1 Introduction
Some SnPh3-containing derivatives involving inorganic oxyacids are reported [1–3]. The important role of the cation on the structure of oxalate SnPh3-containing derivatives has been reported and a cis chelating oxalate has been reported in (Cy2NH2)(C2O4)(SnPh3) [4] and a polymeric structure in (Me4N)(C2O4)(SnPh3) [3]: this shows the key role of the cation in the [C2O4SnPh3]− arrangement. Recently derivatives of general formula (R4N)(XO4)(SnPh3) (R = Cy, H, Bu, Me; X = S, Se) have been reported, in which the role of the counter cation size on the structure has been specified [5]; the structure, in solution, of (Bu4N)(SO4)(SnPh3) is monomeric, while, in (Me4N)(SO4)(SnPh3), SnPh3 residues are still trans-coordinated by oxygens, conferring bipyramidal trigonal environment to the tin atoms.
In the framework of our research work on oxy-anions acting as ligands [6–10], we describe in this paper the synthesis and spectroscopic studies of the 1,1-dimethylguanidinium triphenyltin sulphate.
2 Experimental
[Me2NC(NH)NH2]2H2SO4 and SnPh3Cl are Aldrich chemicals, used without further purification. The title derivative is obtained by mixing in a 1:1 ratio [Me2NC(NH)NH2]2H2SO4 dissolved in a minimum of water and SnPh3Cl dissolved in ethanol (20 ml). The white precipitate obtained is washed with ethanol.
The elemental analyses were performed in the Microanalyses Laboratory of the Inorganic Metallorganic and Analytical Chemistry Department (University of Padua, Italy), the Mössbauer spectrum is obtained as described in [11], while the solid-state 13C and 117Sn NMR spectra were recorded using a Bruker Avance 250 NMR spectrometer, equipped with a broadband MAS probe, operating at 62.90 and 89.15 MHz for 13C and 117Sn nuclei, respectively. Rotors of 4 mm were used, and spinning rates of 7000 and 9000 Hz for 117Sn acquisition and 4000 Hz for 13C acquisition. Adamantane and tetracyclohexyltin were used as external standards for 13C and 117Sn, respectively.
- Microanalytical data: % calculated (% found)
- % C: 46.00(46.45) % H: 4.79(4.67) % N: 8.05(8.76); m.p. > 260 °C; yield: 85%
- Infrared data (cm−1)
- 3371s, 3169s, 3049br νNH3+; 1652s, 1640s δNH3+; 1545m νCN; 1108vs νasSO42−; 616s δasSO42−; 393w δsSO42−; 222m νSnO
- Mössbauer data (mm s−1)
- Q.S. = 3.73; I.S. = 1.47; Γ = 0.94; A% = 1.05
- NMR data [δ (ppm)]
- 13C: 156, CN; 138, Ph: C (i) and C (o); 130 Ph: C (m) and C (p); 38, N–CH3
- 117Sn: −227
3 Discussion
The absence of νs SO42− indicates a Td symmetry for the sulphate [12]. The Q.S. value of 3.73 mm s−1, higher than 3.00 mm s−1, is indicative of a trans-O2SnPh3 stereochemistry about the tin centres [13]. The isotropic 117Sn chemical shift of −227 ppm is consistent with the presence of trans-coordinated Ph3Sn residues [14].
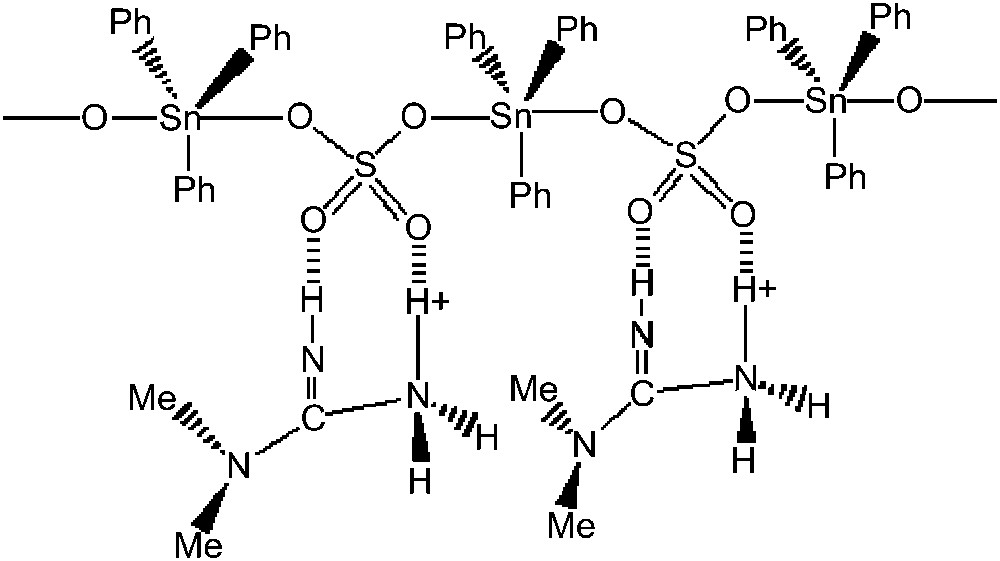
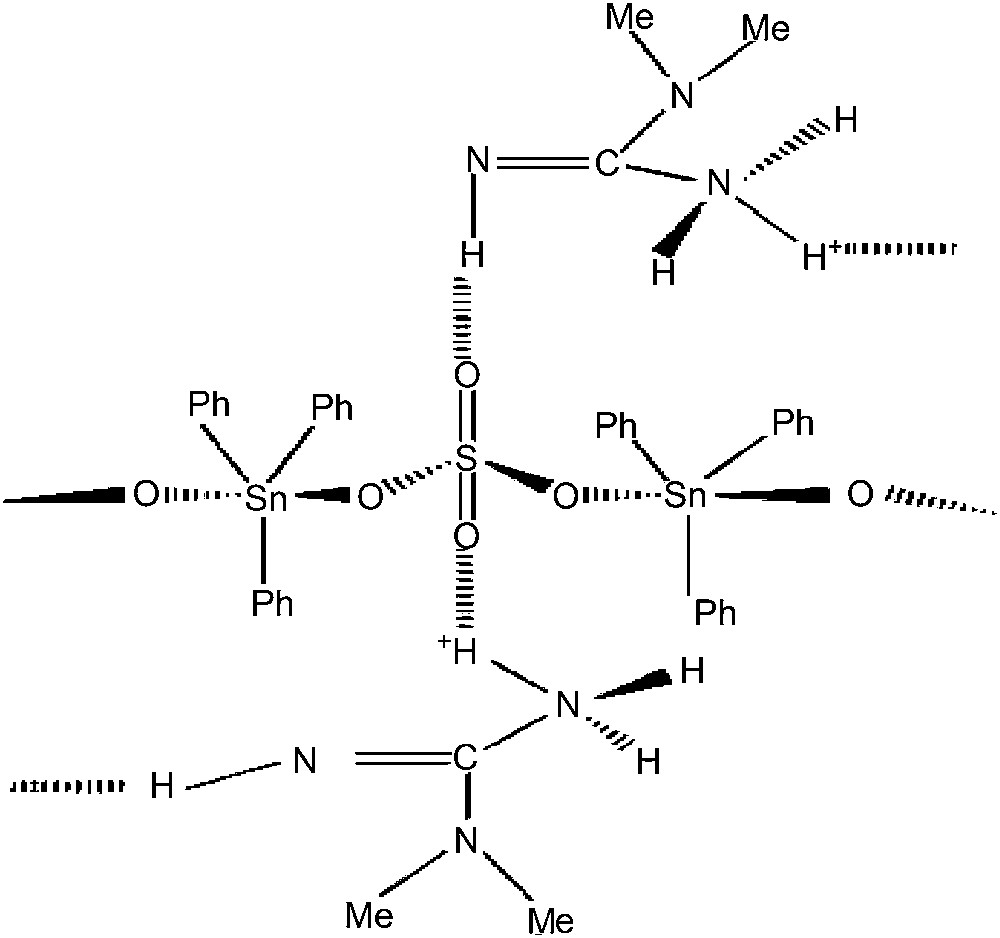
On the basis of these spectroscopic data, the proposed structure is a polymeric chain, in which Ph3Sn residues are trans coordinated, the sulphate behaving as a bidentate ligand, the two other oxygen atoms being involved in NH⋯O hydrogen bonds with the 1,1-dimethylguanidinium (the characteristic absorptions are localized at 3371 and 3169 cm−1 on the infrared spectrum), conferring a Td symmetry to the sulphate. In SO4(SnPh3)(H2O·SnPh3) [15], the sulphate appears as belonging to the Td symmetry group (in this compound, there is no free oxygen atom, three being bonded to the tin center and the fourth one involved in the hydrogen bond linking the two adjacent chains, allowing the anion to appear as belonging to the Td point group). In (R4N)(SnPh3)(SO4) (R = Bu, Me) [5], the sulphate group has a C2v symmetry [5]. The number of the protons linked to N allows us to consider all the non-coordinating oxygen atoms to be involved in H-bonds. While considering the polymeric chains, the cations can be involved in NH⋯O hydrogen bonds in two ways:
- (1) the cations link to oxygen atoms of the same sulphate, allowing the formation of an SO2N2 cycle (Fig. 1);
- (2) the cations link two adjacent polymeric chains, the resulting structure being a three-dimensional network (Fig. 2).
4 Conclusion
In the title compound, the sulphate behaves as a bridging bidentate ligand involving a polymeric chain; H-bonds involving the two other oxygen atoms of the sulphate and the counter cation are noteworthy.


