1. Introduction
Global warming, due to emissions of greenhouse gases such as CO2, is one of the catastrophic phenomena faced by the world today. Many strategies have been devised for the reduction of CO2 emissions; however, CO2 conversion to methanol provides a vital route as it diminishes CO2 concentration on the one hand and produces valuable fuel like methanol on the other [1, 2, 3, 4].
Methanol synthesis is generally carried out by using a mixture of CO2 and syngas at the industrial level [5]. However, pure CO2 hydrogenation to methanol is more preferable than traditional syngas gas route from the environmental point of view. Methanol synthesis by syngas route has been extensively investigated; however, literature on the former route is very limited [6]. The performance of catalysts for pure CO2 conversion to methanol has been affected by various reaction parameters like reaction pressure, temperature, and composition of feed gases. In general, rate of reaction is directly proportional to the reaction temperature. Nevertheless, reaction temperature for CO2 reduction to methanol has not been univocal in the literature. Rate of methanol synthesis by CO2 hydrogenation was increased by increasing reaction temperature and 220 °C was recorded as an optimum reaction temperature [7]. Likewise, according to An et al. [8], 250 °C was reported as the maximum reaction temperature for best methanol selectivity and CO2 conversion. Similarly, both methanol production and CO2 conversion were enhanced by increasing temperature up to 250 °C [9].
Thermodynamically, according to Le Chatelier’s principle, high reaction pressure is favorable for CO2 hydrogenation to methanol. Liu et al. [10] claimed that CO2 hydrogenation to methanol could be synthesized at a low reaction pressure of 20 bar. Another study carried out by Liaw et al. [11] summarized that at 210 °C methanol yield was higher when increasing the total reaction pressure.
CO2 reduction to methanol is also affected by variation of feed gas ratio. From the thermodynamic point of view, H2∕CO2 = 3 is considered favorable for the process. Nevertheless, this factor has also been quite debatable in the literature. Methanol synthesis by CO2 hydrogenation at various feed gas ratios and constant reaction temperature 260 °C and pressure 360 bar over Cu∕ZnO∕Al2O3 catalyst was investigated by Bansode and Urakwa [12]. They observed remarkable increase in the rate of methanol synthesis as well as CO2 conversion by increasing feed gas H2∕CO2 ratio from 3 to 10. Such results were also documented by Kim et al. [13] for CO2 hydrogenation to methanol carried out in a slurry phase reactor. In summary, CO2 reduction to methanol is affected by the magnitude of reaction conditions and hence performance of the catalysts is determined by the reaction medium.
The current work describes CO2 reduction to methanol over Cu/ZrO2 catalysts supported by CNFs. Furthermore, the effect of reaction parameters such as pressure, temperature, and feed gas composition on the performance of the catalysts were also studied.
2. Experimental section
2.1. Catalysts synthesis
Deposition precipitation method was adopted to synthesize CNF-based Cu/ZrO2 catalysts. The detailed procedure for catalysts synthesis has been documented elsewhere [14, 15, 16]. Catalysts Cu⋅ZrO2/CNFs with 15 wt% each of Cu and ZrO2 with CNFs as a support were synthesized. The prepared catalysts were cooled, filtered and dried overnight at 100 °C. The dried catalysts were calcined under N2 flow at 450 °C for 3 h. A 0.5 g of pre-reduced catalysts (reduced in H2 for 6 h with 2000 cm3 h−1 flow rate at 380 °C) was used for reaction studies.
2.2. Variation of reaction parameters
Parr autoclave slurry reactor model, Parr 4593, was employed for the activity studies of the catalysts. The detailed procedure is reported elsewhere [17, 18]. Methanol synthesis via CO2 hydrogenation was first optimized in terms of reaction temperature. Hence, a range of reaction temperatures: 180, 200, 220, and 240 °C, was selected at constant reaction pressure of 30 bar. After an optimized reaction temperature was identified, methanol synthesis was tested at different reaction pressures of 20, 30, 40, and 50 bar at a constant reaction temperature of 220 °C. Similarly, the effect of feed gas ratio was also studied by carrying out methanol synthesis at different H2∕CO2 ratios of 0.8, 1.5, and 3 at 220 °C and 30 bar reaction temperature and pressure respectively. Methanol content was evaluated by using flame ionization detector (FID). Gas chromatography GC Agilent 6890 was employed for the current study.
Methanol synthesis rate was calculated by the following equation:
3. Results and discussions
3.1. Effect of reaction temperature
The rate of methanol synthesis was studied in a slurry reactor at fixed pressure of 30 bar in a range of 180–240 °C. The correlation of reaction temperature and methanol synthesis rate is displayed in Figure 1.
| (reaction 1) |
| (reaction 2) |
| (reaction 3) |
Correlation of reaction temperature and methanol synthesis rate.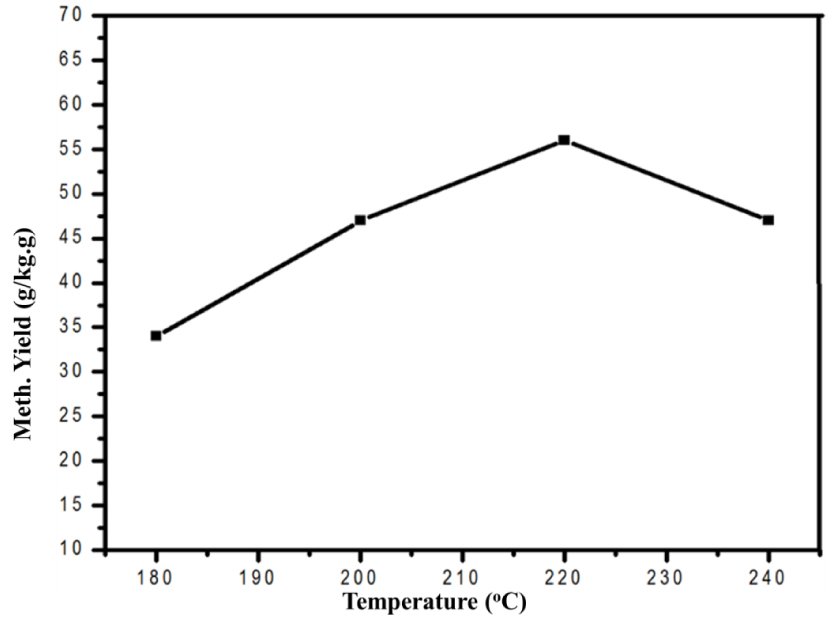
Correlation of reaction pressure and methanol synthesis rate.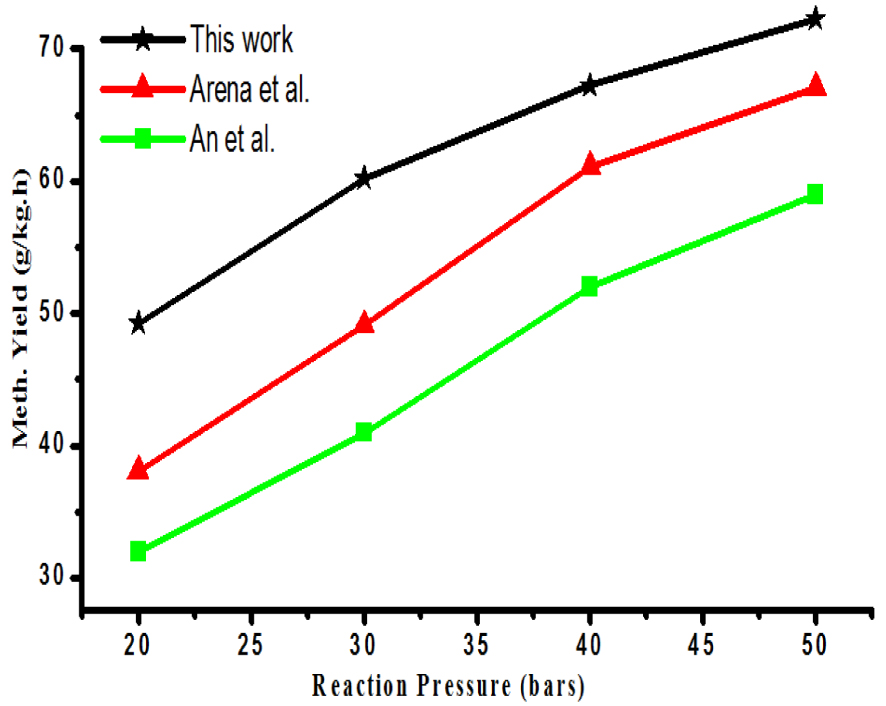
3.2. Effect of pressure
Methanol synthesis by CO2 hydrogenation was also studied at different pressures like 20, 30, and 40 bar. Figure 2 shows the correlation of reaction pressure and methanol synthesis rate. As observed, CO2 reduction to methanol is a molecule decreasing reaction so that it is thermodynamically facilitated at higher pressure. In the current study, a linear relationship was observed between pressure and methanol synthesis rate. Rate of methanol synthesis was increased from 45 to 57 g∕kg⋅h when pressure was increased from 20 to 30 bar. A similar trend of increasing rate was continued with further increase in pressure and maximum rate was obtained with the highest reaction pressure.
The obtained observations were in good agreement with the work of An et al. [8] and Arena et al. [22]. Although the process is favorable at high reaction pressure, too high pressure demands higher requirement of materials strength, high cost of operation and it is associated with safety problems.
3.3. Effect of feed gas composition
Rate of methanol synthesis by CO2 hydrogenation was also studied by varying feed gas composition. Figure 3 displays the rate of methanol synthesis as a function of increasing H2∕CO2 ratio. The methanol synthesis rate was increased from 32 to 41 g∕kg⋅h when the H2∕CO2 ratio was increased from 0.8 to 1.5. A similar trend was observed with further increase in H2∕CO2 ratio. This trend is quite understandable keeping in mind the stoichiometric chemistry of CO2 hydrogenation reaction.
Correlation of H2∕CO2 ratio and methanol synthesis rate.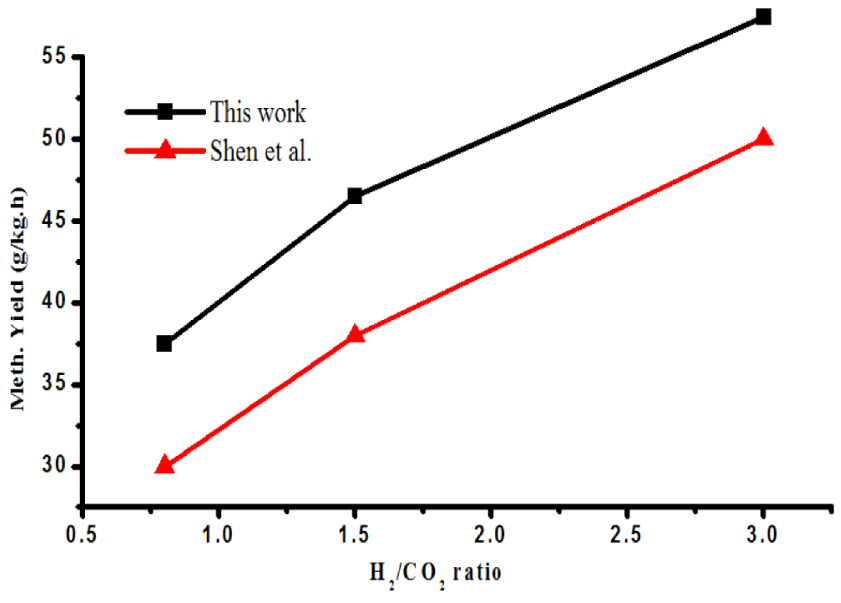
Even though H2∕CO2 ratio of unity has been favorable for formation of CO as the main product, the rate of methanol synthesis was enhanced by increasing H2∕CO2 ratio. Equation (reaction1) rightly justifies this trend, where higher H2∕CO2 ratio was desirable for better methanol yield. This could be one of the reasons of lower activity of catalyst to methanol synthesis with H2∕CO2 ratio <3.0. In addition, Shen et al. [23], reported similar observations for methanol synthesis via CO2 hydrogenation over Cu∕Zr∕Al2O3 catalyst.
4. Conclusion
The current work documented the effect of reaction variables on the CO2 reduction to methanol. Rate of methanol synthesis was significantly altered with variation in reaction temperature. It was initially raised with increasing temperature and the highest rate was observed at 220 °C before it declined with further increase in temperature. Likewise, the influence of reaction pressure was investigated by conducting the methanol synthesis at different magnitudes of total pressure. It was concluded from the pressure variation study that the rate of methanol synthesis progressively enhanced as a function of increasing total pressure. Finally, rate of methanol synthesis was also optimized in terms of feed gas ratio. The study concluded that a linear relationship existed between the rate of methanol synthesis and the increasing H2∕CO2 ratio.
Acknowledgment
The financial support provided by Ministry of Higher Education Malaysia via FRGS No: FRGS/1/2011/SG/UTP/02/13 is appreciated. Support provided by Chemistry Department, Prince Sattam bin Abdulaziz University, Saudi Arabia is also highly appreciated.



 CC-BY 4.0
CC-BY 4.0