1 Introduction
The Cymbopogon genus comprises 56 species [1] among which four species including Cymbopogon giganteus Chiov. grow wild in Ivory Coast. C. giganteus, an aromatic Poaceae, is a perennial and sweet-smelling grass that grows spontaneously in the savannahs of Asian and African tropical regions. It possesses a rhizome-bearing stem and can grow up to 3 m high [2]. Decoctions of the leaves and flowers are used as an effective treatment against skin disorders [3], conjunctiva and migraine [4] and hepatitis [5]. Solvent extracts of C. giganteus have been reported to contain flavonoids [6] and amino acids [7]. The composition of essential oils, obtained from aerial parts of plants growing in different countries, has been investigated: Benin [8], Burkina Faso [9], Mali [10,11], and Cameroon [12]. The composition of two samples of leaf oil from Ivory Coast was briefly investigated; however, only six to eight components were identified [12,13]. The major constituents of C. giganteus oils were p-menthane derivatives such as cis- and trans-p-mentha-2,8-dien-1-ols, cis- and trans-p-mentha-1(7),8-dien-2-ols cis- and trans-isopiperitenols, limonene and carvone. Conversely, the oils differed from sample to sample by the occurrence of different minor components.
The aim of the present work was to get a better insight on the composition of C. giganteus leaf oil from Ivory Coast, using a combination of column chromatography, GC, GC/MS and 13C-NMR following a methodology developed and computerized in our laboratories [14,15].
2 Experimental
2.1 Essential oil
The leaves of C. giganteus (1.8 kg) were harvested in August 2002, near Bouaké (Ivory Coast) and water distilled, using a Clevenger-type apparatus (3 h). The leaf oil (9.6 ml) was the cumulative oil of three independent hydrodistillations (yield = 0.20% v/w).
2.2 Oil fractionation
One part of C. giganteus leaf oil (2 g) was separated by flash chromatography: silica gel, 63–200 μm, elution using pentane with increasing amount of diethyl ether (from 100/0 to 0/100) as eluent. Nineteen fractions were obtained and analyzed by GC/RI and GC/MS or 13C-NMR.
2.3 Analytical GC
GC analyses were carried out using a PerkinElmer Autosystem GC apparatus equipped with dual FID and fused-silica capillary columns (50 m × 0.22 mm i.d., film thickness 0.25 μm), BP-1 (polydimethylsiloxane) and BP-20 (polyethyleneglycol). Carrier gas: helium at 1 ml/min (split 1/50); injector temperature, 250 °C; oven temperature programmed from 60 to 220 °C at 2 °C/min and then held isothermal (20 min); detector temperature, 250 °C.
2.4 GC/MS analysis
Samples were analyzed with a PerkinElmer TurboMass detector (quadrupole), directly coupled to a PerkinElmer Autosystem XL equipped with fused-silica capillary columns (60 m × 0.22 mm i.d., film thickness 0.25 μm), Rtx-1 (polydimethylsiloxane) and Rtx-Wax (polyethyleneglycol). Ion source temperature: 150 °C; energy ionization: 70 eV; electron ionization mass spectra were acquired over the mass range 35–350-Da scan rate. Split: 1/80. Other GC conditions were the same as described under GC.
2.5 13C-NMR analysis
All NMR spectra were recorded on a Bruker AC 200 Fourier transform spectrometer operating at 50.323 MHz for 13C, equipped with a 10 mm (or 5 mm) probe, in deuterated chloroform (CDCl3), with all shifts referred to internal tetramethylsilane (TMS). 13C-NMR spectra were recorded with the following parameters: pulse width (PW), 5 μs (or 3 μs) (flip angle 45°); acquisition time, 1.3 s for 32 K data table with a spectral width (SW) of 12 500 Hz (250 ppm); CPD mode decoupling; digital resolution 0.763 Hz per pt. In a typical procedure, 200 mg (or 70 mg) of the oil were used in 2 ml (or 0.5 ml) of CDCl3. The number of accumulated scans ranged between 2000 and 10 000 for each sample, depending on the amount of oil available. Exponential line broadening multiplication (LB = 1 Hz) of the free induction decay (FID) was applied before Fourier transformation.
2.6 Component identification
Identification of the components in each oil and fractions of chromatography was based: (a) on their GC retention indices (RI) on polar and apolar columns, determined relative to the retention times of a series of n-alkanes with linear interpolation with those of authentic compounds or literature data [16]; (b) on computer matching with laboratory-made and commercial mass spectral libraries [17,18] and comparison of spectra with those of our own library or literature data [19,20]; (c) and by carbon-13 NMR following the methodology developed and computerized in our laboratories [14,15]. The chemical shift of each carbon in the mixture spectrum is compared with those of the spectra of pure compounds listed in a laboratory made computerized data bank. Each compound is identified taking into account (i) the number of identified carbons, (ii) the number of overlapped signals, (iii) the difference of chemical shift of each resonance in the mixture spectrum and in the reference. The structure of 3,9-oxy-p-mentha-1,8(10)-diene (2,3,3a,4,5,7a-hexahydro-6-methyl-3-methylenebenzofurane) (Fig. 1), not present in our MS and 13C-NMR data libraries was determined by extensive NMR studies. Spectral data (1H, 13C, DEPT, 1H-1H-COSY, HSQC) were measured on a fraction of chromatography (80.0% purity on GC). 1H-NMR (400 MHz, CDCl3, δ = ppm, J = Hertz): 5.51 (1H, br s, H-2), 4.95 (1H, q, 2.4 Hz, H-10), 4.91 (1H, q, 2.0 Hz, H-10), 4.37 (1H, dq, 13.3, 1.9 Hz, H-9), 4.24 (1H, dq, 13.3, 2.1 Hz, H-9), 4.28 (1H, br s, H-3), 2.60 (1H, br s, H-4), 1.90 (2H, m, H-6), 1.71 (3H, s, H-7), 1.63 (2H, m, H-5). 13C-NMR, δ = ppm: 152.30 (C-8), 140.78 (C-1), 119.90 (C-2), 103.46 (C-10), 76.17 (C-3), 70.02 (C-9), 41.15 (C-4), 28.18 (C-6), 24.76 (C-5), 23.84 (C-7).
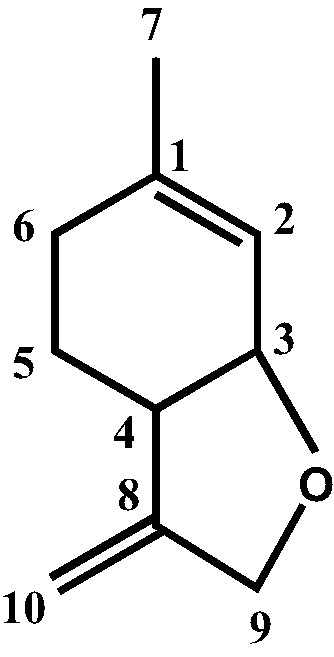
Structure of 3,9-oxy-p-mentha-1,8(10)-diene ((2,3,3a,4,5,7a-hexahydro-6-methyl-3-methylenebenzofurane).
3 Results and discussion
Fractionation on SiO2 of the C. giganteus leaf oil from Ivory Coast led to 19 fractions that were analyzed by GC/RI. Some fractions, selected on the basis of their chromatographic profile were also analyzed by MS and/or 13C-NMR, depending of the available amount of product. The quantitative and qualitative data on the composition of the essential oil, as well as the mode of identification of the individual components, are reported in Table 1. Forty-six constituents, who represented 91.0% of the oil, were identified. The major constituents of the essential oil were: trans-p-mentha-2,8-dien-1-ol (18.4%), cis-p-mentha-1(7),8-dien-2-ol (16.0%), trans-p-mentha-1(7),8-dien-2-ol (15.7%), limonene (12.5%), cis-p-mentha-2,8-dien-2-ol (8.7%), while trans- and cis-isopiperitenols (3.1% and 2.2% respectively), carvone (2.7%) and trans-carveol (2.2%) were present at appreciable contents.
Composition of the essential oil of C. giganteus from Ivory Coast
| Compounds | RIa | RIp | % | Identification |
| Myrcene | 978 | 1157 | 0.1 | RI, MS, 13C-NMR |
| p-Cymene | 1010 | 1267 | 0.3 | RI, MS, 13C-NMR |
| Limonene* | 1020 | 1200 | 12.5 | RI, MS, 13C-NMR |
| 1,8-Cineole* | 1020 | 1209 | 0.1 | RI, 13C-NMR |
| γ-Terpinene | 1045 | 1242 | 0.1 | RI, MS, 13C-NMR |
| p-Cymenene | 1069 | 1430 | 0.2 | RI, MS, 13C-NMR |
| Terpinolene | 1076 | 1278 | 0.1 | RI, MS, 13C-NMR |
| Nonanal | 1078 | 1387 | 0.1 | RI, MS, 13C-NMR |
| Trans-p-mentha-2,8-dien-1-ol | 1102 | 1619 | 18.4 | RI, MS, 13C-NMR |
| Cis-p-mentha-2,8-dien-1-ol | 1114 | 1660 | 8.7 | RI, MS, 13C-NMR |
| Cis-limonene 1,2-oxide | 1118 | 1449 | 0.5 | RI, MS, 13C-NMR |
| Trans-limonene-1,2-oxide | 1121 | 1470 | 0.2 | RI, 13C-NMR |
| Citronellal | 1127 | 1472 | 0.2 | RI, MS, 13C-NMR |
| Trans-p-mentha-1(7),8-dien-2-ol * | 1168 | 1790 | 15.7 | RI, MS, 13C-NMR |
| 3,9-Oxy-mentha-1,8(10)-diene* | 1168 | 1551 | 2.2 | 1H-NMR, 13C-NMR |
| Trans-dihydrocarvone * | 1171 | 1600 | 0.2 | RI, 13C-NMR |
| Cis-dihydroperillaldehyde * | 1171 | 1596 | 0.6 | RI, 13C-NMR |
| Trans-dihydroperillaldehyde | 1174 | 1606 | 1.3 | RI, 13C-NMR |
| Trans-isopiperitenol | 1178 | 1744 | 3.1 | RI, MS, 13C-NMR |
| Decanal | 1183 | 1489 | tr | RI, MS, 13C-NMR |
| Trans-carveol * | 1195 | 1829 | 2.2 | RI, MS, 13C-NMR |
| Cis-isopiperitenol * | 1195 | 1739 | 2.2 | RI, MS, 13C-NMR |
| Cis-p-mentha-1(7),8-dien-2-ol | 1204 | 1885 | 16.0 | RI, MS, 13C-NMR |
| Cis-carveol | 1207 | 1860 | 0.5 | RI, 13C-NMR |
| Carvone | 1213 | 1725 | 2.7 | RI, 13C-NMR |
| 3-Methylbutyl hexanaote * | 1229 | 1450 | 0.1 | RI, MS, 13C-NMR |
| Geraniol* | 1229 | 1840 | 0.4 | RI, 13C-NMR |
| Geranial | 1238 | 1727 | 0.1 | RI, 13C-NMR |
| Perillaldehyde | 1242 | 1779 | 0.4 | RI, 13C-NMR |
| Safrole | 1260 | 1872 | 0.1 | RI, MS, 13C-NMR |
| Thymol | 1262 | 2178 | 0.9 | RI, 13C-NMR |
| Ascaridole | 1273 | 1860 | 0.2 | RI, 13C-NMR |
| β-Elemene | 1384 | 1580 | tr | RI, MS |
| Tetradecane | 1400 | 1400 | tr | RI, MS |
| (E)-β-Caryophyllene | 1415 | 1587 | 0.1 | RI, MS |
| 3-Methylbutyl octanoate | 1423 | 1648 | 0.1 | RI, MS, 13C-NMR |
| Precocene I | 1428 | 2075 | 0.2 | RI, MS, 13C-NMR |
| β-Selinene | 1475 | 1706 | tr | RI, MS |
| δ-Cadinene | 1511 | 1746 | tr | RI, MS |
| Caryophyllene oxide | 1568 | 1980 | 0.1 | RI, MS, 13C-NMR |
| Hexadecane | 1600 | 1600 | tr | RI, MS |
| 2-Phenylethyl hexanoate | 1606 | 2166 | 0.1 | RI, MS |
| Benzyl benzoate | 1719 | 2379 | tr | RI, MS |
| Octadecane | 1800 | 1800 | tr | RI, MS |
| 2-Phenylethyl octanoate | 1814 | 2232 | tr | RI, MS |
| Eicosane | 2000 | 2000 | tr | RI, MS |
| Total | 91.0 |
Although some quantitative differences could be observed, the major components of the investigated sample are qualitatively and quantitatively similar to those already reported in Ivory Coast [12,13] and other countries [8–11]. However, the detailed analysis carried out after fractionation of the bulk sample, gives a better insight on the composition of the C. giganteus leaf oil from Ivory Coast and allows the comparison with oils from other countries. Indeed, 25 compounds were reported for the first time in C. giganteus leaf oil, including oxygenated acyclic monoterpenes (citronellal, geraniol, geranial), oxygenated menthane derivatives (cis- and trans-dihydroperylaldehydes, thymol), phenylpropanoids (safrole), phenylethyl derivatives (hexanoate, octanoate) and oxides (ascaridole, precocene, caryophyllene oxyde) as well as sesquiterpene hydrocarbons (β-elemene (E)-β-caryophyllene, β-selinene, δ-cadinene) and linear aldehydes (nonanal, decanal) and alkanes.
4 Conclusion
The present study confirmed that the composition of the leaf oil of C. giganteus growing wild in Ivory Coast, is dominated by unsaturated alcohols bearing the menthane skeleton (p-menthadienol isomers) as the oils from other countries of West Africa. These compounds have been reported as components of the essential oils of other species of Cymbopogon: Cymbopogon densiflorus from Angola, Congo and Zambia [12,21], and Cymbopogon martinii var sofia from India [22]. However the detailed analysis of a sample from Ivory Coast, by combination of chromatographic (CC, GC) and spectroscopic (MS, 13C-NMR) techniques, allowed the identification of 25 components found for the first time in C. giganteus leaf oil (whatever the origin) including oxygenated monoterpenes, sesquiterpene hydrocarbons, aromatic esters, alkanes and linear aldehydes.
Acknowledgements
The authors thank Professor Laurent Aké Assi for his valuable help in the identification of the plant and they are indebted to Professor Antoine Koffi Ahibo and the ‘Ministère de l'Enseignement supérieur de Côte-d'Ivoire’ for providing a research grant (J.B.B.).


