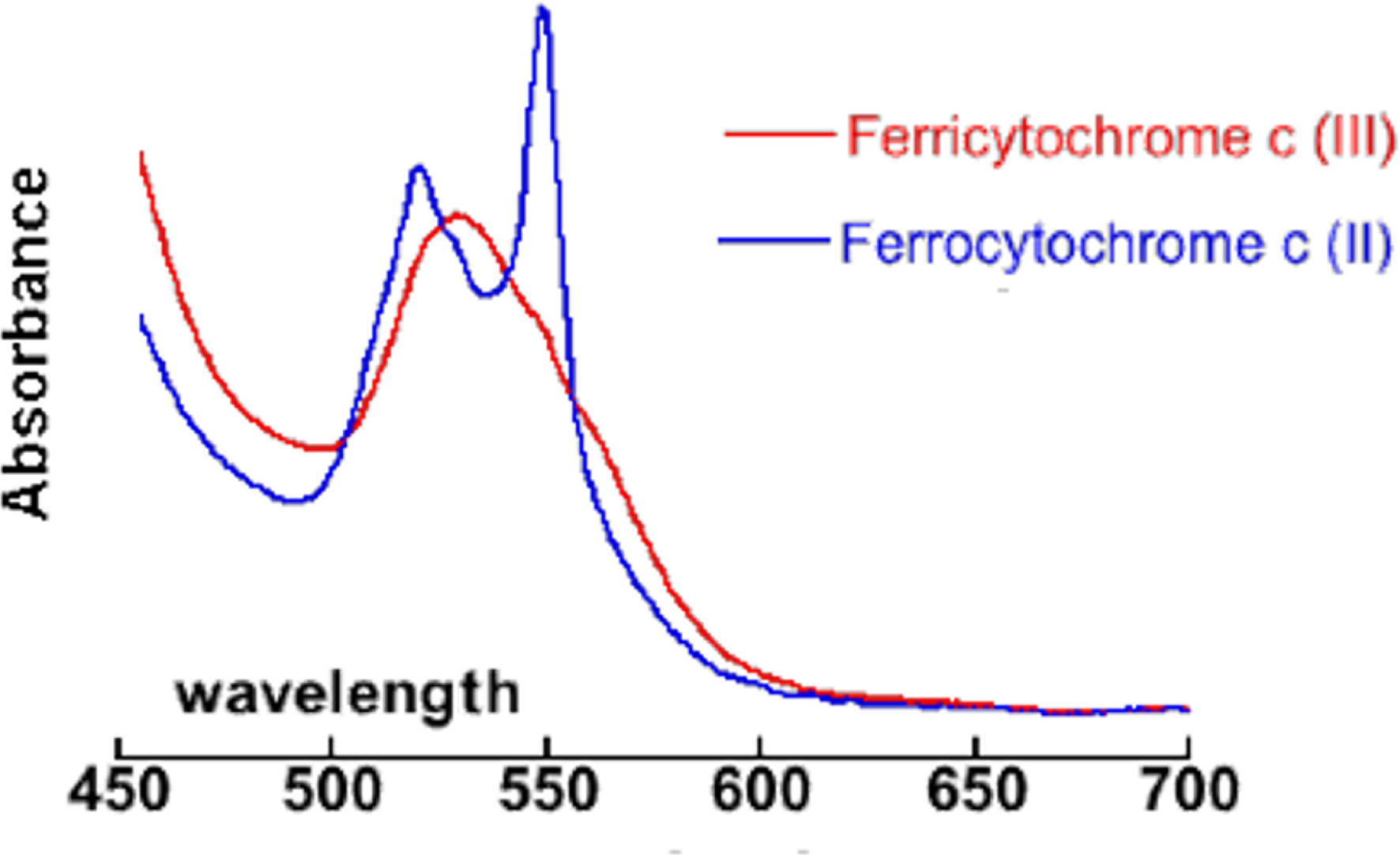
Visible spectrum (wavelength in nm) of ferri- and ferrocytochrome c showing the peak at 550 nm used for quantification.
The activity seen in this assay could be due either to (a) an inhibition of the O2∙− production or (b) to the quenching of the superoxide produced (through a SOD-like activity). We have shown that the SOD mimic Mn1 (see Figure 5) was not an inhibitor of O2∙− production using an electrochemical quantification of ROS (H2O2, ONOO−, NO, NO2−) with a possible back-calculation of the O2∙− produced, which was similar with and without the Mn1 incubation [41]. These results show that the reduction of the O2∙− flow is not due to an inhibition of O2∙− production, but to a direct reaction of Mn1 with O2∙−. Note that the results in electrochemistry and by ferricytochrome c quantification were complementary, as the electrochemistry provides information on the overall O2∙− production, not modified by the SOD mimic Mn1, and cytochrome c assay gives the extracellular flow of O2∙− over a one-hour period, efficiently reduced by the presence of a SOD mimic [41].
3.2. Epithelial intestinal cells as cellular models to estimate intracellular antioxidant and anti-inflammatory effects
Oxidative stress is known to play a key role in triggering inflammation [46] and inflammatory bowel diseases [47]. Intestinal epithelial cells (IECs) have adapted to the presence of commensal bacteria with a limited response to bacterial lipopolysaccharide (LPS) of its regular receptor (TLR4). Low or absent expression of the myeloid differentiation 2 (MD2) receptor, LPS co-receptor to TLR4, underlies LPS tolerance in IECs, and MD2 blood circulating levels have been shown to be associated with LPS responsiveness [48, 49]. The HT29 intestinal epithelial cell line has been stably transformed to overexpress the MD2 protein in the extracellular environment. The HT29-MD2 cell line thus exhibits a high LPS-responsiveness, increased more than 100-fold compared to the HT29 cell line [49, 50]. The release of interleukin 8 (IL8), but also the expression of cyclooxygenase 2 (COX2) and the related secretion of prostaglandin E2 (PGE2), were coordinately stimulated by LPS in HT29 cells expressing MD2. We have also shown that LPS stimulation for six hours induces overexpression of an active mitochondrial MnSOD, but not of the cytosolic CuZnSOD nor CAT [2, 10, 11, 51]. This is in line with LPS challenge–induced ROS production [52] at the mitochondria and clearly incriminates O2∙−. The overexpression of MnSOD has been interpreted as a result of cellular feedback to mitochondrial O2∙− increase induced by LPS. In this cellular model, metal-based antioxidants displaying a SOD-like activity have shown anti-inflammatory activity, as measured by IL8 secretion and COX2 expression after six-hour incubation with LPS [2, 10, 11, 51]. The expression of the MnSOD was reduced when LPS was co-incubated with a SOD mimic [2, 10, 11, 42, 51, 53], showing that competent SOD mimics are able to complement for MnSOD.
A typical result with Mn1 is shown in Figure 3. The anti-inflammatory activity of Mn1 has been compared to the 5-aminosalicyclic acid (5-ASA, see Figure 5), an organic anti-inflammatory agent used as the first therapeutic line in IBD [2, 54]. As shown on Figure 3, 5-ASA is less efficient against inflammation than Mn1. A comparison of its antioxidant activity with other antioxidants, ranging from Mn porphyrins to organic nitroxide is discussed below (Table 1, Section 3.4 and Figure 7), showing that Mn1 is among the best cell-efficient antioxidants.
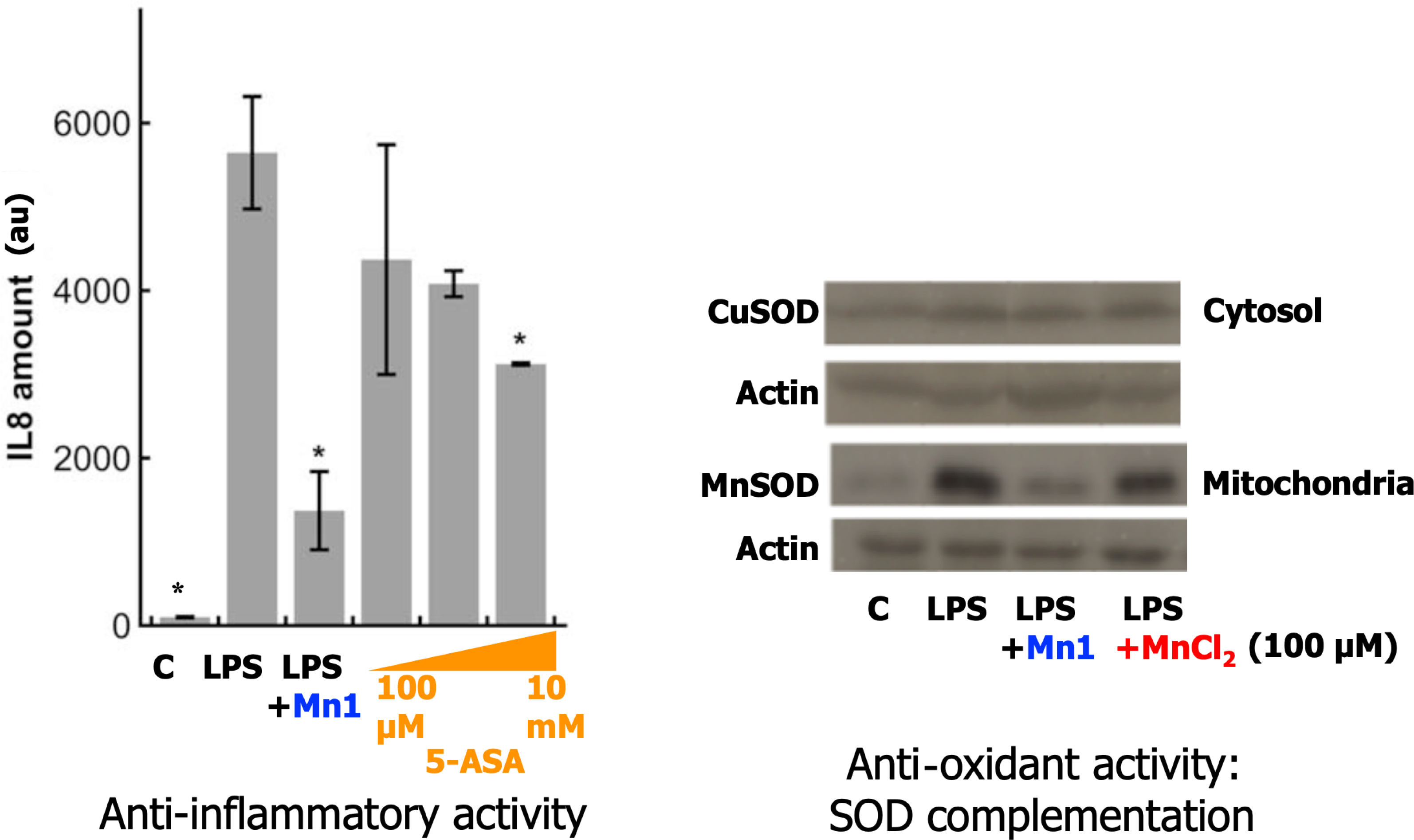
Anti-inflammatory and antioxidant activity of SOD mimic Mn1 and comparison with 5-ASA (see Figure 5 for the structure of Mn1 and 5-ASA). Left: Anti-inflammatory activity in intestinal epithelial cells activated with LPS: comparison with 5-ASA (see Figure 5). IL8 secretion measured by ELISA in supernatants of HT29-MD2 incubated 7 h under several conditions: C: control; LPS (0.1 μg/mL) was added after 1 h of incubation with Mn1 (100 μM), 5 ASA: 100 μM, 1 mM and 10 mM. Data represent means ± SD for 2 replicates, ∗∗ p < 0.01 and ∗ p < 0.05 versus LPS. Right: Antioxidant activity, measured indirectly by overexpression of MnSOD and CuSOD in comparison with actin. Representative experiment in Western blot (WB) after a 7 h incubation under several conditions, LPS (0.1 μg⋅mL−1) added after 1 h and incubated for 6 h: C for control; LPS; LPS + Mn1 (100 μM); LPS + MnCl2 (100 μM). Adapted with permission from [2] Copyright 2017 American Chemical Society. These results are representative of typical experiments, performed many times by different experimentalists in slightly different conditions [2, 10, 11, 51, 53]. Masquer
Anti-inflammatory and antioxidant activity of SOD mimic Mn1 and comparison with 5-ASA (see Figure 5 for the structure of Mn1 and 5-ASA). Left: Anti-inflammatory activity in intestinal epithelial cells activated with LPS: comparison with 5-ASA (see Figure 5). IL8 secretion ... Lire la suite
3.3. HeLa HyPer cells
The detection of ROS using fluorescence probes is one of the most straightforward strategies to correlate the bioactivities of antioxidants with their intrinsic catalytic activities. However, for more reactive ROS such as the hydroxyl radical (∙OH) and O2∙−, commercially available selective probes are scarce. Very interestingly, for H2O2 detection, a genetically encoded ratiometric sensor called HyPer has been developed, in HeLa cells among others [55]. HyPer is a circularly permuted yellow fluorescent protein (cpYFP) emitting at 530 nm, integrated into the regulatory domain of the bacterial H2O2-sensing protein OxyR (regulatory domain of OxyR, OxyR-RD). The oxidation of HyPer thiols by H2O2 induces the formation of disulfide bridges that leads to a modification of the spectral properties of the YFP. More precisely, a ratiometric modification of the excitation spectrum of HyPer, which possesses two excitation maxima at 420 and 488 nm, is induced. Thus, by measuring the normalized ratio of the intensity I(405/530)/I(491/530) by confocal fluorescence microscopy, it is possible to monitor specifically H2O2 concentration in cells (see Figure 4). H2O2, being not as reactive as O2∙−, is able to diffuse across cytoplasmic membrane, mainly through aquaporines [56, 57], and endogenous H2O2 diffuses outside cells. Consequently, when these HeLa HyPer cells are incubated with antioxidants mimicking CAT, the decrease in fluorescence ratio observed may be composite: it may be due to dismutation of endogenous H2O2 inside or outside the cells. For instance, incubation of HeLa HyPer with the recombinant heme CAT has been shown to reduce H2O2 concentration although it does not cross cells membrane [58]. When antioxidants possessing a CAT-like activity [11], including peptidyl Cu complexes (Figure 5) [24], were incubated with HeLa HyPer cells, a clear decrease in HyPer fluorescence has been observed, showing that they were efficient in this biological context [11, 24].
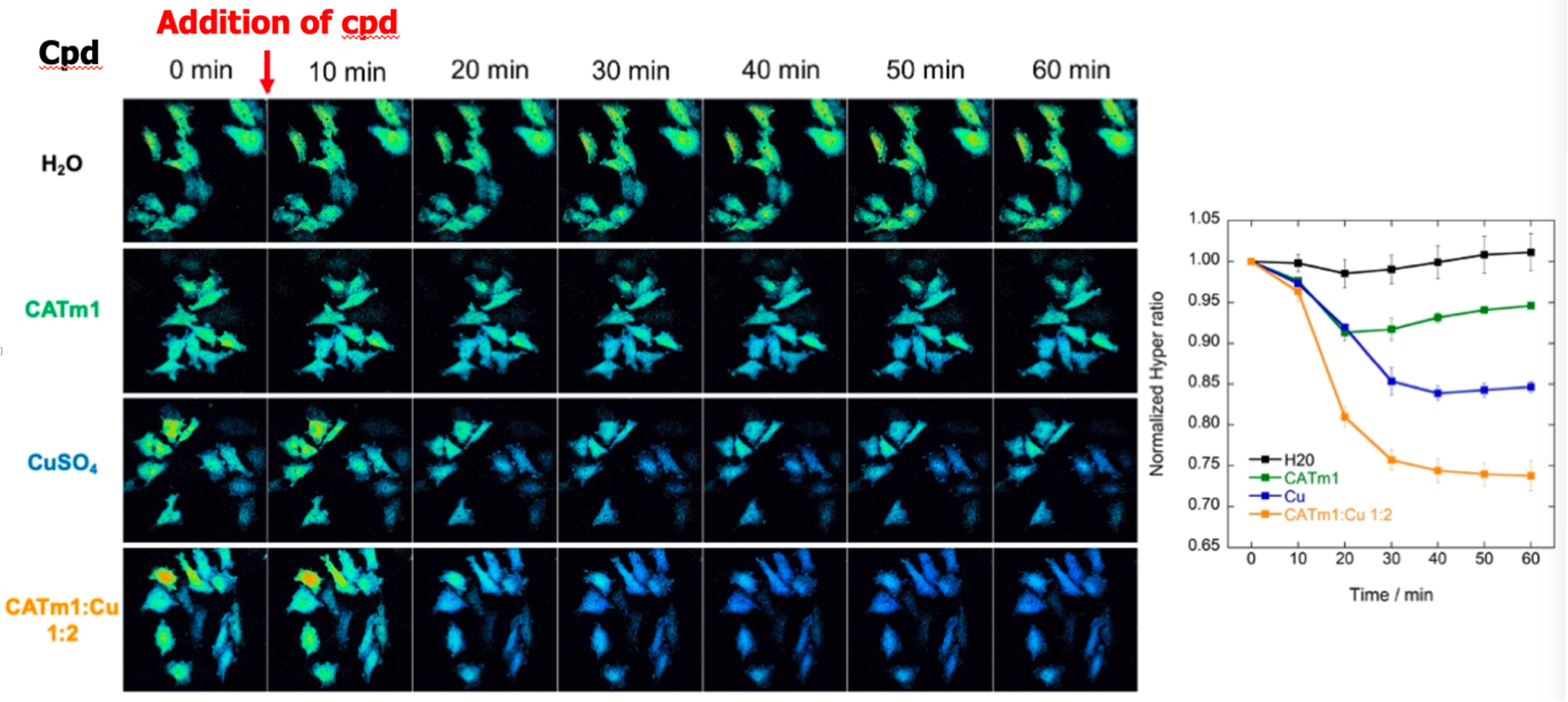
H2O2 levels in HeLa HyPer cells monitored by fluorescence for 60 min after treatment with H2O (control cells), CATm1 peptide (250 μM), CuSO4 (500 μM), and complex CATm1-Cu2+ 1:2 (250 μM). The right panel shows the mean ratio for 2 or 3 independent experiments. Adapted with permission from [24]. Copyright 2021 American Chemical Society.
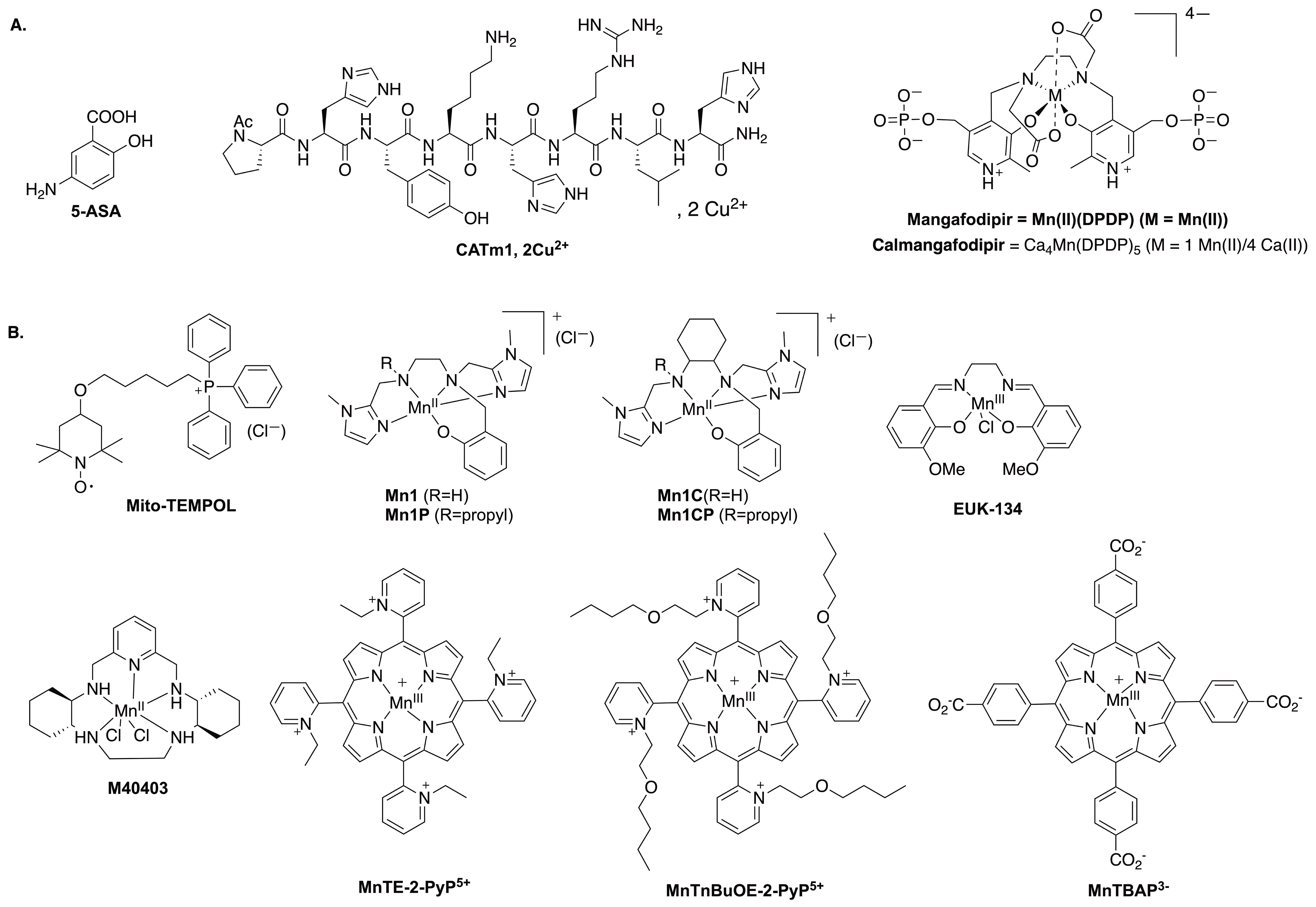
Chemical structures of the molecules discussed in this article. (A) 5-ASA, CATm1, Mangafodipir and Calmangafodipir. (B) Structure of the antioxidants screened by the three-cell assay.
3.4. Conclusion on the three cellular models
We have recently used the above-mentioned extracellular assays to screen a range of antioxidants metal complexes known as SOD or CAT mimics (see Figure 5) [11].
We have compared their intrinsic activities, defined as their catalytic activities measured out of any cellular context, with their bioactivities in these assays (see Table 1).
Intrinsic dismutation kinetic constants and cellular activity of antioxidants
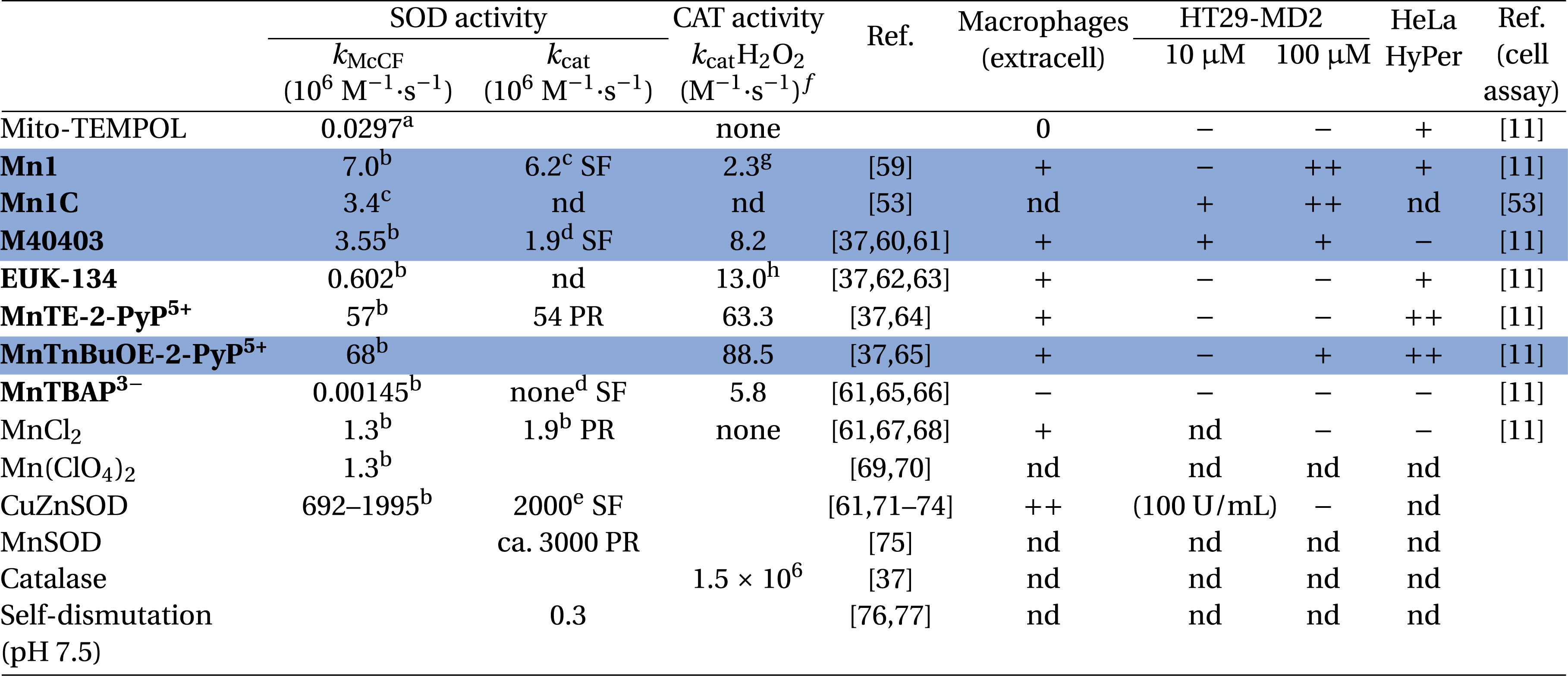 |
kMcCF refers to the rate constant calculated from the McCord and Fridovich assay [36, 70].
aFerricytochrome c, phosphate buffer (50 mM, pH 8). bFerricytochrome c, phosphate buffer (50 mM, pH 7.8). cHEPES (60 mM, pH 7.4). d Phosphate buffer (50 mM, pH 7.4). ePhosphate buffer (50 mM, pH 7.8). fUnless specified, catalytic constants were measured by polarography using a Clark-type electrode in Tris buffer (50 mM, pH 7.8). gHEPES (100 mM, pH 7.4). hValue found for EUK-8 instead of EUK-134 which differs only by the two methoxy substituents. SF means stopped-flow and PR pulse radiolysis. iCellular assays: ++/+/− refers to the level of activity in comparison with activated condition (macrophages and HT29-MD2) or basal H2O2 (HeLa HyPer) and they correspond to the color code in Figure 6. In blue: the best compounds identified as active in the three-cell assay.
Overall, three groups were identified for each of the assay, namely very efficient (++), efficient (+) and weakly active or inactive (−) antioxidants (Figure 6). As anticipated, the less intrinsically active antioxidants proved the most inactive in the cell assays. For instance, MitoTempol and MnTBAP3−, which show a kcat for superoxide activity lower than the rate of self-dismutation of superoxide and that are therefore not true SOD-like catalysts, were found inactive with the RAW model and HT29-MD2 assays. These two are probably able to react once with O2∙− but their molecular structure or redox potential prevent cycling back and catalysis. In other words, they are stoichiometric antioxidants. The observation of their weak cellular activity is probably linked to their stoichiometric nature, as opposed to the higher activity catalytic antioxidants. In the extracellular assay (raw 264.7), the CuZnSOD was found to be very active as expected, but it was not active in the HT29-MDA assay: we can deduce that this assay (HT29-MD2) probes an intracellular antioxidant effect. For the HT29-MD2 assay, only M40403 and MnTnBuOE-2-PyP5+ were found active at both 10 μM and 100 μM, whereas Mn1 was only active at 100 μM. Interestingly, more inert analogues of Mn1, Mn1C and Mn1CP were found to remain active at 10 μM [53], confirming the idea that the lack of activity of Mn1 at 10 μM was due to its dissociation in cells (see below). A further study showed that, when Mn1 dissociates in cells, it leads to the Zn-analogue, which was found inactive both in the HeLa HyPer and HT29-MD2 assays [42]. Another interesting example is the case of MnTnBuOE-2-PyP5+ and MnTE-2-PyP5+, showing a similar intrinsic SOD activity and cell penetration but different bioactivities [11], probably due to different subcellular localizations.
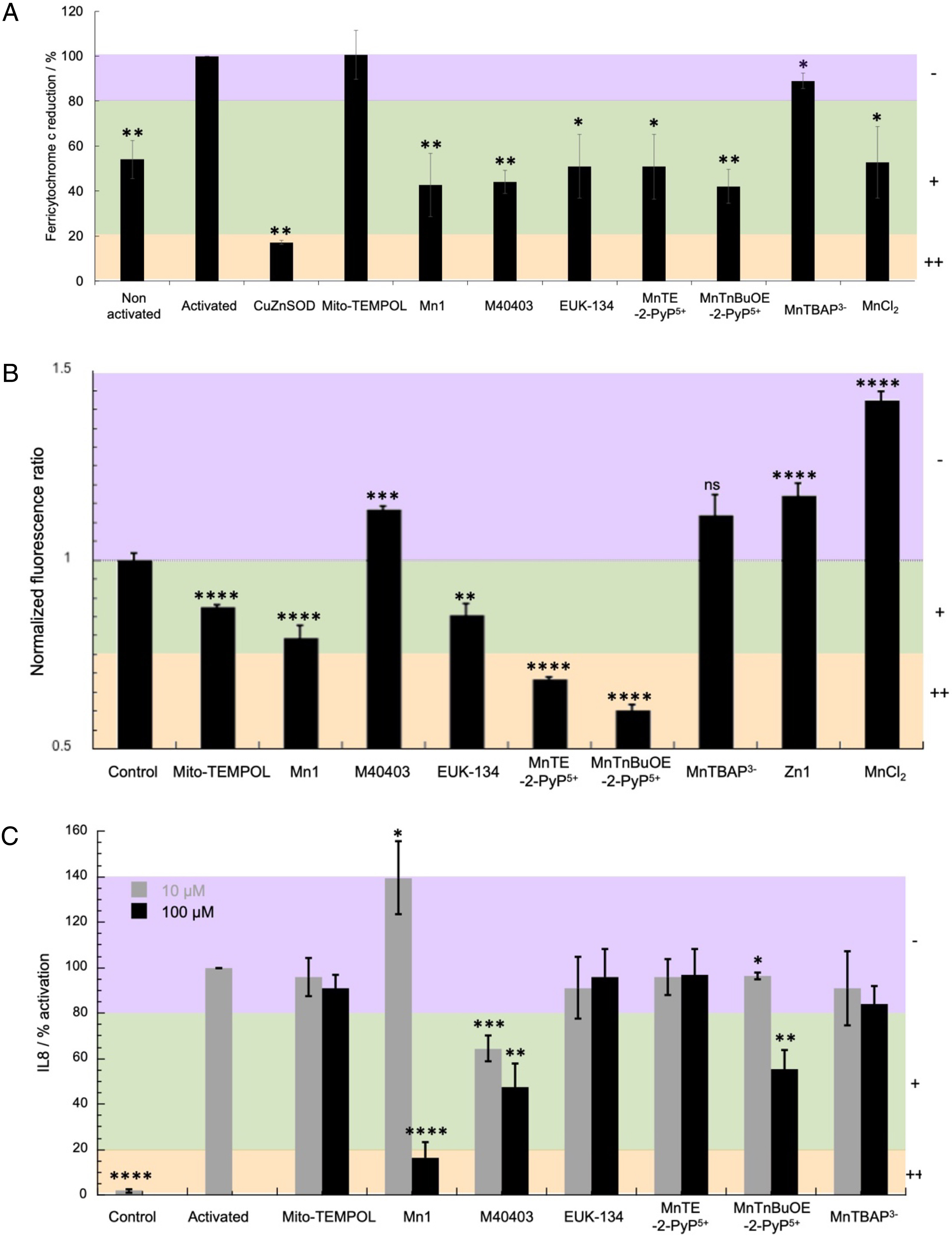
Screening of antioxidants with the three-cellular assay. Adapted with permission from [11]. Copyright 2021 Elsevier. (A) Evaluation of antioxidants on murine macrophages RAW 264.7. Relative values in comparison with activated cells arbitrarily set at 100%. Macrophages were incubated with IFN-γ (1 U⋅mL−1) and LPS (1 ng⋅mL−1) for 24 h at 37 °C in DMEM followed by an incubation with ferricytochrome c (100 μM), PMA (800 nM) and antioxidants (100 μM) at 37 °C for 1 h in a homemade medium devoid of phenol red (see [11] exp. Part). Data are mean ± SEM of three independent experiments with (∗∗) p < 0.01 and (∗) p < 0.05 versus activated cells. (B) H2O2 levels measured in HyPer HeLa cells. Cells were incubated for 1 h at 37 °C in HEPES (30 mM pH 7.5) under 5% CO2 without (control) or with antioxidants at 100 μM. Fluorescence images were recorded after excitation at 405 nm and 491 nm and the ratio of the emission at 530 nm I(491/530)/I(405/530) was measured for several cells. The ratio was arbitrarily set to 1 for control cells, i.e., cells incubated with HEPES only. Data are mean ± SEM of several cells (from 48 to 163 cells) with ∗∗∗∗ p < 0.0001, ∗∗∗ p < 0.001, ∗∗ p < 0.01 and ∗ p < 0.05 versus control cells. ns means non-significant. (C) Quantification of the inflammatory marker IL8 by ELISA after incubation of HT29-MD2 cells with the antioxidants at 10 or 100 μM. Antioxidants were incubated for 6 h at 37 °C in supplemented DMEM in presence of LPS (0.1 μg⋅mL−1) except for control where neither antioxidants nor LPS were added. The absorbance measured for activated cells was arbitrary set to 100%. Data represent mean ± SEM of two independent experiments for MnTnBuOE-2-PyP5+ and M40403 and three independent experiments for Mito-TEMPOL, Mn1, EUK-134, MnTE-2-PyP5+ and MnTBAP3− with ∗∗∗∗ p < 0.0001, ∗∗∗ p < 0.001, ∗∗ p < 0.01 and ∗ p < 0.05 versus activated cells. Masquer
Screening of antioxidants with the three-cellular assay. Adapted with permission from [11]. Copyright 2021 Elsevier. (A) Evaluation of antioxidants on murine macrophages RAW 264.7. Relative values in comparison with activated cells arbitrarily set at 100%. Macrophages were incubated with IFN-γ ... Lire la suite
Overall, the best compounds identified as active in the three-cell assay are those highlighted in blue in Table 1.
As we have already discussed in other cases [2, 78, 79], the overall efficacy of a metal-based compound relies on several parameters. Figure 7 shows radar plots revealing the link between intrinsic activity (bottom part) and cellular activities (upper part) determined in the three-cellular assays. The choice of axis was guided by the idea of maximizing the differences in the series explored, with favorable effects leading to a larger value on the axis (see caption of Figure 7 for details).
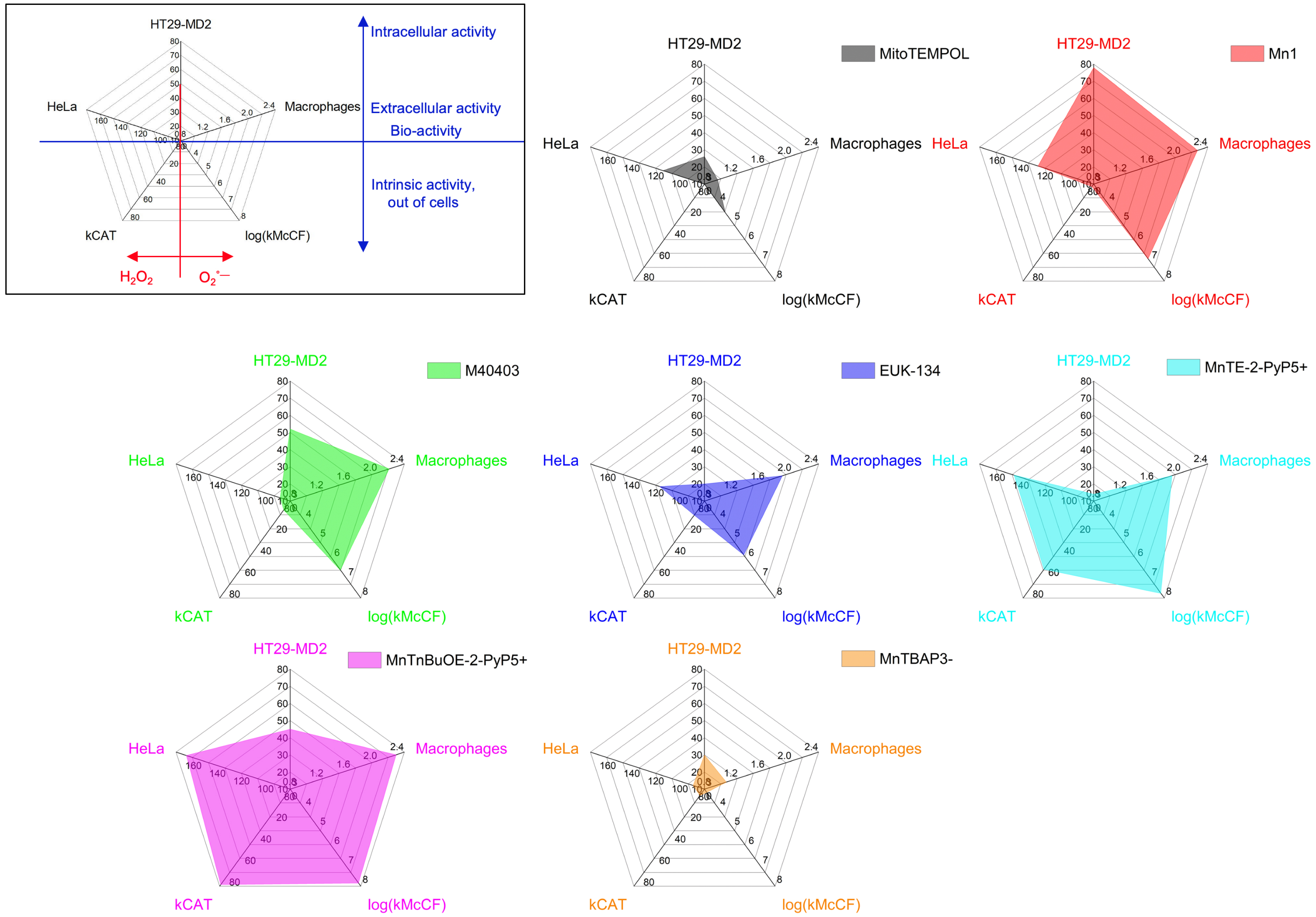
Performances of metal-based antioxidants compared using a radar representation for MitoTEMPOL, Mn1, M40403, EUK-134, MnTBAP3−, MnTnBuOE-2-PyP5+, and MnTE-2-PyP5+ after incubation at 100 μM. Upper-left radar: parameters involved in this representation. Axis c1: HeLa, 100/F (F = normalized fluorescence ratio, see Figure 6B). Axis c2: Macrophages, 100/F-red (F-red: % of ferricytochome c reduction, see Figure 6A). Axis c3: HT29-MD2, 100-I (I = IL8: % of activation by LPS, see Figure 6C).
The cellular assays have been chosen for their complementarity, with the macrophage assay measuring the effect on the superoxide extracellular flow, HeLa Hyper measuring the effect on H2O2, and HT29-MD2 measuring an intracellular effect on inflammation induced by oxidative stress.
As shown on the upper-left insert in Figure 7, the upper part of the pentagons deciphers the biological effects on cells, whether extracellular (HeLa and macrophages, middle part of the pentagon) or intracellular (HT29-MD2 cell assay, at the apex of the pentagon), linked to oxidative stress in general.
The lower part reveals the intrinsic SOD and CAT activity. The left side of these pentagons is associated to the catalase-type activities (intrinsic and in the HeLa cell assay) and the right side with the anti-superoxide activities (intrinsic and in the macrophage assay).
The larger surface in the lower area, the higher the intrinsic activities. The larger the surface in the upper area, the better the activity in the three cells model. The larger the overall surface, the higher the antioxidants activities, intrinsic and in cells.
As shown by the analysis of Table 1 (see above), a low intrinsic activity leads to a weak cellular activity—see Mito-TEMPOL and MnTBAP3− with the very small chart surface. For all compounds, the macrophage and HeLa cell assays, which probe a bioactivity in an extracellular medium, mirror well the intrinsic catalytic activities since cell penetration is not required. However, a large intrinsic activity (lower part of the pentagon) does not necessarily go with a high intracellular activity (HT29-MD2, apex of the pentagon). A loss of activity when going to cells or biological environment in general can be due to a low bioavailability, because of a weak penetration, poor stability in cells (see above, discussion in the case of Mn1), or formation of a less active ternary complex with cellular ligands.
MnTnBuOE-2-PyP5+ is the most active antioxidant followed by MnTE-2-PyP5+ and Mn1 and this reflects on the surfaces of their pentagons being larger. The two porphyrins MnTnBuOE-2-PyP5+ and MnTE-2-PyP5+ are associated with a high intrinsic catalase and SOD activity. They show a similar good activity in the HeLa and macrophage assays but a weaker one in the HT29-MD2 assay. Note that the difference in activity between MnTnBuOE-2-PyP5+, MnTE-2-PyP5+ and MnTBAP3− cannot be ascribed to a difference in cell penetration. Indeed, MnTBAP3−, anticipated to penetrate weakly due to its high negative charge, was paradoxically penetrating better in HT29-MD2 than MnTnBuOE-2-PyP5+ and MnTE-2-PyP5+ (see Figure 8). Mn1 was the most penetrating molecule.
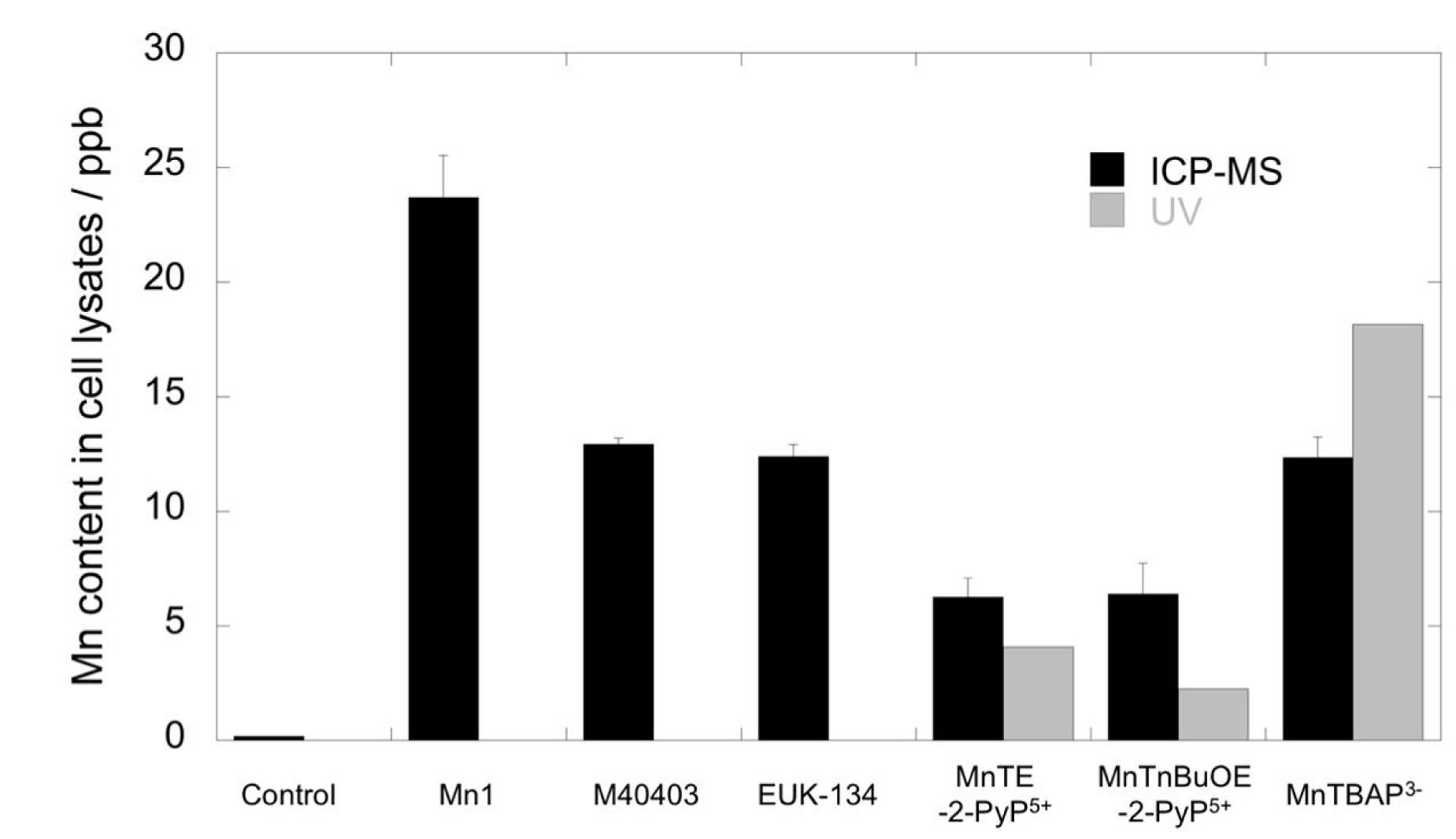
Quantification by inductively coupled plasma mass spectrometry (ICP-MS) (black columns) and UV spectrometry (gray columns, only for porphyrins) of Mn content in cell lysates of HT29-MD2 stimulated cells, incubated with or without compounds at 100 μM in DMEM supplemented with 10% heat-inactivated FBS and LPS (0.1 μg/mL). Values are given for a single experiment. For ICP-MS, values were corrected according to the Zn content of control cells to compare the data. Reprinted with permission from [11]. Copyright 2021 Elsevier.
Mn1 has a very weak catalase activity and good SOD activity: this is shown by the surface on the chart representation being shifted to the right side. In contrast, the activity of MnTnBuOE-2-PyP5+, MnTE-2-PyP5+ is more balanced between CAT and SOD, with a left-right symmetry of the filled inner surface.
These results on cells stress the importance of characterizing the bioactivity within cellular models. Of course, a compound needs to show a good intrinsic activity to be active in cells. But other parameters are at play, such as cell penetration, localization, stability, and inertia, in link with speciation in cells.
Other studies on Mn porphyrins have demonstrated that these compounds upregulate MnSOD, CAT, glutaredoxins (GRXs), peroxiredoxins (PRDXs) and other antioxidants. These proteins are under control of NRF2, a transcription factor known to activate genes through the antioxidant response element (ARE). NRF2 is continuously produced in cells and binds to KEAP1 (Kelch-like ECH-associated protein, ECH = enoyl-coenzyme A (CoA) hydratase) with a particular conformation that promotes its continuous degradation by the proteasome (see Figure 9). The modification of the Cys in KEAP1 (upon oxidative or chemical stress induced by electrophiles for instance) leads to an inhibition of NRF2 degradation: the newly synthesized NRF2 accumulates in the cytosol and translocates to the nucleus where it activates genes leading notably to an overexpression of SOD, CAT, glutathione reductase, heme-oxygenase (HO-1), and NAD(P)H dehydrogenase quinone (NQO1) [80]. Note that a similar activation of the ARE has also been proposed for nitroxides [81] which react stoichiometrically with O2∙−.
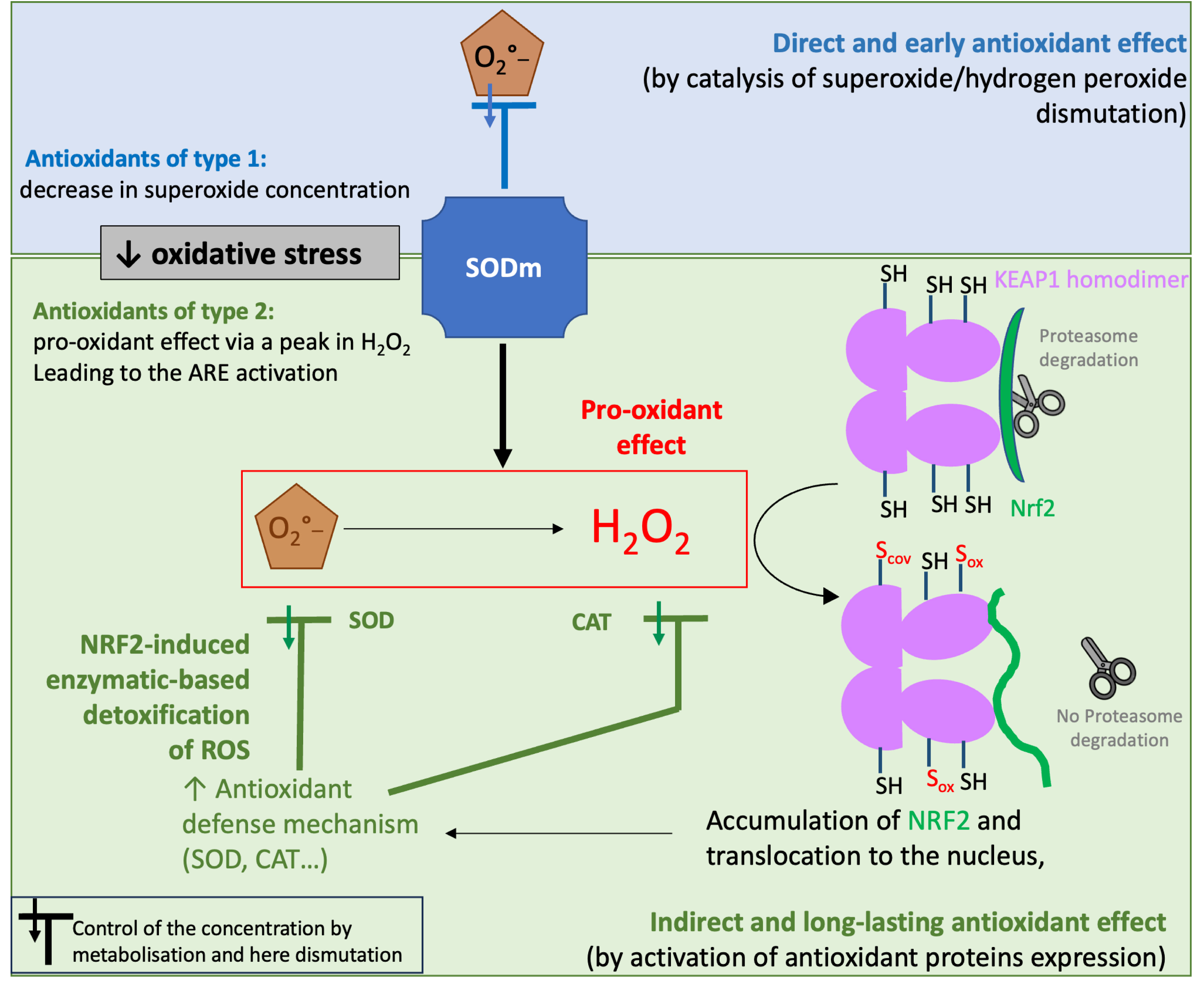
Schematic representation of the two possible pathways on cellular activity of SOD mimics. Pathway 1 corresponds to the direct effect of SOD mimics, with their ability to catalytically dismutate superoxide. A similar pathway could be ascribed to CAT mimics as well as they reduce H2O2. Pathway 2 is associated to a pro-oxidant effect of SOD mimics: as they produce H2O2, they can activate the NRF2-signaling ARE pathway involving the biosynthesis of antioxidant enzymes such as SOD. (Adapted with permission from [9].) Copyright 2022 Elsevier. Masquer
Schematic representation of the two possible pathways on cellular activity of SOD mimics. Pathway 1 corresponds to the direct effect of SOD mimics, with their ability to catalytically dismutate superoxide. A similar pathway could be ascribed to CAT mimics as ... Lire la suite
SOD mimics can then act through two pathways (see Figure 9) [9]: (A) by reducing the O2∙− concentration and (B) by increasing H2O2 concentration leading to an induction of the ARE. The balance between these two pathways could depend on the speed of dismutation (kcat, intrinsic activity) in comparison with the cellular capacity to detoxify H2O2 and also on the H2O2 cell content. A fast dismutation delivers a high shot of H2O2 concentration activating the antioxidant cellular response (pathway B, pro-oxidant). Pathway A may occur as soon as the SOD mimics enter the cells or the mitochondria but is also prone to decrease in time due to the SOD mimic degradation. In contrast, Pathway B requires time to activate protein synthesis (from 30 min to several hours), but this has a long-lasting effect as it works by boosting the cellular antioxidant feedback. We have recently shown by a proteomic cysteine-redoxomic analyses in HT29-MD2 that LPS challenge generates an early oxidative stress (15 min time-scale) leading to inflammation (hour time-scale) with a cellular-feedback activating the antioxidant response. These effects are mitigated by Mn1 with an early limitation of the cysteine oxidations as early as 15 min [82].
In a way, compounds commonly known as SOD mimics can be separated into two classes [9]: (1) compounds directly acting on O2∙−, by catalyzing its dismutation, are truly mimicking SOD activity and should show an early cellular activity and (2) compounds, such as porphyrins, acting in fact as pro-oxidants, activate the cellular antioxidant enzymatic arsenal through transcription factors, such as NRF2, that promote gene expression of antioxidant proteins (SOD, CAT…). Their action takes longer to activate, as it depends on cellular protein biosynthesis, but also lasts longer.
3.5. Of cells and mice
3.5.1. Mice models in link with inflammatory bowel diseases
It has been shown that inflammation and oxidative stress are strongly intertwined in inflammatory bowels diseases (IBDs) [83]. In addition, IBDs are associated with a weakening of the antioxidant defenses: the content and/or activity of SODs was shown significantly reduced in plasma [84, 85, 86] or in tissues homogenates from resection specimen of IBD patients as compared to healthy subjects [87, 88]. In this context, managing oxidative stress using SOD mimics is thus an interesting therapeutic alternative to existing treatments [89, 90]. Toward this goal, we have used an IBD mouse model in which colitis is induced by administration of 2,4-dinitrobenzenesulfonic acid (DNBS). Starting the same day as DNBS administration, Mn1 was administered daily by gavage in a basic buffer, to try to limit metal decoordination in the acidic stomach environment. Interestingly, mice treated with Mn1 showed a significantly reduced weight loss in comparison with untreated mice, supporting the idea that accelerating superoxide dismutation is a valuable strategy against inflammatory pathologies [2]. Recently, we have shown that the anti-inflammatory activity of Mn1 could be preserved in this mice colitis model, without using the buffer, when Mn1 was loaded in bacteria (called “chemically modified bacteria”) [91].
3.5.2. Side effects of anticancerous agents: mitigation of neuropathy by SOD mimics
As already mentioned, low levels of ROS play important roles in normal cells and are involved in the regulation of cellular signaling and proliferation [92]. Higher levels of ROS along with deregulation of antioxidant pathways have been observed in cancer cells [92, 93]. The differences in cellular redox states between normal and cancer cells and the role of ROS and their regulation in tumorigenesis have received much attention to explore and develop new therapeutic and preventive cancer-targeted strategies [94]. In the context of anticancer therapy, Mn-porphyrins SOD mimics have been studied in clinical trials for radioprotection of normal tissue and organ and for chemosensitization of cancer cells, their anticancer activity has been reviewed elsewhere [95, 96].
Clinically used chemotherapeutics are characterized by dose-limiting off-target toxicities. Treatment with Oxaliplatin (Oxa), a reference drug in the treatment of digestive tract tumors, especially colorectal cancer and gastric cancer, is associated with the development of peripheral neuropathy (Oxa-induced peripheral neuropathy, OIPN) in a majority of patients in its acute form, which may evolve into a chronic form severely impacting the patients’ quality of life. OIPN is a frequent cause of treatment arrest and a major hurdle limiting Oxa’s clinical use with no clinically effective treatment or prevention therapy [97, 98].
OIPN affects nerves and induces damages in peripheral nerves and dorsal root ganglia, causing pain, impaired sensations, or involuntary movements. The associated molecular mechanisms are complex and were shown to involve neuroinflammation, oxidative stress, and mitochondrial dysfunction. Antioxidants and modulators of redox balance have been studied as adjuvants in Oxa treatments in in vivo studies and were shown to have neuroprotective effects [99, 100, 101]. Mangafodipir (see Figure 5) is a Mn(II) complex developed for its activity as a contrast agent in magnetic resonance imaging (MRI), associated with the release of Mn(II) from the ligand, but which also shows SOD-like activity [102, 103]. In this context, the first phase III neuroprotection trial in patients with colorectal cancer involved calmangafodipir (CaM; PledOx®; PledPharma AB/Egetis Therapeutics AB, see Figure 5), although negative results were reported [104].
We recently explored the relevance of SOD mimics derived from Mn1 in combination treatments with Oxa in reducing OIPN [105, 106]. In vitro, antitumoral activity was assessed by the viability and ROS production of tumor cell lines (CT16, HCT116, HT29) and murine fibroblasts (NIH3T3). In vivo, a murine model of colorectal cancer with subcutaneous injection of CT26 cells in Balb/c mice was used. The tumoral growth was monitored, and OIPN was primarily assessed using a behavioral Von Frey test reflecting nociception and mechanical sensitivity (tactile pressure on the paw). In vivo, the association of Mn1 derivatives to Oxa did not counteract Oxa’s antitumoral activity, significantly decreased OIPN, and improved global clinical tolerance of Oxa. Additional effects were measured by cold plate tests, specific functional neuromuscular assays, and electron microscopy analyses of sciatic nerves. Further studies are on the way to characterize Pt accumulation in organs, peripheral nervous system, and tumors by inductively coupled plasma mass spectrometry (ICP-MS), and neuropathy markers by immunohistology (hind paws, sciatic nerves). Our studies showed that Mn1 derivatives are promising in preventing the appearance of sensitive axonal neuropathy and neuromuscular disorders induced by Oxa [105, 106], as they reduce Oxa-induced neurosensitive and neuromuscular alterations in vivo and prevent Oxa-induced peripheral demyelination. We are now developing covalent conjugates between MnSOD mimics and Oxa under the shape of Pt(IV) prodrugs and evaluating them for antitumoral activity and neuropathy prevention in mice models.
4. Speciation in cells: complex integrity and physical form
As exemplified by the results reported above, a key information when it comes to the design of bioactive metal complexes and rationalization of their bioactivity is their speciation. Speciation of an element is defined by the International Union of Pure and Applied Chemistry (IUPAC) as its distribution among defined chemical species, including molecular structure or physical form [107, 108]. Determining the integrity of a metal complex within a biological system, or its physical form (solute, precipitate), is part of this description and several examples are given below.
Because it is not easy to anticipate which molecular structure is active in the biological environment, it is important to explore experimentally the nature of metal-based species present in cells. The recent development of analytical and imaging techniques [109, 110], including X-ray fluorescence [9, 111, 112, 113], nano secondary ion mass spectrometry (nanoSIMS) [109, 110], ICP-MS and its laser ablation version for imaging (LA-ICP-MS) [109, 114, 115], ion mobility spectrometry coupled to mass spectrometry (IMS-MS) [42], Energy Dispersive X-ray (EDS or EDX) [116], or laser-induced breakdown spectroscopy (LIBS) [117], have bolstered our capacity of picturing the cellular content and distribution of metal based species and metal ions. We here delineate two original ways of examining the integrity of metal-complexes in cells, through X-fluorescence imaging and mass-based approaches. Molecular and chemical design tools have also been developed by chemists to improved stability and inertia of metal compounds [118, 119, 120, 121, 122]. In most cases, ligand exchange is being detrimental to the activity, but it can be also favorable and purposely designed, with a carrier metal complex delivering an active ligand, protected by metal coordination before reaching its target and then released in the desired cellular environment [123, 124].
4.1. Exploring complex integrity and speciation by “unconventional” methods
4.1.1. X-ray fluorescence imaging
Exploring speciation in cells using a single imaging technique in a single experiment has the advantage of limiting sample manipulation (lysis, extraction, dilution, etc.), that could lead to metal decoordination, as much as possible. One original possibility is to use X-ray fluorescence (XRF), which enables the detection of heavy elements such as metal ions and imaging them using raster scanning of cells or biological tissue sections. If the ligand is appended with a heavy substituent, it is possible to map this heavy element as well as the metal ion and thus determine whether they colocalize in μ-XRF or not. This has been done for instance with a Br-substituted ligand [125].
We have functionalized a Mn-based SOD mimic with a rhenium-based moiety (see Figure 10). This type of Re-based complexes has been developed by us [126] and others, as UV–vis fluorescent probes. Interestingly, they also show IR and Raman characteristic absorptions and rhenium (Re) can be detected using X-fluorescence [113, 127]. These probes are thus multimodal probes active in UV–vis fluorescence, IR, Raman, and X-fluorescence. The distribution of the Mn in cells incubated with Mn1 is homogeneous in the cell (not shown) [2] and is different from that of Mn1Re with an accumulation into perinuclear organelles (see Figure 10) [10]. The Re-probe is positively charged and hydrophobic: it may drive Mn1Re into lipid-rich organelles like mitochondria characterized by a negative potential [126]. The bioactivity in the HT29-MD2 assay (described above) of Mn1 and Mn1Re was similar, although Mn1Re was overall more cell-penetrant than Mn1. Their anti-inflammatory activity is thought to be associated to a reduced LPS-induced mitochondrial oxidative stress. Interestingly, both Mn1 and Mn1Re showed the same quantity accumulated in mitochondria, in line with their similar bioactivity and that bioactivity being associated with an effect in mitochondria.
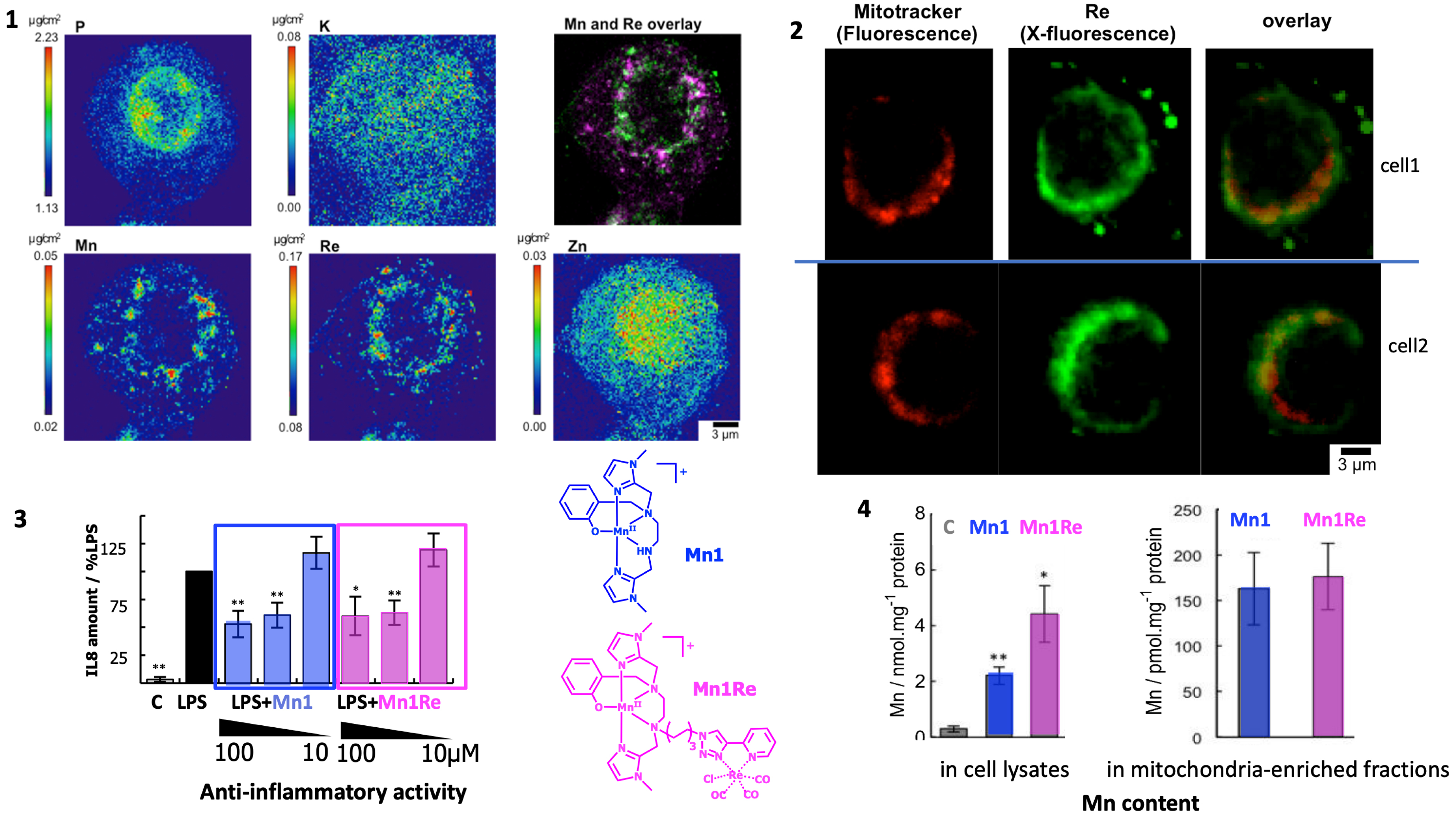
From ref [10]. (1) Elemental distribution of P, K, Mn, Re, and Zn in an HT29-MD2 cell incubated with Mn1Re. The phosphorus (P, Kα-line), and zinc (Zn, Kßz-line, and not Kα to avoid overlapping with Re Lα) maps are used to identify the nucleus area. The overlay (top right) corresponds to the manganese (Mn) (Kα-line) (magenta) and rhenium (Re) (Lß-line that does not overlap with other lines) (green) maps. The regions corresponding to an overlap of both elements are displayed in white. Intestinal epithelial cells HT29-MD2 were incubated for 2 h with Mn1Re (100 mM, 0.02% DMSO) before cryofixation and freeze-drying. Images were recorded on the 2-ID-D beamline of the APS synchrotron (excitation at 12.0 keV; integration time 2 s per pixel; pixel size 200 nm). (2) Confocal fluorescence images of HT29-MD2 of two different cells incubated with a mitochondrial marker (Mitotracker™, fluorescence) and X-fluorescence maps of Mn1Re (Re, X-fluorescence). Intestinal epithelial cells HT29-MD2 were incubated for 1.5 h with Mn1Re (100 μM, 0.02% DMSO) before the addition of Mitotracker™ deep red (200 nM, 30 min), washing with 50 mM EDTA, cryofixation and freeze-drying. Confocal fluorescence images of Mitotracker™ deep red (ex 633 nm, em 645–750 nm) were recorded with open pinhole. X-fluorescence (Re) images were recorded on the Nanoscopium beamline of SOLEIL synchrotron (excitation at 14.1 keV; integration time 2.4 s/pixel; pixel size 300 nm). Scale bar 3 μm. (3) C: control HT29-MD2 cells, Mn1/Mn1Re: HT29-MD2 cells incubated with Mn1/Mn1Re (10 and 100 μM, 6 h). IL8 secretion in HT29-MD2 cells. Intestinal epithelial cells HT29-MD2 were incubated for 7 h under different conditions indicated in the figure. LPS (0.1 μg⋅mL−1) was added at the end of the first hour. Data represent means +/− SEM for 7–10 independent experiments. (∗) p < 0.05, (∗∗) p < 0.01 vs. LPS condition. (4) C-control cells, Mn1/Mn1Re-HT29-MD2 cells incubated with Mn1/Mn1Re (100 μM, 6 h). Total Mn content was determined in acid-digested cell lysates by titration using EPR. Data represent mean +/− SEM for 4 independent experiments. (∗) p < 0.05, (∗∗) p < 0.01 versus C. Total Mn content in mitochondria-enriched fractions was determined by ICP-MS. Mitochondria were isolated using a mitochondria isolation kit (Thermo Fisher Scientific). Data represent mean +/− SEM for 7 independent experiments; see [11] for details. Schemes in blue and pink: molecular structure of Mn1 and Mn1Re mentioned in this figure. Masquer
From ref [10]. (1) Elemental distribution of P, K, Mn, Re, and Zn in an HT29-MD2 cell incubated with Mn1Re. The phosphorus (P, Kα-line), and zinc (Zn, Kßz-line, and not Kα to avoid overlapping with Re Lα) maps are used ... Lire la suite
4.1.2. Metal speciation studied by mass spectrometry (MS) and ion mobility spectrometry (IMS)
The detection and quantification of exogenous metal complexes are crucial to understand their activity in biological environments. In this regard, Mn(II) complexes are a challenge because of their low association constants, high lability, and rapid metal exchange [2, 128, 129]. We have recently shown that liquid chromatography coupled to mass spectrometry (LC-MS) in cell lysates can be used to detect and quantify Mn-based SOD mimics as long as they are not too labile, with the simple use of a calibration curve [53]. For the more labile ones, metal ions leaking from the LC-MS detection system were detrimental, with an Mn–Zn exchange, especially at low concentrations of the Mn complexes. As direct infusion showed no metal exchange, the leakage of metal ions from the separation part (LC) was incriminated to be responsible for the metal ion exchange (Mn–Zn) in the complex. Another separation technique, namely ion mobility, was then explored to separate labile metal complexes of the first transition series.
Ion mobility spectrometry (IMS) was first developed in the 1970s to detect traces of organic molecules in gas phase [130]. This technique uses separation according to the size of the molecule within an electric field and within a gas flow that pushes the molecules forward or backward depending on set-up. Their velocity is linked to their collision cross section (CCS). In IMS-MS, the detection is achieved by mass spectrometry. IMS-MS is a powerful technique used to characterize differences in folding of proteins upon ligand binding [131] or of peptides upon metal ion binding [132].
Recently, we have shown that IMS-MS is powerful enough to discriminate between very similar low molecular weight (LMW) ligands folding around metal ions across the first metal transition series [42]: ion mobility correlates well with ion radius (Figure 11). Then, IMS-MS was efficiently used to detect these labile metal complexes in cell lysates. They were quantified using a calibration curve with a standard made of a heavy ligand (with six 13C) and a Co(II), metal ion chosen to lead to the least metal exchanges. The use of this heavy ligand-based Co complex, added just before IMS-MS analysis, allowed us to probe the metal exchange that occurred in the analytical system rather than in the cell lysates [42].
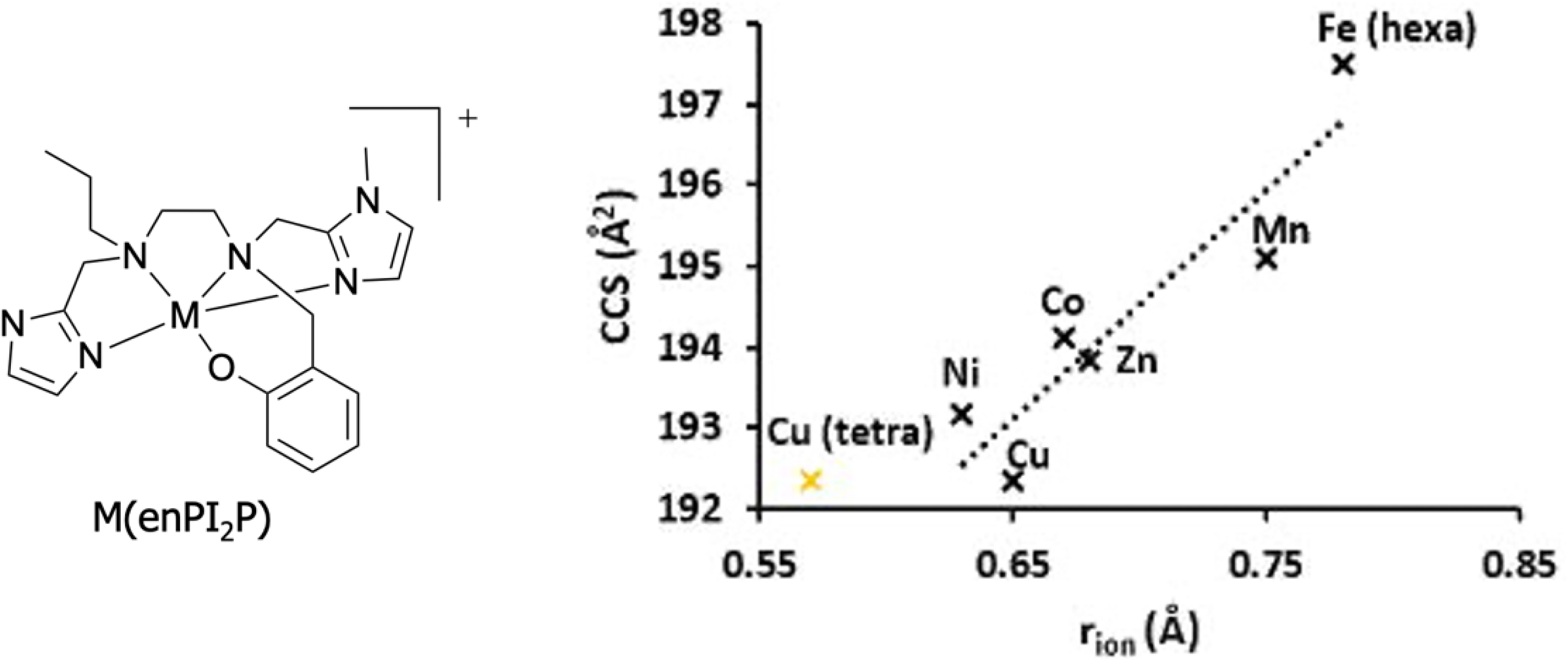
Collision cross-section of complexes M(enPI2P) (M(II) coordinated by ligand enPI2P) in Å2 plotted against the ionic radius of metal cations in Å. The rion of any M(II) corresponds to a high-spin pentacoordination, except for Fe(II) (high-spin hexacoordination). The rion for Cu(II) is also shown (in yellow) for a high-spin tetracoordination. The rion values were taken from http://abulafia.mt.ic.ac.uk/shannon/ptable.php. Adapted with permission from [42]. Copyright 2022 Wiley.
In the cellular context, there are a lot of Lewis bases that can coordinate to endogenous metal ions. The Irvin–Williams series [133] describes the range of association constants for divalent cations of the first transition row associated with unconstrained ligands. They show a trend of increasing association constant from left (Mn(II)) to right (Cu(II) and Zn(II)). Interestingly, Giedroc and Lisher have proposed a very enlightening idea, classifying cations as “competitive” (Zn(II), Cu(I), the redox state in cells) or “bioavailable” (Mn(II) but also, to a weaker extent, Fe(II)) [129]. Cu(I) and Zn(II) are more tightly controlled in cells than Mn(II). They show a higher association constant for most biological ligands. The existence of a pool of loosely bound Cu(I) and Zn(II) is very elusive, if only because hard to define. With a concentration of ca. 10−19 M estimated for this pool, as defined by the threshold of detection by endogenous metal sensors [134, 135, 136], this is not even one ion in a 2 pL cell, a typical volume for one eukaryotic cell. In contrast, the loosely bound pool is very abundant in Mn(II) (10−5 M corresponding to more than 10 millions Mn(II) ions in 2 pL) [129].
In a mixture where several metal ions M and several ligands L are competing, the primarily formed LM complex(es) are those showing the highest KLM association constants. The ligands and metals ions remaining after this pairing are those less prone to associate. Hence, paradoxically, the stability threshold in cells is more stringent for Cu-complexes, which will find strong ligand competitors in cells, than for Mn-complexes, which will in contrast find Mn(II) highly bioavailable in cells. Furthermore, the loosely bound abundant pool of Mn(II) will contribute to shift the equilibrium M + L = LM toward the formation of the complex L–Mn(II). To that extent, and paradoxically, complexes of Mn(II), such as Mn1, Mn1P, Mn1C and Mn1CP, are probably ideal to be used in cells, as the ligand is weak enough not to compete with endogenous ligands for Cu or Zn (proteins), and probably strong enough to possibly remove Mn from the loosely bound endogenous Mn pool.
4.2. Improving inertia against metal exchange
As stated earlier, the use of metal complexes involving coordination bonds raises specific difficulties in terms of stability in biological contexts, associated with metal exchanges, ligand addition (into a ternary complex for instance) or ligand exchanges, which is in addition to questions raised by other metabolization pathways common with organic molecules. This can clearly impact the pharmacokinetics properties of metal complexes–based drugs. Indeed, the first metabolization pathway of such drugs is metal decoordination, in particular for complexes involving an open-chain ligand. Dissociation may occur via different pathways, such as spontaneous decoordination and acid-catalyzed or metal-assisted decomplexation involving exchange with a competitive metal ion [128]. Thermodynamic stability and kinetic inertness are thus key parameters to take into account when designing complexes for biological applications. In order to improve the biological properties of Mn(II)-based SOD mimics, inspired by the work of Tóth et al. [121], we have increased the inertness of the complexes by rigidifying the ligand central structure. To do so, the central ethane diamine bridge has been replaced by a trans cyclohexyl-1,2-diamine [53]. We expected that a weaker elasticity of the ligand, as defined by Comba [137], would reduce the kinetics of metal decoordination by limiting the opening–closing breathing. Indeed, we have shown that the rigidified ligand led to complexes with slower metal exchanges with divalent competitive cations [129] such as Cu2+, Zn2+, Ni2+, and Co2+. Very interestingly, this improved inertness was associated with similar to better anti-inflammatory and antioxidant properties in the HT29-MD2 cell line. These activities were also retained at lower concentration (10 μM, Mn1C, Mn1CP), than what is observed for the complex built with the unconstrained ligand (100 μM, Mn1). Increasing the inertness of complexes is consequently a valuable strategy to improve their bioactivities [53].
4.3. Equilibrium between soluble and insoluble phases
In addition to soluble species, it has also been observed that accumulated metals can form dense phases in biological systems, suggesting the formation of insoluble phases. This is particularly striking in the case of metal-accumulation diseases, such as hemochromatosis, i.e., iron (Fe) overload, or Wilson diseases, i.e., copper overload. In the pathological contexts of metal overload, tissues that accumulate significant quantities of metals, such as the liver, generally present dense deposits of a few to few hundred nanometers. In the case of Fe, these phases can be identified as ferritin, the iron storage protein, resulting in the formation of crystalline objects around 8 nm, containing different crystalline phases [138]. However, not all metal ions undergo finely tuned biomineralization, and other transition metals could, when they accumulate, result in less organized phases, with differences in size, crystallinity, and composition. For example, in Wilson disease, while dense areas can easily be observed by transmission electron microscopy imaging, the diversity of the observed structures is important, and does not allow their nature to be clearly identified [139].
Interestingly, in a recent work published with Gazeau and Carn, we elucidated that exogenous metal ions could be subjected to precipitation as insoluble phases [140, 141]. These observations stem from the initial description of the biodegradation of gold (Au) nanoparticles inside cell lysosomes. Not only was it found that Au nanoparticles evolve and dissolve over time, but that the released metal ions recrystallize under a unique ultrastructure, forming curved leaves composed of self-assembled nanometer-sized Au clusters. This was surprising as Au particles have been considered inert or non-degradable. However, this result was consistent with some old literature dedicated to soluble ionic Au compounds used for the therapy of rheumatoid arthritis [142]. In patients’ biopsies and in animals, similar Au assemblies were observed due to Au accumulating in the tissues during treatment. These findings suggest that repeated exposure to an exogenous element in a soluble form can trigger its precipitation inside tissues as new solid phases with a common structure, regardless of the initial speciation of the metal (soluble, nanoparticular Au), the cell type considered, and the host species.
These phases present brand new challenges for speciation, as they present complex composition (mixed amorphous and crystalline phases incorporating endogenous elements, metabolites, or proteins) with complex reactivity, that can varies with the surrounding biological or chemical environment. They might also be highly sensitive to minor changes in their chemical environment, and particularly to change in the redox environment. Indeed, in the case of gold nanoparticles’ biodegradation, oxidative stress was a key element to enable gold nanoparticles dissolution, and inhibition of intralysosomal ROS production was found to slow down degradation process [140]. Thus, lysosomal change in redox potential or pH might be of major importance in biodissolution processes, but also to the formation of insoluble phases.
If soluble–insoluble phase equilibrium raises new challenges to describe metals speciation, it also offers new opportunities for their characterization, as their density allows their observation using transmission or scanning electron microscopy, even if caution is called for as these highly energetic beams can destabilize the samples while observing [141, 143].
This phenomenon of metal ions precipitation in biological matrices provides food for thought when considering how cells can deal with metal ions that are essential for their metabolism but become toxic at high concentration, such as iron and copper. Interestingly, it also raises questions about the metabolization of metal ions that are naturally present in ultra–trace levels, but become elevated due to exposure resulting from therapeutic, occupational, accidental or pollution-related activities. Indeed, precipitation could be a way for the cells to mitigate metal ion reactivity, limiting the proportion of chemically active atoms to the atoms accessible at the surface (which can be different from all of the surface atoms because of protein adsorption on the dense phase), thus also moderating their potential adverse effects.
5. Conclusion
Because speciation matters, integrating the design of metal complexes meant to be bioactive with their study in a cellular context is quite important and fruitful toward the development of therapeutic application for metal complexes. For this integrated approach, the terms cellular inorganic chemistry or inorganic chemobiology can be coined. It goes with a particular interest in subcellular metal imaging. In addition, as paramagnetic species such as metal centres perform activation of paramagnetic dioxygen, there is a strong intertwining between biochemistry of metal ions and that of dioxygen. In addition, metalloproteins and metal complexes are used to combat pathologies related to oxidative stress.
As it encompasses chemical conception, characterization, and cell studies, including chemical analyses and evaluation of bioactivities, this approach in inorganic chemical biology requires skills in organic (ligands) and inorganic (complexes) syntheses, physical and analytical chemistries in intricate biological media, cell biology and bioimaging, with special interests in monitoring metal distribution and cell state, and more specifically redox state.
In this article we have described first the cellular models that we have developed for evaluating the bioactivities of these complexes. Those three cellular models can be used for the screening of metal-based antioxidants, to have a better idea of their propensity to act on superoxide or hydrogen peroxide concentration, and of their ability to be an efficient antioxidant in a biological context. The second part deals with the nature of the metal species in cells and of their subcellular distribution. The question of the integrity of metal complexes is intrinsically linked to the broader question of metal speciation, which aims to determine the complete array of metal-based species present, including insoluble phases formed by precipitation. Interestingly for the design of Mn-complexes, it is possible to play with the inertia to enhance bioactivity through a higher in-cell stability.
Abbreviations
| ARE | Antioxidant response element |
| CaM | Calmangafodipir |
| CAT | Catalase |
| COX2 | Cyclooxygenase 2 |
| Cys | cysteine |
| DMEM | Dulbecco’s modified eagle medium |
| DNBS | 2,4-dinitrobenzenesulfonic acid |
| EDS/EDX | Energy-dispersive X-ray spectroscopy |
| EUK-134 | See Figure 5 |
| FBS | fetal bovine serum |
| GRX | glutaredoxin |
| HT29(-MD2) | Human colon cancer cell line (stably transfected to over express MD2, the co-receptor of TLR4 for LPS) |
| HeLa | Cancerous cell line |
| HEPES buffer | 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, pKa (25 °C) = 7.48 |
| HO | Heme oxygenase |
| HyPer | Hydrogen peroxide sensor |
| IBD | Inflammatory bowel diseases |
| ICP-MS/LA-ICP-MS | Inductively coupled plasma mass spectrometry/laser ablation inductively coupled plasma mass spectrometry |
| IEC | Intestinal epithelial cells |
| IFN-γ | Interferon γ |
| IL8 | Interleukin 8 |
| IUPAC | International union of pure and applied chemistry |
| IMS/MS | Ion mobility spectrometry coupled to mass spectrometry |
| KEAP1 | Kelch-like ECH-associated protein |
| LC | Liquid chromatography |
| LIBS | Laser-induced breakdown spectroscopy |
| LPS | Lipopolysaccharide |
| LMW | Low molecular weight |
| MitoTempol | See Figure 5 |
| MD2 | Myeloid differentiation 2 (receptor) |
| Mn1, Mn1C, Mn1CP, MnTxxx, M40403 | See Figure 5 |
| MS | Mass spectrometry |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide |
| NAD(P) | Nicotinamide adenine dinucleotide (phosphate) |
| (i)NOS | (inductive) NO-synthase |
| NOX | NADPH oxidase |
| NQO-1 | NAD(P)H Quinone Dehydrogenase 1 |
| NRF2 | Nuclear factor (erythroid-derived 2)-like 2 |
| OIPN | Oxa-induced peripheral neuropathy |
| Oxa | OxaliPt |
| OxyR(-RD) | Bacterial peroxide sensor (regulatory domain) |
| PDRX | peroxiredoxin |
| PMA | Phorbol 12-myristate 13-acetate |
| PGE2 | Prostaglandin E2 |
| RAW 264.7 | Murine macrophages |
| ROS | Reactive oxygen species |
| Shh | Sonic hedgehog |
| SIMS | Secondary ion mass spectrometry |
| SOD, CuZnSOD and MnSOD | Superoxide dismutase, Copper-Zinc superoxide dismutase, manganese superoxide dismutase; SOD1: SOD cytosolic (Cu-Zn); SOD2: MnSOD, mitochondrial |
| TLR4 | Toll-Like-Receptor 4 |
| Tris buffer | 2-amino-2-(hydroxymethyl) propane-1,3-diol, pKa (20 °C) = 8.3 |
| XRF | X-fluorescence |
| (cp)YFP | (circularly permuted) yellow fluorescent protein |
Declaration of interests
The authors do not work for, advise, own shares in, or receive funds from any organization that could benefit from this article, and have declared no affiliations other than their research organizations.
Funding
The authors want to acknowledge ENS-PSL, PSL University, CNRS, MITI-CNRS, Sorbonne University (SU) and the other bodies that funded this work. ANR-15-CE07-0027 MAGIC; ANR-16-CE18-0017-01 SATIN; ANR-16-CE07-0025 Metallopepzyme; DEI20151234413 (Fondation pour la recherche médicale 2016), and BACTMAN and ANACOMDA (Mission pour les initiatives transverses et interdisciplinaires MITI-CNRS), the Association François Aupetit (AFA, research fellowship 2015), ANR 21-CE18-0053-02 MOBIDIC, ANR 20-CE07-0039-01-CATMAN, ANR 23-CE23 METALINFY; Idex PSL Qlife project Main ANR Q-life ANR-17-CONV-0005; CEFIPRA project no. 6505-1; EMERGENCE SU EMRG-24 TOTEM; ANR-22-PEBI-0003 (PEPR).
Acknowledgements
Servier Medical Art, licensed under CC BY 4.0, is acknowledged for cell image used in TOC.
1 There has been so far no protective system identified for HO∙: this radical reacts with a rate only limited by diffusion, meaning that the first encounter with a molecule leads to an efficient reaction (H-abstraction notably) and there is no possibility for this radical to diffuse to a protective system.





 CC-BY 4.0
CC-BY 4.0