Tout ce que le cœur désire peut toujours se réduire à la figure de l’eau (Paul Claudel, extract from Positions et propositions).
1 Introduction
Some facts: a jellyfish is made of 99.9 wt% of water and only 0.1 wt% of solid matter [1], a composition very similar to that of the cerebrospinal fluid [2]. This nicely demonstrates the importance of water molecules for biological activity. We review here the possibility to generate water clusters inside inorganic containers. This is expected to lead to a new type of nanochemistry being of crucial importance for several aspects of interdisciplinary topics. In this context a series of spherical, soluble and a large number of pore-containing nanocapsules (nanosponges) displaying unprecedented high-charge and internal-surface variations were synthesized. These offer an ideal inorganic capsule system for investigating associated structures of a variety of encapsulated water assemblies.
2 Hard or soft water?
Our nanocapsules are based on a very robust fundamental skeleton {Pentagon}12{Linker}30, which can be modified in various routes [3]. The pentagonal unit (P) is based on an aggregate of six Mo-atoms in the form of a central pentagonal {O=MoVI(H2O)O5} bipyramid sharing its equatorial edges with five {O=MoVI(H2O)O4} octahedra. The linker (L) may be either a monomeric {O=M(H2O)O4} octahedral unit or a dimeric edge-sharing octahedral moiety {(MoV=O)(μ-O)2(MoV=O)X} with X = HCOO– (FORM), CH3COO– (ACET), H2PO2– (HYPO), SO42– (SUL) or PO43– (PHOS) ions. As shown in Fig. 1, the ‘coexistence’ in aqueous solutions of these two building blocks induces a spontaneous self-assembly reaction leading to a spherical {P12L30} perforated nanocapsule displaying Ih point-group symmetry.
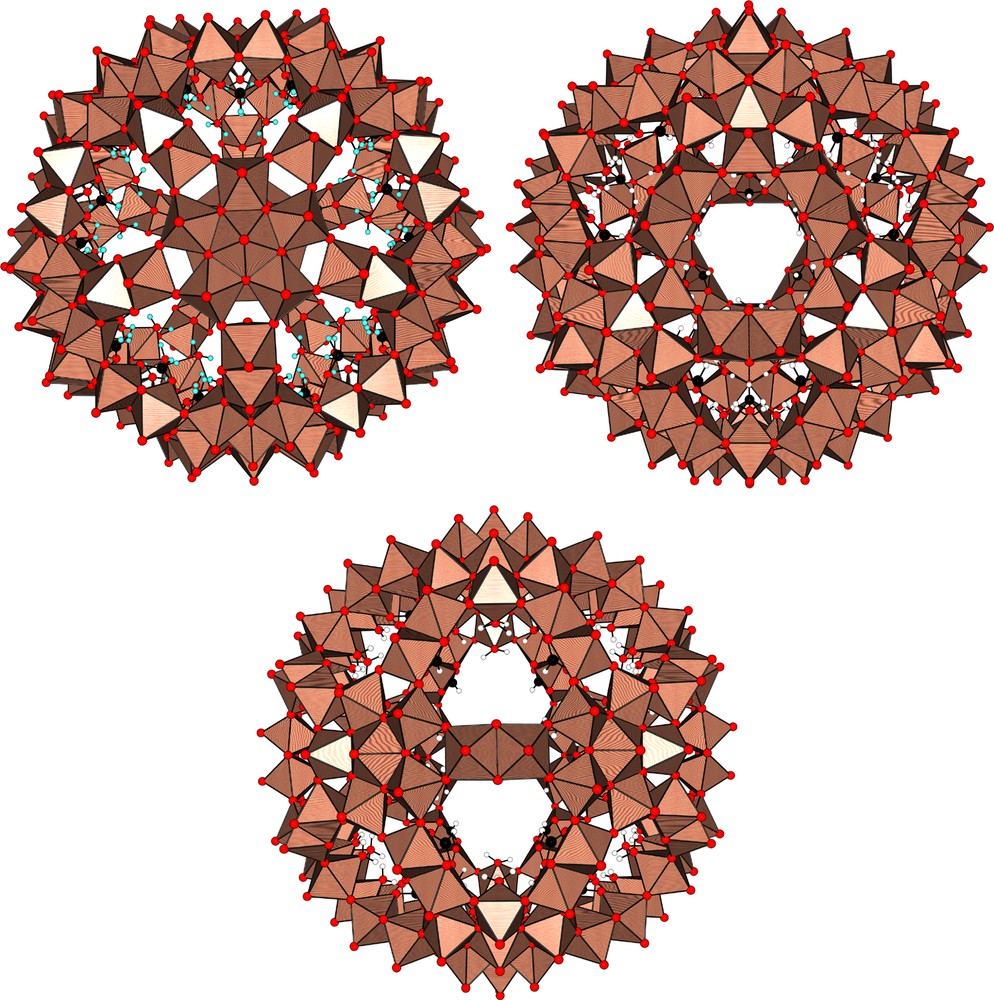
A nanocapsule displaying Ih (532) point-group symmetry. Top left: view along the C5 symmetry axis showing the pentagonal P–unit. Top right: view along the C3 symmetry axis showing one of the 20 {Mo9O9} pores/rings. Bottom: view along the C2 symmetry axis showing the di-octahedral linker L. The full stoichiometry of this nanocapsule is [P12L30]x–, with x = 42 for a monovalent linker-based ligand (formate, acetate, hypophosphite) and x = 72 for a divalent one (sulphate).
One of the major features of a nanocapsule with (mixed) SO42–/H2PO2– ligands having pores closed by guanidinium cations [4], lies in the encapsulation of a structurally highly organized {H2O}100 assembly inside the cluster cavity. This can be described by three concentric shells having radii of 3.84–4.04, 6.51–6.83 and 7.56–7.88 Å and spanned by 20, 20, and 60 molecules, respectively [5]. The structure (Fig. 2) may be described as a central dodecahedron of water molecules (12 five-membered rings) with each of its 20 vertices decorated by a water molecule. This {H2O}20 aggregate is ‘encapsulated’ in a strongly distorted {H20}60 rhombicosidodecahedron (12 boat-shaped six-membered rings). Interestingly, exactly the same arrangement was found to occur, as the central core of a {H2O}280 cluster obtained according to molecular dynamics calculations [6]. Thanks to the options of the highly efficient PACHA algorithm [7], it was possible to unravel the intramolecular H-bond pattern in these types of supramolecular water assemblies. It may be shown that the perfectly H-bonded dodecahedron of 20 water molecules (Fig. 2, with a total of 30 hydrogen bonds) is characterized by a mean H–bond energy <EHB> = –23.3 kJ mol–1, a typical value found in crystalline ice for instance [8]. By adding the 20 other water molecules onto the vertices, we get a {H2O}40 water cluster characterized by 50 H–bonds (Fig. 2) and <EHB> = –29.7 kJ mol–1. There is thus a clear anisotropy of the H–bond energy in this system. Finally, after the ‘capping’ with the 60 remaining water molecules (Fig. 2), we get <EHB> = –28.9 kJ mol–1 for this {H2O}60 cluster. The picture emerging from this detailed analysis, is a heart of ice-like dodecahedral water clusters wrapped in a still stiffer water shell. This obviously demonstrates the very clear knowledge that may be attained on these H-bonded assemblies.

The {H2O}100 water cluster extracted from its {Mo132} type nanocontainer (see text). Top left: the central {H2O}20 dodecahedron displaying 30 intramolecular hydrogen bonds. Top right: the fully H-bonded vertex-decorated {H2O}40 dodecahedron (50 hydrogen bonds). Bottom left: the complete {H2O}100 cluster with its 170 intramolecular hydrogen bonds. Bottom right: the formal decomposition of the {H2O}100 cluster into its three shells of Platonic and Archimedean solids, {H2O}60 (yellow) and two {H2O}20 (blue and green). In the [{(Mo)Mo5O21(H2O)4CH3COO}12{MoVO(H2O)}30] nanocapsule, only the {H2O}40 cluster was found.
But the level of knowledge on the hydrogen bonding in the mentioned nanocapsule is marginal in comparison to that obtained on the basis of the capsule-size and internal-surface variation options [3, 9–12]. Accordingly, by just performing very basic aqueous chemistry, we may get a deep insight into the structural response of the encapsulated water assemblies due to geometrical and/or chemical perturbations. This was clearly demonstrated [9, 12] by considering another capsule in the compound (NH4)42[P12(FORM)30]·30 NaCOOH·250 H2O [10]. Here the substitution of the guanidinium cations of the former compound by ammonium and sodium ions and of hypophosphite/sulphate bridges (see above) by formate ions leads to a completely amorphous central {H2O}59 water assembly (Fig. 3). A possible explanation for such an observation may be the release of the osmotic pressure exerted by the guanidinium ions unable to enter the central cavity (roughly estimated as Π ~ 19 MPa = 190 bar, i.e. the pressure at a sea-depth of 1900 m). Accordingly, owing to their smaller sizes, sodium and ammonium ions are able to enter the inside of the cavity, passing through the unobstructed {Mo9O9} pores, which leads to a disorganization of central water clusters. For this new water cluster, the mean H–bond energy is found to be <EHB> = –17(6) kJ mol–1. This 30% reduction in H-bond strength relative to ice polymorphs points to a clear slackening of the H–bond network in this kind of amorphous assembly.
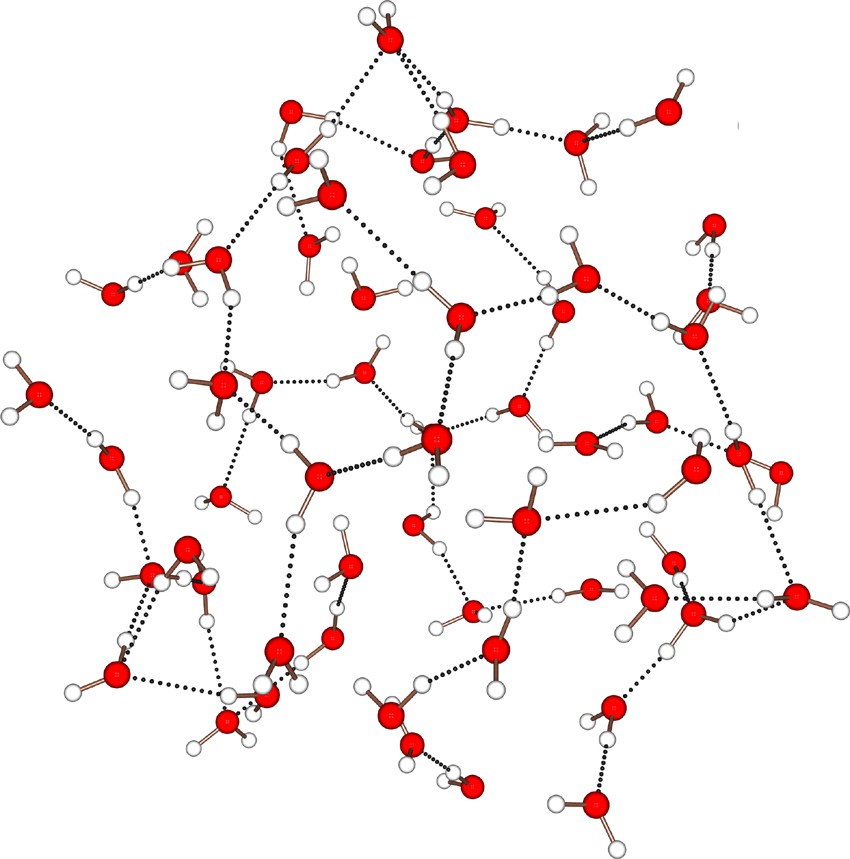
The amorphous {H2O}59 water cluster with chain-type fragments extracted from its {Mo132} type nanocontainer (see text).
3 Playing the nanocapsule game
The quite easy modification of water structures in inorganic nanocapsules suggests that other kinds of water assemblies may be characterized by playing with the size and/or the charge of the inorganic shell. The charge effect was demonstrated by synthesizing nanocapsules displaying either only acetate-, hypophosphite-, or sulphate-based linkers. This allows us to tune the charge from 42– to 72– in a stepwise manner. Accordingly, for the purely monovalent case (hypophosphite-based linker), [P12(HYPO)30]42– type capsules with a central water cluster with 80 molecules [11,12], instead of 100 observed in the above mentioned mixed [P12(SUL)10(HYPO)20]52– nanocapsule [12], can be obtained. As shown in Fig. 4, this new cluster displays a pentagonal dodecahedron which is spanned by 20 water molecules trapped in the 20 trigonal holes (note the presence of 30 PH2 groups) of the perforated internal surface of the nanocapsule and are hydrogen-bonded to 60 water molecules spanning the vertices of a distorted rhombicosidodecahedron.

Top: the encapsulated water cluster of [P12(HYPO)30]42– with the inner {H2O}60 shell (yellow) together with the 20 H2O molecules (red) – embedded in the perforated internal cluster-shell surface (left) – forms a novel completely hydrogen-bonded {H2O}80 cluster with an average O···O separation of approximately 2.70 Å (turquoise; right) [12]. Shown additionally in both cases: the shell envelope spanned by the 30P(H2) centres (lilac). Bottom: the structure of the spherical cluster [P12(SUL)30]72– showing the encapsulated water and NH4+ (left). A related segment (right) together with some representative average interatomic distances [Å] is also shown, see [12] (Mo atoms light blue; O red; SO42– green tetrahedra; inner {H2O}20 shell segment blue; outer {H2O}60 shell yellow).
On the other hand, the [P12(SUL)30]72– capsules displaying a very high negative charge attract surrounding counter-cations being literally drawn inside the central cavity. Thus if only ammonium ions are present in the solution we get a central expanded {H2O}20 dodecahedron (incorporating two ammonium ions) forming hydrogen bonds to a second shell comprising a strongly distorted {H2O}60 rhombicosidodecahedron (practically identical to the third shell of the previously discussed {H2O}100 cluster) [12] (Fig. 4). In the presence of larger cations unable to enter the cavity (such as dimethylammonium [(CH3)2NH2]+) we observed for a mixed [P12(ACET)6(SUL)24]66– nanocapsule an uptake of sodium ions (Fig. 5), leading as in the above mentioned formate case to a not well-defined central water cluster [12]. A more pronounced disorganization of the water assembly was observed for the nanocapsule [P12(ACET)12(SUL)18]60– containing more hydrophobic ligands [12], and correspondingly in the [P12(ACET)30]42– nanocapsule, too [13] (the corresponding water cluster was not studied in great detail at that time).
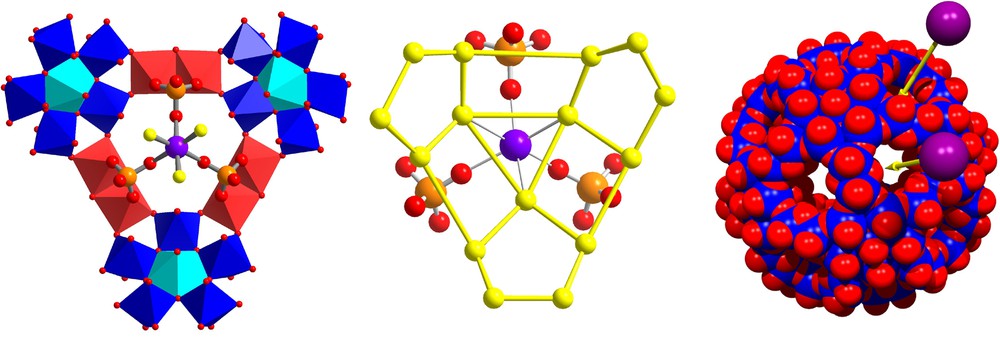
A segment of the [P12(ACET)6(SUL)24]66– structure [12] below the pore area viewed from the cluster centre is shown in two representations. Left: an (under occupied) encapsulated Na+ ion ‘coordinated’ to three SO42– ligands and three (correspondingly under occupied) H2O molecules; the {Na(SO4)3(H2O)3} group is shown together with three {(Mo)Mo5}(blue polyhedra and central turquoise pentagonal bipyramid) and three {Mo2} building units (red polyhedra). Middle: the same {Na(SO4)3(H2O)3} group without the Mo-based building units but shown in relation to a segment of the incomplete H2O shell with the three coordinating H2O molecules in the centre. Right: modelling of the uptake of small cations through the {Mo9O9} pores of a highly negatively charged nanocapsule.
Yet another possibility is to play with the cavity size by switching from a di-octahedral linker to a mononuclear spacer {O=MoV(H2O)}3+ associated to a {MoVIMoVI5O21(H2O)4(CH3COO)} pentagonal unit [12, 14]. Owing to the much smaller size of the central cavity, the rhombicosidodecahedron spanned by water molecules becomes completely squeezed out leaving an encapsulated {H2O}40 cluster based on a central pentagonal dodecahedral assembly embedded in a larger dodecahedron of water molecules (Fig. 2). All of these new water clusters are currently under investigation with a view to unravelling their detailed H–bond networks via the PACHA algorithm. Similar studies on the hydration shells of the {Mo154} [3, 15], the {Mo176} type big wheels [16–18], and on the water structure in the {Mo368} nano-hedgehog [19] are also under investigation (Fig. 6). At last, well-defined water clusters may also be reproducibly synthesized and characterized by using nanoporous frameworks as containers [20–21]. This clearly demonstrates that we are currently able to treat on exactly the same theoretical grounds both molecular complexes and 3D–networks and allows us to begin to tackle a general question: “Is water templating nanoporous materials?”
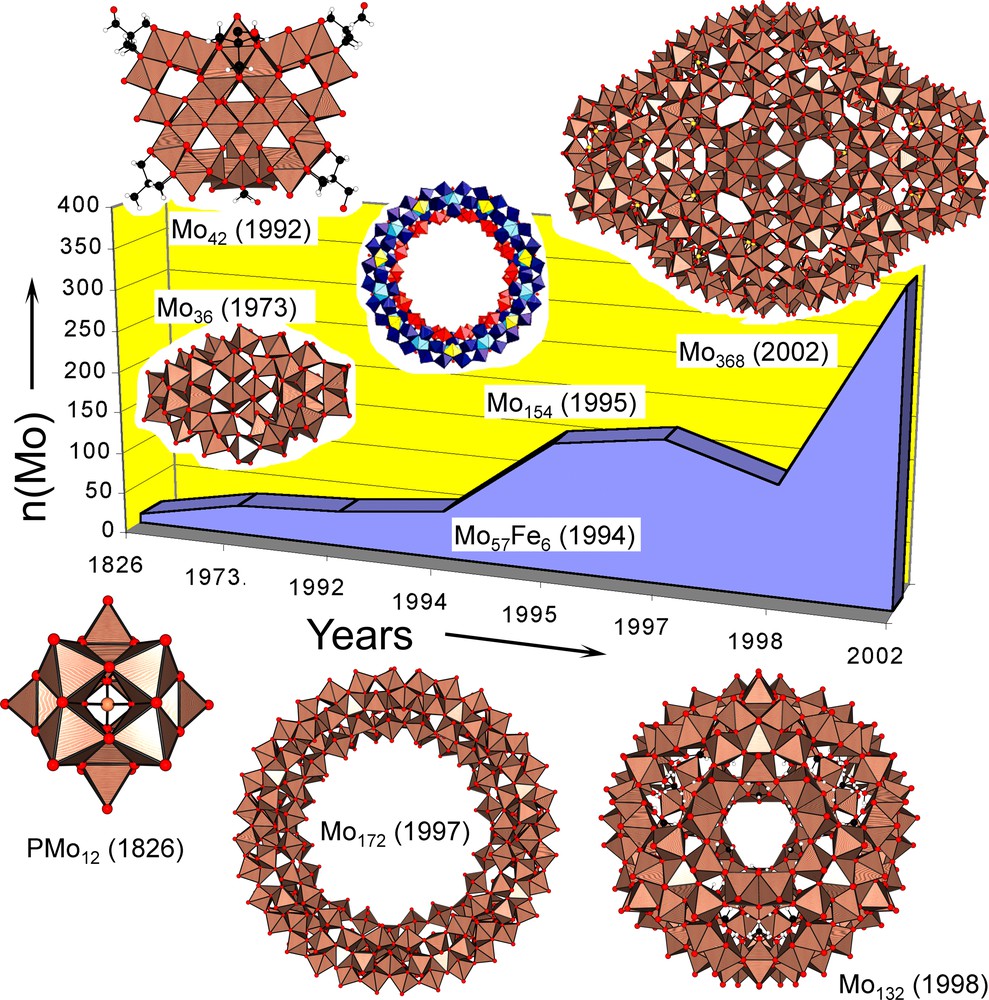
Mo-oxide-based nanoobjects synthesized since the discovery of the Keggin heteropolyanion in 1826. Most of these structures are heavily hydrated in the solid state, while providing fascinating structural models for a wide variety of water molecule assemblies with or without encapsulated counter-cations. The {Mo248} cluster is not shown. See A. Müller et al., Nature 397 (1999) 48.
4 Conclusion and perspectives
The fundamental route, based on the changeable properties of nanocontainers with their robust skeleton kept intact, provides the possibility to study segments of liquids. Accordingly, in our nanocapsules, water assemblies with a variety of ingredients are found under well-defined reproducible conditions. This contrasts strongly with larger water assemblies that can practically only be studied by mass-spectroscopy and can in any case not sophisticatedly be investigated in liquid water itself. As liquid water has a short-range order, evidenced by X-ray or neutron diffraction via radial distribution curves, it is a very interesting situation that the discussed encapsulated water clusters provide good quantitative models for the liquid water structure with its LDW and HDW components [9]. Interestingly, molecular dynamics calculations supported our experimentally observed structures and PACHA algorithm supported the fact that a condition for the versatility of water structures is: hydrogen bonding is neither too weak nor too strong.
The possibility ‘to study’ encapsulated electrolyte ingredients is significant as these are of interest for biochemical aspects: simple inorganic ions play a crucial role in stabilizing and destabilizing proteins (cf. Hofmeister series with reference to an optimum stabilization of biomacromolecules requiring an anion with relatively high charge density and a cation with low charge density) [22]. Correspondingly, it is also possible to study the influence of ions on water structures in confined spaces [12], i.e. in capsules in general, in the sense of the categories ‘order makers’ and ‘disorder makers’ [23]. Na+ for instance destroys partly the above discussed water cluster, while NH4+ may be integrated. As it is also possible to modulate the hydrophilicity/hydrophobicity of the internal cavity, novel assemblies with novel high-density water assemblies can be studied. This opens new perspectives in connection with the well-known hydrophobic effect [22, 24] that is of fundamental importance for biological structures and processes.
In summary, based on the use of synthetic nanocontainers to construct different well-defined structures of rather large water aggregates, including those with electrolytes, we are currently denying the statement found recently in the literature: Liquid water and biological systems: the most important problem in science that hardly anyone wants to solve [25].


