1. Introduction
Waxy crude oil accounts for a large proportion of crude oil resources, and its characteristics are high viscosity and high pour point. It is these properties that cause waxy crude oil to have many problems during production, separation, transportation, and refining [1, 2, 3]. With the progress of society, the demand for oil continues to increase, while the reserves of light crude oil continue to decrease, and even have passed a turning point [4, 5]. In order to meet the market’s consumption of crude oil, especially some developing countries with great development prospects in China and India, the extraction of heavy oil is particularly important. In recent years, heavy oil catalytic hydrothermal cracking technology has emerged, and the development prospects are bright. The hydrothermal cracking reaction of heavy oil with a catalyst converts the large molecules in the heavy oil into small molecules, and reduces the sulfur content in the heavy oil. It is currently very promising for heavy oil mining. After years of accumulated research, this group has also achieved results in the selection and preparation of catalysts in heavy oil catalytic hydrothermal cracking technology [6, 7, 8, 9, 10]. Considering the heavy oil exploitation environment, carbon dioxide flooding and water flooding are difficult to achieve; so chemical flooding has become a good choice [11, 12, 13].
The key is to solve the problem of low fluidity caused by colloid and asphaltene. The proportion of colloid and asphaltene in crude oil is not dominant, but it has an important impact on crude oil. Colloids and asphaltenes have complex structures, which are composed of five and/or six aromatic fused rings. These aromatic rings or branched chains are connected with heteroatoms such as nitrogen, oxygen, and sulfur. It plays an important role in the physical and chemical properties of crude oil [14]. Existing studies have proved that the interaction of π–π bonds between colloidal and asphaltene macromolecules leads to the aggregation of macromolecules, which is one of the reasons for the high viscosity of heavy oil. Researchers have used magnetic fields to influence the aggregation of colloidal and asphaltene macromolecules [15, 16, 17].
In order to solve the problems encountered in the recovery of crude oil, viscosity reducers have been developed. So far, the viscosity reducers are divided into four categories: EVA and its modifiers, poly (meth) acrylate series, malic acid anhydride copolymers, and nitrogen-containing polymers [18, 19]. However, there are many polymer molecular chains, large molecular weight, and harsh production conditions, which lead to polymer depressants being limited in practical applications. In view of the above forms, small-molecule crude oil pour point depressants have been proposed. Such depressants have a small molecular weight, so that they are easily to prepare with guaranteed quality. Taking into account the status quo of sustainable development, the problem of heavy oil viscosity and difficult flow must be solved, and environmental protection requirements must be considered. Many researchers have made good progress in selecting non-polluting chemical raw materials and producing products that are green to the environment or that are within the environmental tolerance range [20, 21, 22, 23]. Chen et al. used waste glass to synthesize polymethacrylate and polymethacrylamide which have been used in heavy oil mining [24, 25]. Zhang et al. used vegetable oil fatty acids and methylol triamide to synthesize crude oil flow improvers [26]. The raw materials used in these methods are non-polluting and have a wide range of acquisition methods. In this work, barium alkylbenzene sulfonate is synthesized by a simple chemical neutralization reaction, and the viscosity reduction and condensation reduction evaluation experiments are performed in L8401 crude oil. DSC and FTIR characterization methods were used to analyze and propose the mechanism of its action on crude oil.
Synthesis route of barium dodecylbenzenesulfonate (BaDBS).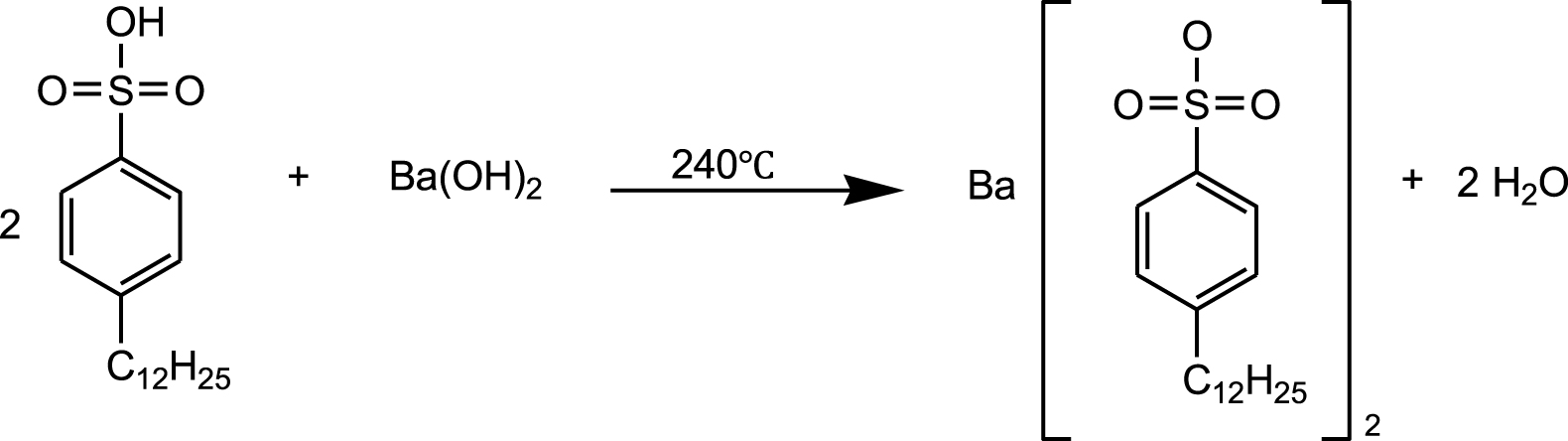
Nomenclature for the reaction of different acids with different alkali or elemental metal
| Reactants | Products | Name |
|---|---|---|
| Dodecylbenzenesulfonic acid + barium hydroxide | Barium dodecylbenzenesulfonate | BaDBS |
| Acetylbenzenesulfonic acid + barium hydroxide | Barium hexadecylbenzenesulfonate | BaHBS |
| Octadecylbenzenesulfonic acid + barium hydroxide | Barium octadecylbenzenesulfonate | BaOBS |
2. Experiment
2.1. Materials
All chemical reagents used in this experiment are commercially available with analytical grade without further purification before use. The L8401 crude oil sample utilized in the experiment was collected from the Nanyang Oilfield of China.
2.2. Determination of physical parameters
The density of crude oil is measured according to GB/T 1884-2000. At 30 °C, the density of L8401 is 0.961 g/cm3. The pour point was measured according to SY/T 2541-2009. The pour point of L8401 is 26.2 °C. Determination of the four components of petroleum asphaltene according to NB/SH/T0509-2010: the saturated hydrocarbon content of oil sample L8401 is 34.12%, the aromatic hydrocarbon content is 25.24%, the colloid content is 24.98%, and the asphaltene content is 15.66%. Viscosity of crude oil sample was measured according to ASTM D97-96 at a certain temperature.
2.3. Synthesis of oil-soluble viscosity reducer
Dodecylbenzenesulfonic acid, acetylbenzenesulfonic acid, or octadecylbenzenesulfonic acid and n-octyl alcohol were added in a flask with the molar ratio of 1:3; excess barium hydroxide was added. The mixture was heated for 6 hours at 280 °C by using a heating jacket until the solution became neutral and the reaction completed then. A solution extract was obtained by centrifugation and the target product was named barium alkylbenzene sulfonate. The chemical reaction equation of baryum alkylbenzene sulfonate is shown in Figure 1. Other alkylbenzenesulfonates can be synthesized in similar conditions. The reactants, products, and abbreviations of alkylbenzene sulfonates are shown in Table 1.
2.4. Differential scanning calorimetry analysis
The wax precipitation point and amount of crude oil were measured according to SY/T 0545-2012 by different scanning calorimetry (DSC) analysis, which was performed on a Mettler-Toledo DSC822e DSC (Switzerland) in a nitrogen (N2) atmosphere at a flow rate of 70 mL/min at a scan rate of 10 °C/min between −70 and −20 °C.
2.5. Optical microscopy and FTIR characterization
The saturated hydrocarbon component was separated from crude oil using the standard method of SY/T 5119-2016 for morphology of wax crystal study [12]. The crystal morphology of the wax was observed using a BX41-POLYMPUS polarizing microscope. A small amount of wax crystal was loaded onto the glass slide inside a copper stage with a central window. Samples were initially heated to 50 °C and then cooled to 15 °C for 5 min. During the measurement, the temperature of the copper stage was controlled at 15 °C in a circulating bath. The crude oil uses the Fourier infrared spectrometer to select the liquid film method, and the measurement spectral range is 4000–500 cm−1 at 4 cm−1 interval.
Effect of concentration on the viscosity of crude oil L8401.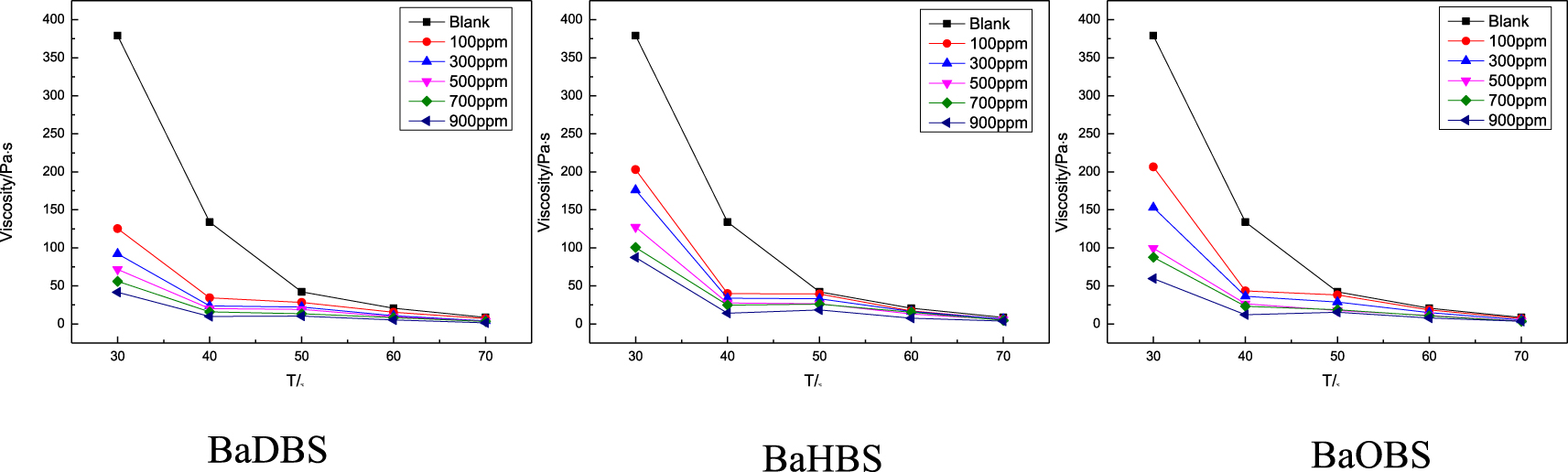
3. Result and discussion
3.1. Viscosity reduction
At 50 °C, the viscosity of crude oil is very low, and the flowability of crude oil depends not only on the concentration of crude oil flow improver, but also on temperature. As shown in Figure 2, when the temperature is lower than 50 °C, the crude oil flow improver has a good viscosity reduction effect, and then the viscosity–temperature curve tends to be level. The efficiency increases with the dosage. In the presence of 900 mg/L BaDBS, BaHBS or BaOBS, the viscosity reduction ratio reaches to 89.0%, 76.9%, and 84.23% at 30 °C. After adding barium dodecylbenzenesulfonate (BaDBS), barium hexadecylbenzenesulfonate (BaHBS), and barium octadecylbenzenesulfonate (BaHBS), the viscosity of L8401 at 50 °C decreased to 10.355, 18.338, and 15.526 Pa⋅s, respectively. It can be seen from Figure 2 that crude oil is very sensitive to temperature. Beyond 50 °C, the viscosity reduction effect of crude oil flow improver is obviously weakened.
Pour point reduction on L8401 with addition agent
| Concentration (mg/L) | 𝛥P/°C | ||
|---|---|---|---|
| BaDBS | BaHBS | BaOBS | |
| 100 | 6 | 9 | 7 |
| 300 | 14 | 10 | 9 |
| 500 | 18 | 11 | 15 |
| 700 | 13 | 12 | 16 |
| 900 | 18 | 13 | 17 |
The wax precipitation peak temperature and wax precautions point of crude oil L8401 with and without BaDBS.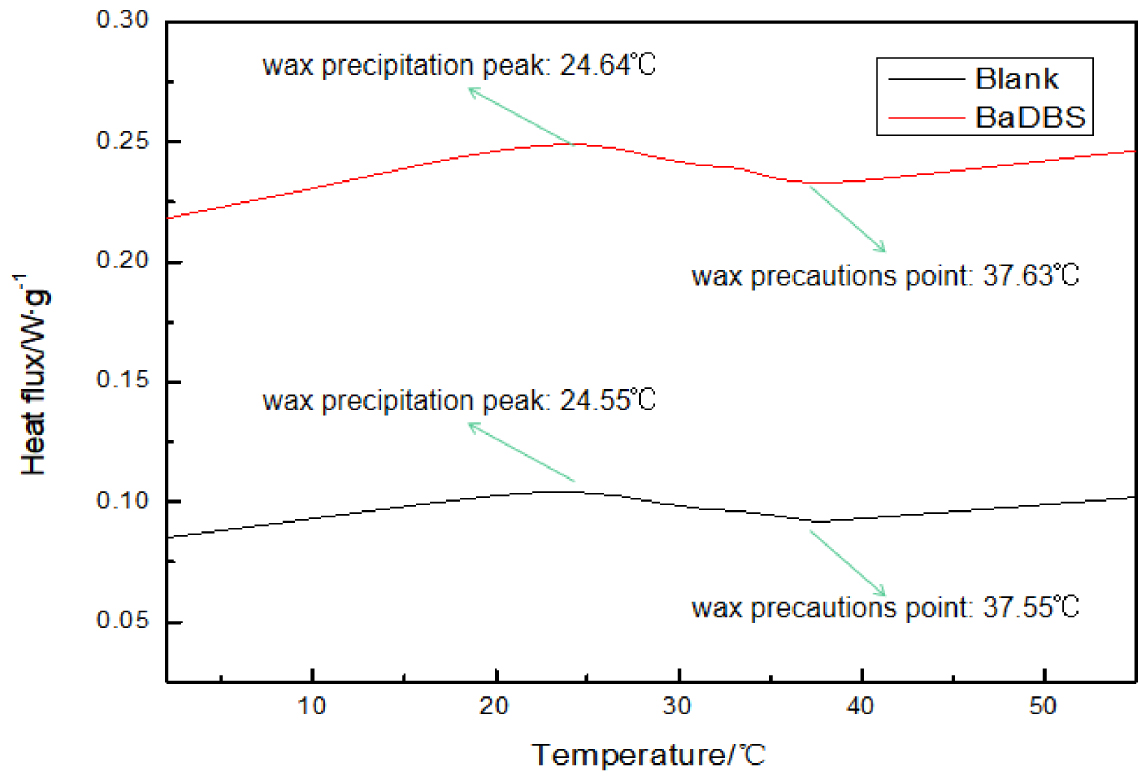
Wax crystals in saturated hydrocarbon without (left) and with BaDBS (right).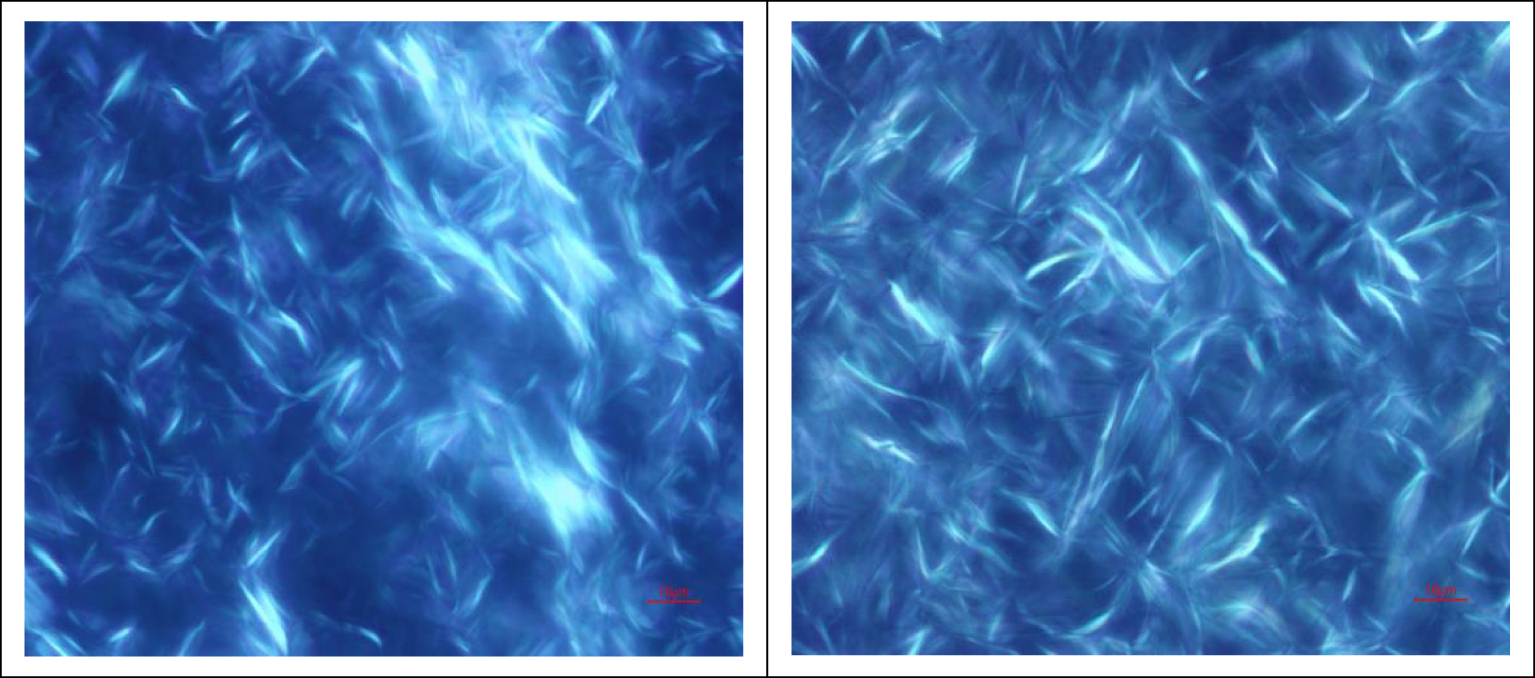
3.2. Pour point depressing (PPD)
The pour point depressant (PPD) effect after adding pour point depressant is shown in Table 2. In general, BaDBS has the best pour point depressing effect. The influence of additive concentration on pour point of crude oil sample L8401 was studied in the range from 100 to 900 mg/L. When the dosage is 900 mg/L, the pour point can be reduced to 18 °C. In addition, the depressant effect is also related to the chain length of alkylbenzene sulfonate: the shorter the alkyl chain length, the lower the pour point.
3.3. Differential scanning calorimeter analysis
BaDBS was added to heavy oil at 900 mg/L and stirred at 70 °C, then cooled to room temperature for DSC analysis. The result is displayed in Figure 3. After the wax precipitation peak temperature and wax precautions point of the oil sample rose from 24.55 °C and 37.55 °C to 24.64 °C and 37.63 °C, respectively, which means that BaDBS can promote the crystallization of wax. Moreover, after BaDBS addition, more heat is released during the cooling process, which means that more wax is precipitated.
3.4. Wax crystal microscope analysis
Figure 4 shows that the viscous oil wax crystals after BaDBS addition are very different from those without additives. The lengths of the wax crystals increase with the additive, and the number of wax crystals also increases. After addition, the wax crystals can form a three-dimensional (3D) network structure [27]. This structure can catch the small molecules of oil droplets and hinder the oil flow as the wax crystals grow. BaDBS not only acts as a nucleus for wax crystallization, it also accelerates crystallization. In addition, the composition of crude oil, resin, and asphaltenes will affect the precipitation of wax [28, 29]. The lower the light fraction in crude oil, the higher the wax-out point. Resin and asphaltenes will also affect the wax-out point: resin can adsorb on the surface of wax crystals to prevent wax crystals from growing further, and asphaltenes can become the center of wax crystals. Together they affect wax crystallization. Asphaltenes in the aggregated state are conducive to the reduction of the waxing point, while the dispersed asphaltenes will increase the waxing point. BaDBS can disperse with asphaltenes and cause the crude oil to wax in advance.
FTIR peak intensity for heavy oil without or with additive
| Wave number/cm−1 | Blank oil peak intensity transmittance/% | Additive oil peak intensity transmittance/% |
|---|---|---|
| 3405 | 14.13513 | 10.73066 |
| 2923 | 0.03438 | 0.03873 |
| 2852 | 0.65020 | 0.98867 |
| 1634 | 52.08981 | 40.63515 |
| 1462 | 13.27312 | 2.80308 |
| 1376 | 31.39136 | 28.20525 |
| 1031 | 85.41202 | 74.19017 |
| 721 | 53.36972 | 42.75475 |
3.5. FTIR spectrum analysis
Untreated oil samples and oil samples after addition were characterized by FTIR spectroscopy. The result is shown in Figure 5. It shows the presence of a wide peak at 3405 cm−1 corresponding to –OH (hydroxyl), –COOH (carboxyl), and –NH2 (amino) stretching which is evidence to prove the existence of hydrogen bond. Those polar groups exist on macromolecules of colloid, asphaltene, and resin, and the viscosity of heavy oil is higher because these groups interact to form hydrogen bonds. Persisting characteristic –CH3 (methyl) and –CH2 (methylene) stretching peaks were present at 2923, 2852, 1460, 1376, and 721 cm−1. The C=C stretching (aromatic ring) and NHR in-plane bending appeared at 1634 cm−1. The presence of R–OH (fatty alcohol), Ar–O–R, and RCH2–NH2 were indicated by the C–O–C (aromatics ether) and C–N (fatty amine) stretching at 1031 cm−1. It can be seen from Table 3 that except for the peak transmittances at 2923 and 2852 cm−1, the peak transmittances with the additive are lower than those of the blank oil samples.
3.6. Mechanism
FTIR with 900 mg/L and without BaDBS of crude oil L8401.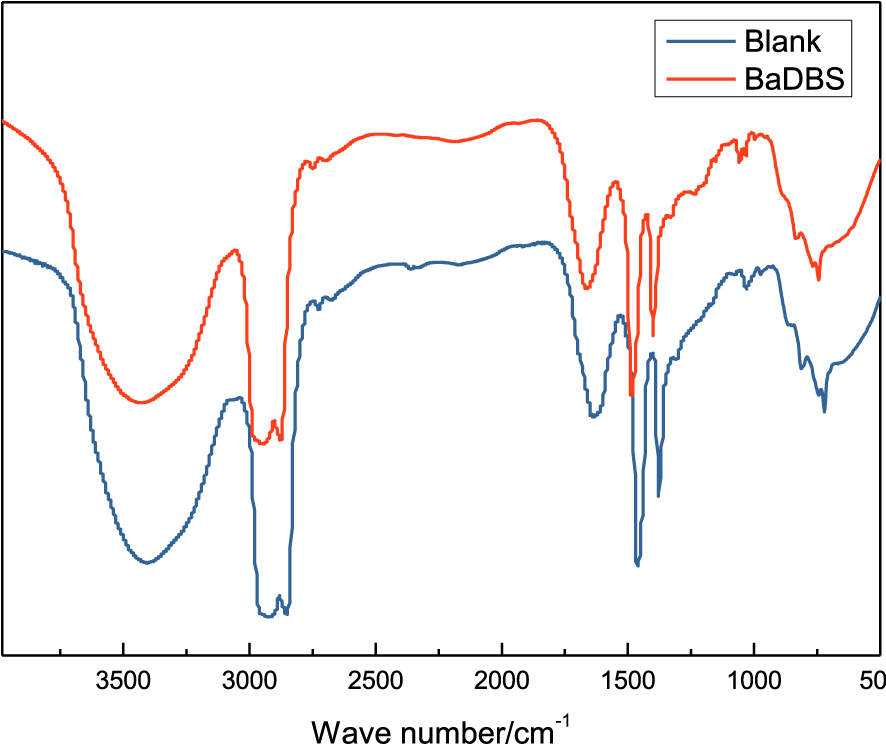
Mechanism study on viscosity reduction of alkylbenzene sulfonate.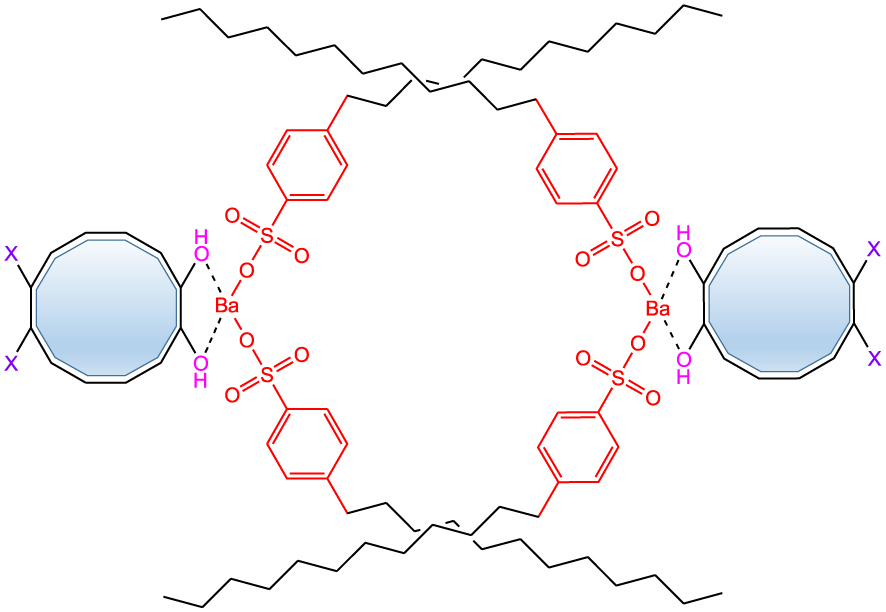
Asphaltene and resin in crude oil contain a large number of aromatic rings, which form planar stacks by intermolecular forces, and resins are gathered together by hydrogen bonds and adsorbed on asphaltenes. This force is the van der Waals force and hydrogen bond. Therefore, it is not difficult to destroy this force. In this study, we found that the flow improver can promote early crystallization of the wax. The alkyl chain of the flow improver acts as a crystal nucleus, so that the wax molecules in the crude oil are adsorbed on the crystal nucleus and continue to grow. The condensed ring in colloidal asphaltene and resin contains a large number of heteroatoms, and the metal of the flow improver can form a complex with these heteroatoms through coordination bonds (as shown in Figure 6), weakening the interaction between the molecules of the colloidal asphaltene and resin condensed ring, and the rotation and twisting of the alkyl chain disrupt the stacking of the molecular structure of the asphaltene and resin plane. The combined effect of the above structures leads to a reduction in the pour point and viscosity of crude oil.
4. Conclusion
In this article, three kinds of barium salts of alkylbenzene sulfonates with different lengths of alkyl chains were synthesized from alkylbenzene sulfonic acid and barium hydroxide. BaDBS at 900 mg/L can reduce the viscosity by 89.0% and depress the pour point by 5 °C. Optical microscopy revealed the eutectic effect of crude oil flow improver with saturated hydrocarbon in heavy oil. The action mechanism of BaDBS might be that the alkyl chain could be adsorbed and co-recrystallized with paraffin chains in oil, modifying the size and crystal shape of wax crystal, while the metal ions in the alkylbenzene sulfonate complex with the heteroatoms in the colloidal asphaltene and resin in the heavy oil, cleaving the hydrogen bonding between the macromolecules, causing the macromolecules to be dispersed in the heavy oil. This work will benefit the research in related fields.



 CC-BY 4.0
CC-BY 4.0