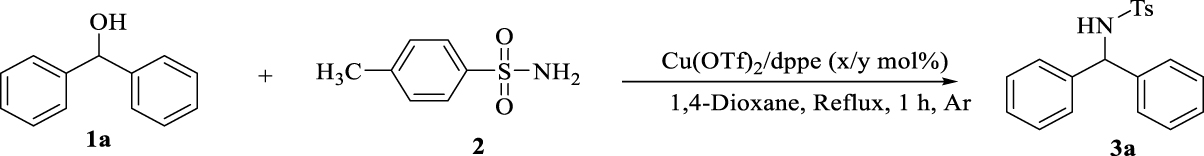
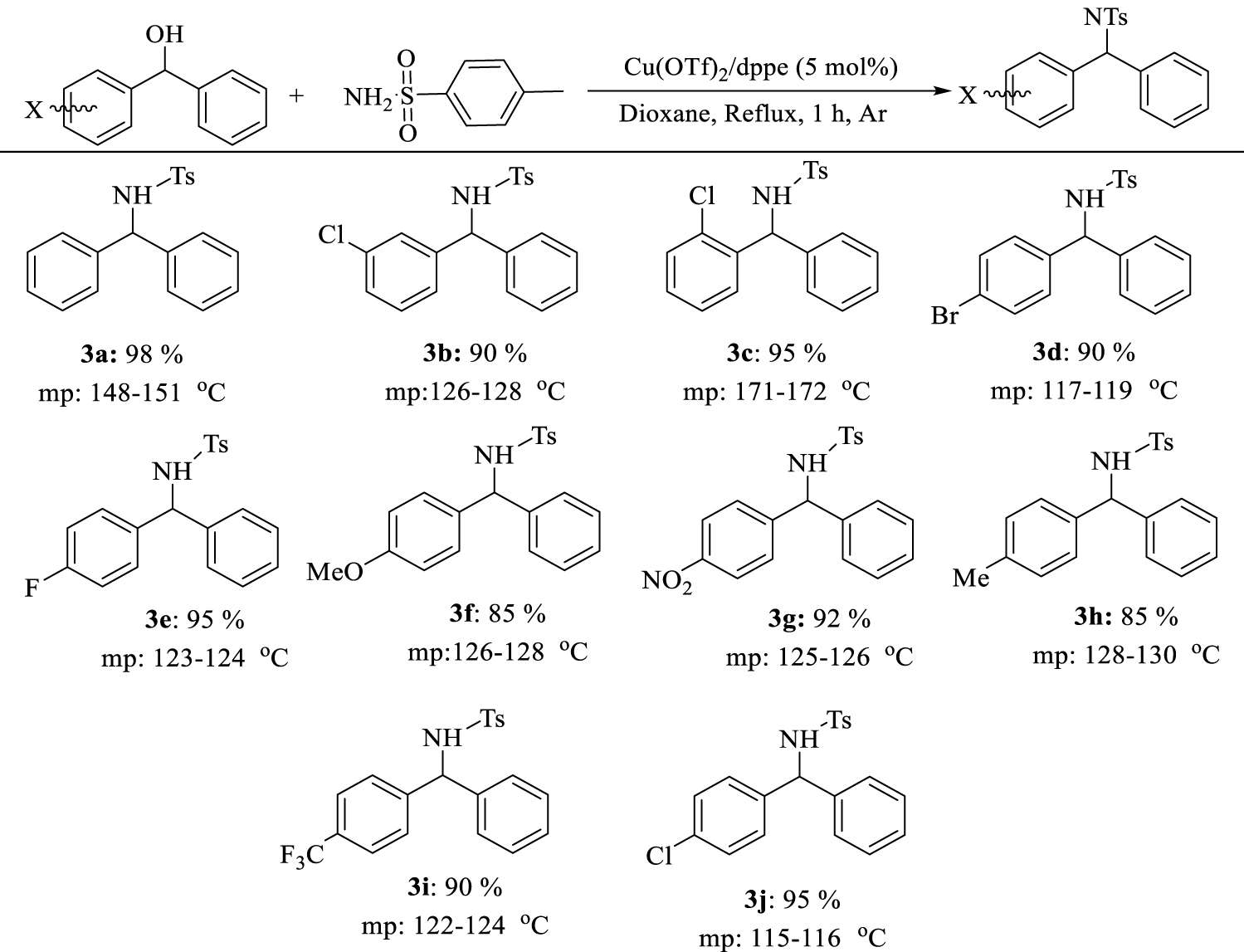
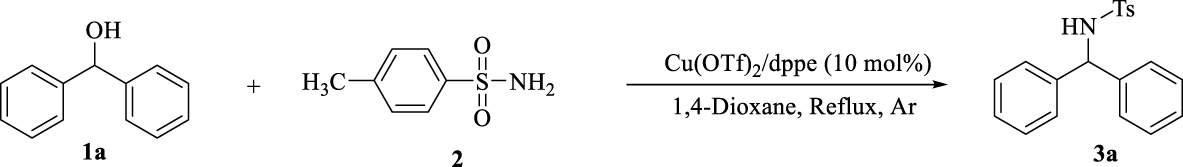1. Introduction
Amines play an important role in biological [1, 2], pharmacological [3] and agricultural [4] activities. Hence the synthesis of substituted amines (the structural active motif of several drugs such as cetirizine [5], sertraline [6], rasagiline [7] and rivastigmine [8]) has been attracting increasing attention.
Different protocols for the conversion of alcohols to amines.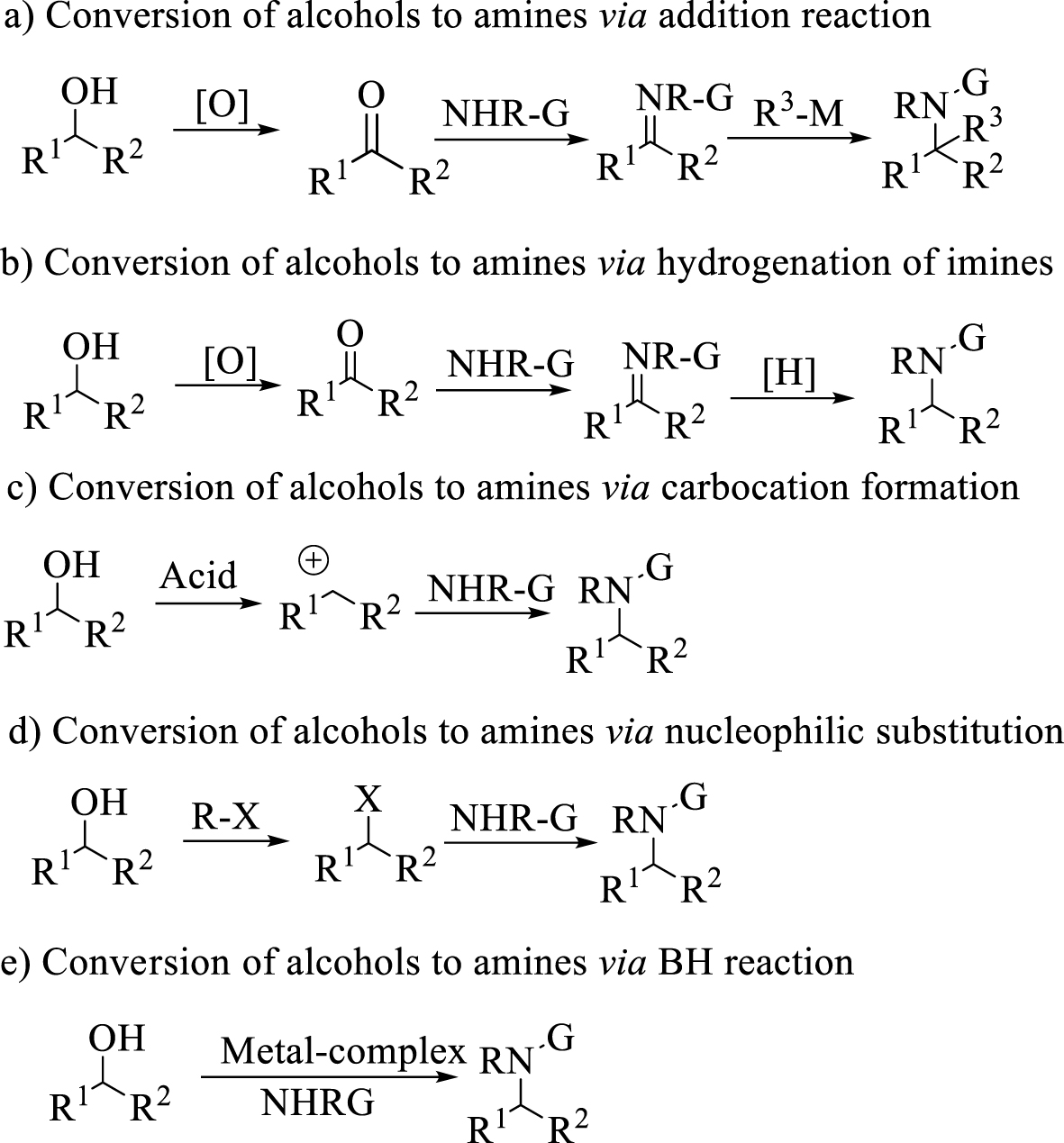
Alcohols are usually inexpensive substrates and available sources to prepare amines. They are usually converted to aldehydes [9], ketones [10], imines [11] or other related compounds before reacting with nucleophiles. Alongside all advances in rhodium catalyst addition of the organometallic reagents to imines producing diarylamine [12], numerous methods were reported in the literature, based on hydrogenation of imines and enamines [13, 14], amination of ketones and amination of alkyl halides [15] (Scheme 1). Amongst them, hydrogenation reactions are much more important in research or industry, and the use of either hydrogen gas or transfer hydrogenation reduction with Brønsted [16] or Lewis catalysts [17, 18, 19] and transition metal complexes of Ir, Pd, Rh is well established [20, 21, 22]. Notwithstanding many reports on these reactions, these multistep pathways often suffer from the instability of the intermediates, low atom economy and the production of byproducts. In addition, the drawbacks of Ir-, Pd-, Rh-based catalysts such as toxicity, high cost and metal leaching problems [23] have limited their broad utilization for these reactions. Using protocols which provide advantages regarding these limitations on an industrial scale is going to be a challenge.
In recent years, a green, economical and environmentally friendly protocol has been designed for producing substituted amines through a borrowing hydrogen reaction (BH methodology) [24] without the need for additional hydrogen sources. The main point of this reaction can be temporary storage of hydrogen, which is released from the nucleophilic substrate to the electrophilic metal catalyst during the gentle oxidation process. After the conversion of the oxidized intermediate to the C=X double bond intermediate, the metal-hydride catalyst returned hydrogen to the C=X double bond which is more electrophilic than the initial substrate. Hence, the development of an efficient catalytic BH methodology has attracted more attention in recent years. Furthermore, because of the stability of the metal-hydrid formed with second and third-row transition metal catalysts, which prevents return of the activated hydrogen [25], these metals are not suitable for BH methodology. Therefore, developing the use of active metals in these reactions has been much highlighted. In 2013, Singh and co-workers reported the conversion of primary benzylic alcohols to N-alkyl amines using Fe(II) phthalocyanine as catalyst [26]. In 2014, Feringa and Barta reported (cyclopentadienone)iron carbonyl as precatalyst for direct coupling of alcohols and amines through BH methodology [27]. In 2014, Zhao and coworkers reported a catalytic method for the amination of alcohols in the presence of an iridium complex and phosphoric acid. In 2018 Sunoj and co-workers reported the amination of alcohols in the presence of iridium-diamine complex and phosphoric acid [28]. In 2019, Barta and co-workers reported direct amination of benzyl alcohols using NH3 in the presence of Ni(OTf)2∕dcpp catalyst [29]. In 2019, Hofmann and Hultzsch also reported the N-alkylation of anilines with benzylic alcohols using the nitrile-ligated Knölker’s complex [30].
Preparation of diarylamines using p-toluene sulfonamides and benzhydrol derivatives in the presence of copper/bisphosphine complexes.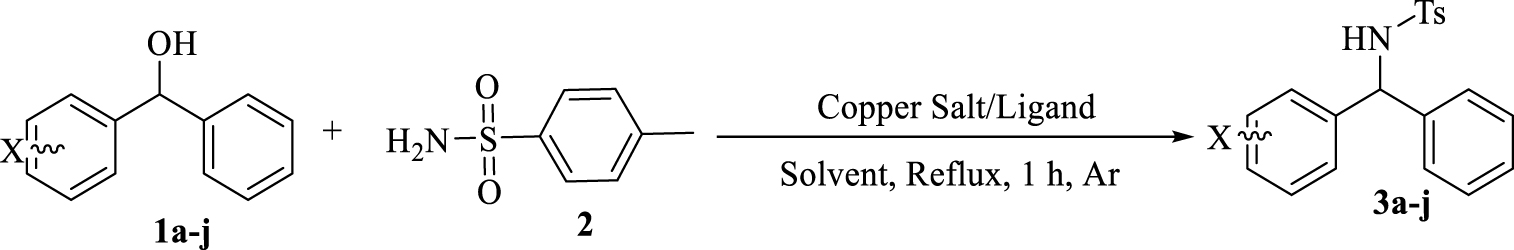
In this context, for the preparation of diarylamines in a clean, suitable and attractive BH methodology, we report a one-step reaction within benzhydrol derivatives and p-toluene sulphonamide in the presence of copper-bisphosphine complexes. This methodology benefits from the use of readily available and low cost copper salts, required no additional acids, bases or activation reagents to activate the alcohol, no hydrogen source and results in the formation of H2O as an only byproduct after 1 h (Scheme 2).
2. Experimental
2.1. Materials
All chemicals were purchased from Sigma-Aldrich or Merck Chemicals. Diethyl ether, tetrahydrofuran, and 1, 4-dioxane were distilled under nitrogen from benzophenone/sodium before use. Copper salts were dried overnight at 120 °C. Benzhydrol derivatives were prepared according to previously reported procedures [31]. 1H NMR and 13C NMR spectra were recorded on BrukerAvIII HD-500 MHz using TMS as an internal standard.
2.2. General procedure
The borrowing hydrogen process is achieved in a sealed Schlenk tube under argon atmosphere. Benzhydrol derivative (0.1 mmol), p-toluene sulfonamide (0.1 mmol), Cu(OTf)2 (5 mol%) and dppe (5 mol%) were dissolved in dried 1,4-dioxane (0.5 mL). The mixture was stirred for 1 h under reflux. After cooling to room temperature, the reaction mixture was passed through a short silica column (eluent ethyl acetate), and then the solvent was removed under reduced pressure. The obtained residue was purified by column chromatography (EtOAc–PE, 1:10) to afford the diarylated amines:
N-[(phenyl)phenylmethyl]-4- methylbenzenesulfonamide(3a)
White solid; yield: 346 mg (98%); mp 148–151 °C [32]: 1H NMR (500 MHz, CDCl3): δ 7.59 (d, J = 8.0 Hz, 2 H), 7.20–7.25 (m, 6 H), 7.10-7.17 (m, 6 H), 5.57 (d, J = 7.2 Hz, 1 H), 5.34 (d, J = 7.1 Hz, 1 H), 2.38 (s, 3 H); 13C NMR (100 MHz, CDCl3): 142.1, 139.5, 128.3, 127.5 (4 C), 127.3, 126.5 (2 C), 126.2 (4 C), 126.1 (2 C), 60.3, 20.4.
N-((4-chlorophenyl)(phenyl)methyl)-4-methylbenzenesulfonamide(3b)
White solid; yield: 367 mg (95%) mp 115–116 °C [33]. 1H NMR (500 MHz, CDCl3): δ 7.56 (d, J = 8.1 Hz, 2 H), 7.23–7.245 (m, 3 H), 7.16–7.23 (m, 4 H), 7.04–7.11 (m, 4 H), 5.54 (d, J = 6.9 Hz, 1 H), 5.10 (d, J = 6.9 Hz, 1 H), 2.42 (s, 3 H). 13C NMR (100 MHz, CDCl3) 141.2, 139.8, 139.6, 137.2, 133.1, 128.4, 127.6 (2 C), 127.6, 126.8, 126.1 (2 C), 59.6, 20.3.
N-((3-chlorophenyl)(phenyl)methyl)-4-methylbenzenesulfonamide(3c)
White solid; yield: 348 mg (90%); mp 126–128 °C [34].
N-((2-chlorophenyl)(phenyl)methyl)-4-methylbenzenesulfonamide(3d)
White solid; yield: 367 mg (95%) m.p. 171–172 °C [33]. 1H NMR (500 MHz, CDCl3): δ 7.62 (d, J = 8.2 Hz, 2H), 7.32 – 7.37 (m, 1H), 7.20 – 7.25 (m, 4H), 7.13 – 7.18 (m, 4H), 7.07 (dd, J = 7.0, 2.2 Hz, 2H), 5.92 (d, J = 7.2 Hz, 1H), 5.31 (d, J = 7.2 Hz, 1H), 2.38 (s, 3H).
N-((4-bromophenyl)(phenyl)methyl)-4-methylbenzenesulfonamide(3e)
White solid; yield: 389 mg (90%); mp 117–119 °C [35]. 1H NMR (500 MHz, CDCl3): δ 7.54 (d, J = 8.2 Hz, 2H), 7.32 (d, J = 8.5 Hz, 2H), 7.20 – 7.23 (m, 3H), 7.14 (d, J = 8.0 Hz, 2H), 7.05 (dd, J = 6.5, 2.9 Hz, 2H), 7.00 (d, J = 8.4 Hz, 2H), 5.52 (d, J = 7.1 Hz, 1H), 5.21 (d, J = 7.1 Hz, 1H), 2.39 (s, 3H).
N-((4-fluorophenyl)(phenyl)methyl)-4-methylbenzenesulfonamide(3f)
White solid; yield: 353 mg (95%); mp 123–124 °C [36]. 1H NMR (500 MHz, CDCl3): δ 7.55 (d, J = 8.3 Hz, 2H), 7.17 – 7.21 (m, 3H), 7.02 – 7.07 (m, 4H), 6.92 – 6.96 (m, 4H), 5.54 (d, J = 7.1 Hz, 1H), 5.16 (d, J = 7.0 Hz, 1H), 2.39 (s, 3H).
N-((4-methoxyphenyl)(phenyl)methyl)-4-methylbenzenesulfonamide(3g)
White solid; yield: 326 mg (85%); mp 126–128 °C [37].
4-methyl-N-((4-nitrophenyl)(phenyl)methyl) benzenesulfonamide(3h)
Light yellow solid; yield: 367 mg (92%); mp 125–126 °C [38]. 1H NMR (500 MHz, CDCl3): δ 7.58 (d, J = 8.1 Hz, 2H-Ar), 7.22–7.26 (m, 3H, Ar-H), 7.22–7.16 (m, 4H),7.10–7.04 (m, 4H) 5.55 (d, J = 6.9 Hz, 1H, NCH), 5.01 (d, J = 6.9 Hz, 1H, HN), 2.42 (s, 3H).
4-methyl-N-(phenyl(p-tolyl)methyl) benzenesulfonamide(3i)
White solid; yield: 312 mg (85%); mp 128–130 °C [33]. 1H NMR (500 MHz, CDCl3): δ 7.54 – 7.59 (m, 2H), 7.17 – 7.23 (m, 3H), 7.14 (d, J = 8.0 Hz, 2H), 7.08 – 7.12 (m, 2H), 6.99 (d, J = 8.1, 8.1 Hz, 4H), 5.52 (d, J = 7.0 Hz, 1H), 5.06 (d, J = 6.9 Hz, 1H), 2.38 (s, 3H), 2.28 (s, 3H).
4-methyl-N-(phenyl(4-(trifluoromethyl)phenyl) methyl)benzenesulfonamide(3j)
White solid; yield: 379 mg (90%); mp 122–124 °C [39]. 1H NMR (500 MHz, CDCl3): δ 2.35 (s, 3H), 5.34 (d, J = 7.2 Hz, 1H), 5.59 (d, J = 7.2 Hz, 1H), 7.01 – 7.05 (m, 2H), 7.10 (d, J = 8.0 Hz, 2H), 7.23 (s, 1H), 7.19 – 7.23 (m, 3H), 7.25 (s, 1H), 7.42 (d, J = 8.2 Hz, 2H), 7.51 (d, J = 8.3 Hz, 2H).
3. Results and discussion
Initially, the model reaction was carried out using benzhydrol (1a) (0.1 mmol), p-toluene sulphonamide (2) (0.1 mmol) in refluxing 1,4-dioxane under argon atmosphere with the trace formation of the desired product (3a) after 10 h. When the reaction was performed in presence of Cu(OTf)2, the rate of reaction increased and yields of 30% were obtained after 10 h.
In the next run, the reaction was done in the presence of Cu(OTf)2 (5 mol%), dppe (5 mol%) which allowed the rate and yield of reaction to significantly improve (98%) after 1 h. We optimized the conditions, screening numerous ligands and copper salts, solvents, the ratio of substrates, the amount of copper salts and ligands and also using a base to significantly decrease the reaction time. As the results showed (Table 1), the reactions performed using dppm, dppp and dppb show no significant conversion, while in the presence of PPh3, the desired product was obtained with 80% yield. Using (rac)-binap and (rac)-segphos the products were prepared with 85 and 90% yields respectively (Table 1, entries 2–7). Surprisingly, other copper salts could not promote the reaction (entries 8–13). The reaction also did not proceed in the presence of base, even non-coordinating bases (Table 1, entry 14). Under basic conditions, the formed metal-hydride/ H(OTf) catalyst becomes inactive and the reaction does not proceed.
| Entry a | Copper Salt | Ligand | Yield (%)b |
|---|---|---|---|
| 1 | Cu(OTf)2 | dppe | 98 |
| 2 | Cu(OTf)2 | dppm | trace |
| 3 | Cu(OTf)2 | dppp | trace |
| 4 | Cu(OTf)2 | dppb | trace |
| 5 | Cu(OTf)2 | PPh 3 | 80 |
| 6 | Cu(OTf)2 | (rac)-binap | 85 |
| 7 | Cu(OTf)2 | (rac)-segphos | 90 |
| 8c | Cu(OAC)2 | dppe | - |
| 9 | CuCl2 | dppe | - |
| 10 | CuCl | dppe | - |
| 11 | CuI | dppe | - |
| 12 | Cu(NO3)2.3H2O | dppe | - |
| 13 | Cu2O | dppe | - |
| 14d | Cu(OTf)2 | dppe | - |
aReaction conditions: benzhydrol (0.1 mmol), p-toluenesulfonamide (0.1 mmol), Dioxane (0.5 mL). bIsolated yields. cEntries 8–13 after 24 h, dKOH or K2CO3 were used.
Next, we investigated the effect of the solvent. In general, the BH reaction in common organic solvents progresses with low conversion towards the desired product. This can be due to insufficient imine reduction. The degree of stability of metal-hydride intermediate is crucial, which is directly affected by the solvent. If the metal-hydride intermediate is too stable, it cannot return hydride easily. If it is not stable enough, it cannot enter the catalytic cycle. Therefore, it appears that a weakly coordinating solvent may stabilize the metal-hydride intermediate to enhance the imine reduction step. As outlined in Table 2, no desired product was observed in tetrahydrofuran and water, while the reaction proceeded with high yields in dichloromethane or toluene (90% and 95%, respectively), but not as rapidly as in 1,4-dioxane which furnished the desired product in both excellent yield and rate.
The influence of the ratio of the substrate was evaluated on the yield and the rate of reaction. Increasing the amount of benzhydrol has no notable effect on the yield, but the reactivity and the rate of reaction decreased and unreacted alcohol was observed (Table 3). Next we studied the effect of the loading amounts of the catalytic system with the best result obtained using Cu(OTf)2 and dppe (1:1, 5 mol% of benzhydrol) (Table 4, entry 2). Subsequently, no reaction occurred at room temperature.
| Entry | Solvent | Yield (%) b |
|---|---|---|
| 1 | Tetrahydrofuran | - |
| 2 | H2O | - |
| 3 | Dichloromethane | 90c |
| 4 | Toluene | 95d |
| 5 | 1, 4-Dioxane | 98 |
aReaction conditions: benzhydrol (0.1 mmol), p-toluene sulfonamide (0.1 mmol), dppe, Cu(OTf)2, solvent (0.5 mL). cAfter 5 h). dAfter 2 h.
The proposed BH mechanism.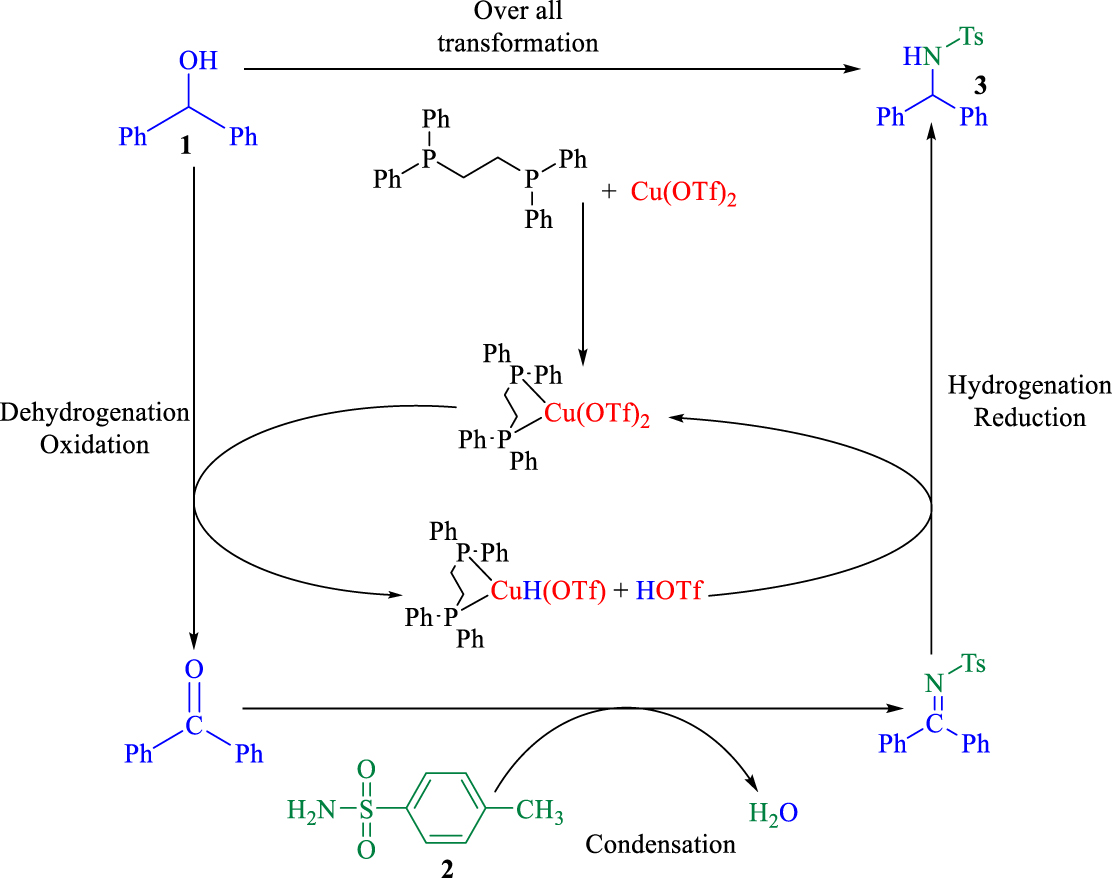
| Entry | Ratio (1a to 2) | Time of reaction (h) |
|---|---|---|
| 1 | 1:1 | 1 |
| 2 | 2:1 | 2 |
| 3 | 3:1 | 3 |
| 5 | 4:1 | 5 |
aReaction conditions: benzhydrol (a), p-toluene sulfonamide (b), dppe, Cu(OTf)2, dioxane (0.5 mL).
| Entry | x | y | Yield (%)b |
|---|---|---|---|
| 1 | 2 | 2 | 80 |
| 2 | 5 | 5 | 98 |
| 3 | 10 | 10 | 60 |
| 4 | 20 | 20 | 53 |
| 5 | 10 | 20 | 70 |
aReaction conditions: Benzhydrol (0.1 mmol), p-toluenesulfonamide (0.1 mmol), Cu(OTf)2 (x), dppe (y), dioxane (0.5 mL).
We extended the optimum conditions to different benzhydrol derivatives. The results are presented in Table 5 and different substituted diarylamines were obtained in high to excellent yields (90–98%) under the optimal reaction conditions.
Under optimized condition, a BH mechanism is suggested in Scheme 3. In the presence of dppe-Cu(OTf)2 catalyst, benzhydrol is converted during the oxidation step to benzophenone which is more reactive in the nucleophilic reaction, resulting in generation of the dppe-Cu(OTf)-H/ H(OTf) catalyst. Then, benzophenone immediately reacted with the amide and an imine intermediate was produced.
In the reductive step, the hydride was returned from the dppe-Cu(OTf)-H/ H(OTf) catalyst to the imine so that the desired product and Cu(OTf)2 catalyst were obtained. Due to the quick formation of the imine intermediate in the catalytic cycle, side reactions were completely suppressed and water is the only by-product [40]. Therefore, this methodology is a highly efficient and atom-economic method producing highly valuable amines for pharmaceutical and synthetic chemistry.
4. Conclusions
In conclusion, we developed a gentle, convenient, dynamically and kinetically efficient amidation of α-branched alcohols using p-toluene sulfonamide and benzhydrol derivatives in the presence of bisphosphine ligated Cu(OTf)2 as a good, first row-transition metal complex catalyst for BH methodology. Various substituted benzhydrols have been assembled effectively under racemic conditions with excellent yields in short reaction times. Further studies, including the development of an enantioselective procedure, are being actively pursued using other conditions by our research group.
Acknowledgments
We are grateful to the University of Kurdistan, Iran Research Councils for the support of this work.
Supplementary data
Supporting information for this article is available on the journal’s website under https://doi.org/10.5802/crchim.5 or from the author.




 CC-BY 4.0
CC-BY 4.0