1. Introduction
Compounds of transition metal oxides find many applications in numerous industries, such as in lithium rechargeable batteries and heterogeneous catalysis. Zinc pyrovanadate Zn3V2O7(OH)2·2H2O is a layered compound with interesting crystal structure feature. It is prepared first by a hydrothermal technique [1]. Before that, copper pyrovanadate Cu3V2O7(OH)2·2H2O was prepared by a solution method at room pressure [2,3]. Both of them show similar layered crystalline structures, although they crystallize in different crystal systems. Copper pyrovanadate adopts a monoclinic lattice in contrast to zinc pyrovanadate, which adopts a hexagonal lattice. Their crystalline structures consist of MO6 octahedral (M = Zn or Cu) layers joined by two tetrahedral VO4 units along the c axis. The two tetrahedral VO4s have one common oxygen and they form pyrovanadate group V2O7. A mixture of zinc and copper pyrovanadate phases (CuxZn1−x)3V2O7(OH)2·2H2O (x = 1.0, 0.95, 0.90, 0.70, and 0.60), isostructural to monoclinic copper pyrovanadate (Volborthite), were recently reported [4,5]. They are prepared by using a solution method, where long reaction time is needed (2 days). Recently, new simple chimie douce method, at normal pressure, successfully leads to zinc pyrovanadate with a short reaction time (15 min) and high yield [6]. The objective of the present study is to incorporate copper in the zinc pyrovanadate lattice using the same method and to investigate the effect of copper on the zinc pyrovanadate crystalline structure.
2. Experimental
In the present preparation, the same method as in Ref. [6] was used. Different molar ratios of Cu/Zn: 0.9/2.1, 1.5/1.5 and 2.1/0.9, which correspond to 30, 50 and 70 at.% Cu were used. About 0.250 g of V2O5 (BDH, England) was mixed with about 3 mL of 30 vol.% H2O2 (Fluka). An exothermic reaction took place immediately. After a few minutes, before the mixture dried, about 400 mL of distilled water was added and heated until boiling. A yellow orange clear solution with pH 2 was obtained. At the same time amount of Cu(NO3)2·2.5H2O (ACROS Organics) and Zn(NO3)2·6H2O (Rasayan laboratory) were dissolved in 50 mL of distilled water and added to the boiling vanadium oxide solution (∼100 °C). The mass ratios Cu(NO3)2·2.5H2O/Zn(NO3)2·6H2O used were: 0.288 g/0.859 g (for 30 at.% Cu), 0.480 g/0.613 g (for 50 at.% Cu) and 0.671 g/0.368 g (for 70 at.% Cu). When ammonium hydroxide NH4OH 10% was added, a precipitate was obtained at pH ≈ 6. The stirring was maintained for 15 min. The products were dried at room temperature and analyzed with a powder X-ray Philips 1710 diffractometer and a JEOL JSM-840A scanning electron microscope equipped with an Oxford EDAX. The effects of temperature on the weight loss were investigated by thermal analyses TGA (2960 Universal TA Instruments, under normal atmosphere and with a heating rate of 10 °C min−1, between room temperature and 600 °C).
3. Results and discussion
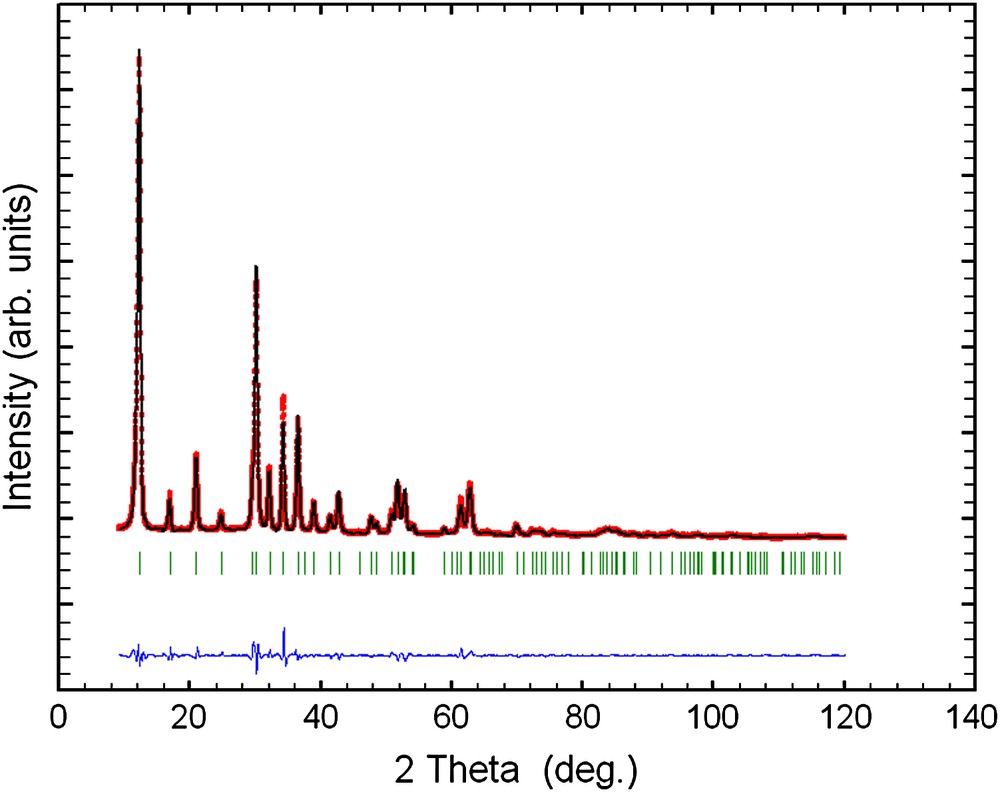
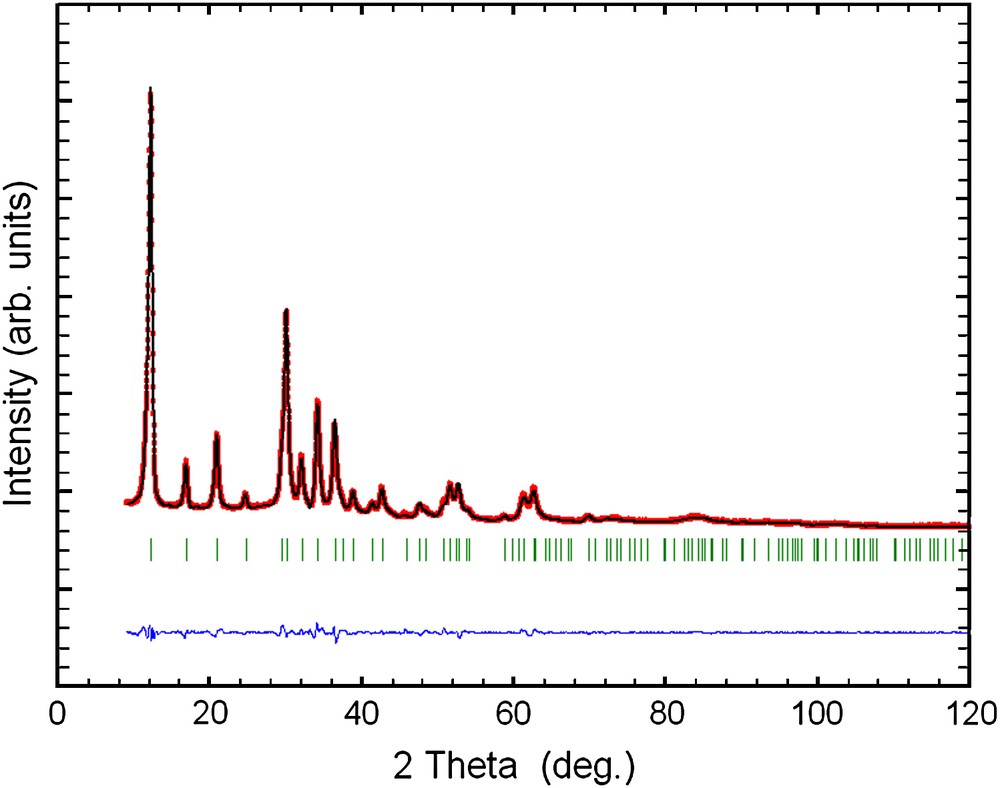
EDAX analyses of the as-prepared samples show that the initial compositions of zinc and copper used are maintained in the final products (Table 1). Also, the present reactions show high yields of products (more than 90%). The as-prepared samples were analyzed by powder X-ray diffraction (Fig. 1). All diffraction peaks present on the powder X-ray diffraction patterns of 30 and 50 at.% Cu-containing samples are indexed by the hexagonal lattice of zinc pyrovanadate. The sample containing 70 at.% Cu presents a different pattern where new peaks appear. The attempts to index this pattern were unsuccessful, although several tests were carried out with different known indexing programs. Thus, the purity of the sample becomes questionable. A careful inspection of the pattern indicates that the present phase is a mixture of both hexagonal zinc pyrovanadate and monoclinic copper pyrovanadate. This is clear in Fig. 2. The powder X-ray diffraction pattern of 70 at.% Cu-containing sample was successfully fitted (Rp = 11.6 and Rwp = 8.5) using the Rietveld analysis (profile matching with constant scale factor) available in FULLPROF program [8]. The unit cells and space groups of both zinc pyrovanadate and copper pyrovanadate were used as input data in the program. This indicates that the hexagonal structure of zinc pyrovanadate is unstable to accommodate high amount of copper such as 70 at.% Cu. It is worth noting that the mixture (CuxZn1−x)3V2O7(OH)2·2H2O (x = 0.70), with similar composition, 70 at.% Cu, and prepared using a different solution method, was reported to be a single-phase isostructural to monoclinic copper pyrovanadate [4]. Fig. 3 shows micrographs of scanning electron microscope of 30 and 50 at.% Cu-containing samples. They show homogeneous particles with platelet shape. Thermogravimetry analysis (TGA) curves for the as-prepared samples, 30 and 50 at.% Cu, are very similar (Fig. 4). They show about three different slopes which probably are related to three different types of water: adsorbed water (about 0.7H2O), which is lost first when the samples are heated from room temperature to 150 °C, followed by bonded water (about 2H2O), which is lost between 150 °C and 250 °C; at the end, above 250 °C, hydroxyl groups, participating in the crystalline structure, are lost (about 1H2O). This will lead to structural transformation and probably formation of zinc and copper orthovanadates [7]. From EDAX analysis and TGA study, the chemical formulae of both 30 and 50 at.% Cu-containing samples are CuZn2V2O7(OH)2·2.8H2O and Cu1.5Zn1.5V2O7(OH)2·2.7H2O, respectively. To study the effect of copper incorporation on the crystalline structure of zinc pyrovanadate, the crystal structures of as-prepared samples were investigated by the Rietveld profile analysis using the FULLPROF program [8]. The structural parameters of zinc pyrovanadate [1] were used as input data. The Pseudo-Voigt function was used to describe individual line profiles. The background was refined as linear interpolation between a set of background points with refinable heights. The parameters refined include the scale factor, overall B factor, zero point correction, cell parameters, isotropic thermal and positional parameters for all atoms, and three coefficients to describe the angular dependence of line breadths, asymmetry factors and shape parameters. In the refinement, the displacement parameters for the same atoms were constrained to vary in the same manner. The occupancy factor values for copper and zinc were taken from EDAX analysis. The refinement converges to Rp = 7.60% and Rwp = 8.60% in the case of 30 at.% Cu-containing sample and Rp = 7.30%; Rwp = 7.10% in the case of 50 at.% Cu-containing sample. Table 2 shows the details of the Rietveld refinement. Figs. 5 and 6 show the final Rietveld plot. Table 3 shows the refined fractional atomic coordinates and Table 4 shows the bond distances and angles, respectively, for 30 and 50 at.% Cu-containing samples. For comparison, the bond distances and angles of pure zinc pyrovanadate are also displayed (Table 4). Similar to zinc pyrovanadate, the Cu-containing samples show only one type of both tetrahedral VO4 and octahedral MO6 (M = Zn or Cu) groups. The VO4 has one common oxygen O3 along the c axe (Fig. 7a and b) that form the pyrovanadate group V2O7. The other three oxygens O2 of VO4 assure connection between tetrahedral and octahedral layers. Oxygen O1 is only common to octahedral MO6. This structure left empty cavities which were occupied by water molecules (Fig. 7c); the water oxygen position in the present refinement was found to be different from the water oxygen position reported in the zinc pyrovanadate structure [1]. Two molecules of water are present in each unit cell cavity. This is consistent with the TGA curve, which shows loss of two bonded molecules of water by unit formula, between 150 °C and 250 °C. The bond distances and angles comparison (Table 4) shows The V–O bonds' distances of the Cu-containing samples become shorter and a noticeable decrease in the V–O2 bond distance can be seen: V–O2 in 30 at.% Cu-containing sample is 1.582 Å, which is much shorter compared to pure zinc pyrovanadate 1.695 Å and it decreases further for the 50 at.% Cu-containing sample (1.573 Å). The V–O bond distance calculated from ionic radii of vanadium V+5 and O−2 in tetrahedral coordination is 1.735 Å [9]. This indicates that as copper substitutes zinc in the zinc pyrovanadate lattice, the V–O2 bond distance becomes much shorter, which increases the distortion in the tetrahedral VO4. The octahedral MO6 for 30 at.% Cu-containing sample shows similar M–O1 bond distance as zinc pyrovanadate; however, the bond distance M–O2 increases from 2.23 Å to 2.29 Å. Probably this is the cause of the Jahn–Teller effect. Also the angle O2–M–O2 has a higher value of 97.4° compared to pure zinc pyrovanadate sample (94.4°). The sample with 50 at.% Cu shows a larger distortion of the MO6 octahedron, in which both M–O1 and M–O2 bond distances are changed: the M–O2 bonds become longer (2.29 Å), which is similar to the M–O2 bond distance value in the 30 at.% Cu-containing sample, and the M–O1 bond distance becomes shorter (1.85 Å), compared to pure zinc pyrovanadate (1.94 Å). The Jahn–Teller effect in the present sample is more accentuated, which should be expected as the amount of copper in the sample is higher. Also the O2–M–O2 angles are changed, while one O2–M–O2 is increased, from 97.4° for 30 at.% Cu-containing sample to 99.7° for 50 at.% Cu-containing sample, the other one O2–M–O2 is decreased, from 82.6° for 30 at.% Cu-containing sample to 80.3° for 50 at.% Cu-containing sample. This indicates that the incorporation of copper in the zinc pyrovanadate lattice induces distortion in both tetrahedral and octahedral groups. The octahedral groups are more distorted (both bond distances and angles are changed) compared to tetrahedral groups, where only bond distances are changed. This distortion increases with a larger amount of copper. This explains why the X-ray diffraction pattern of 70 at.% Cu-containing sample shows a mixture of phases. It seems that 70 at.% Cu amount has exceeded the limit of copper allowed by the zinc pyrovanadate lattice. The incapacity of the zinc pyrovanadate hexagonal lattice to incorporate more copper is essentially related to the structure distortion. This is probably mainly due to the Jahn–Teller effect. For this reason pure copper pyrovanadate, although its structural features is similar to that of hexagonal zinc pyrovanadate, adopts the lower symmetry lattice, a monoclinic lattice that seems to tolerate octahedral distortion much better compared to the hexagonal lattice.
EDAX analysis of as-prepared samples
| Experiments | Elements | Initial at.% | Product at.% |
| 1 | Zn | 70 | 69 |
| Cu | 30 | 31 | |
| 2 | Zn | 50 | 48 |
| Cu | 50 | 52 | |
| 3 | Zn | 30 | 27 |
| Cu | 70 | 72 |
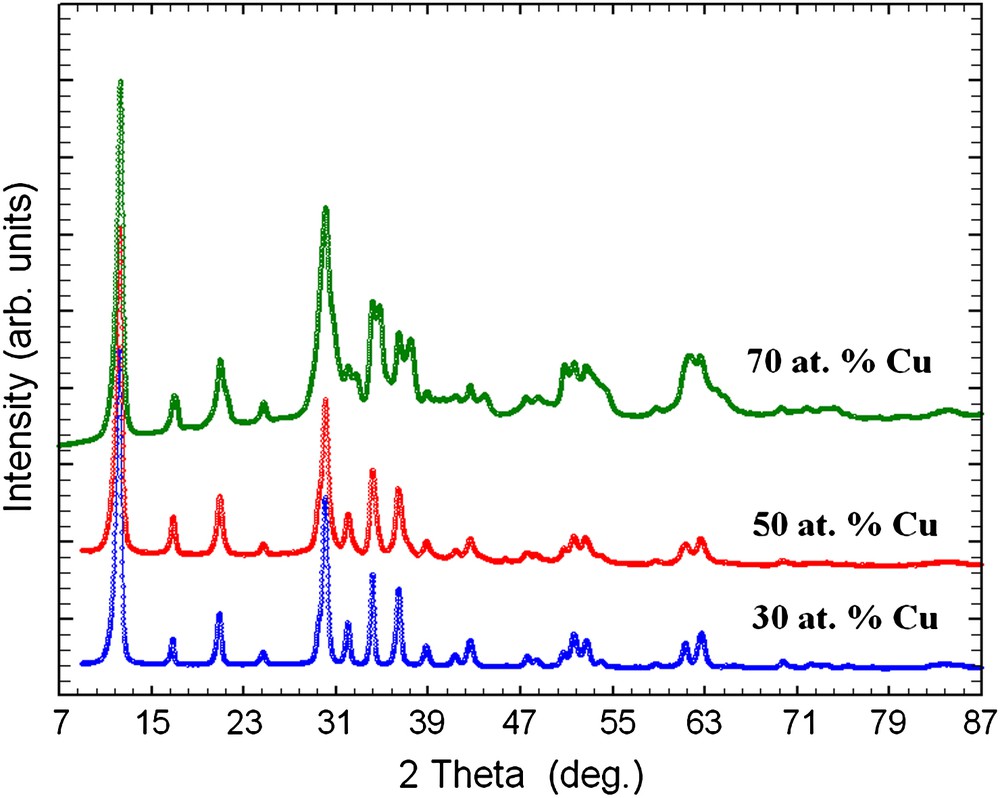
Powder X-ray diffraction of as-prepared samples with different amounts of copper.

Rietveld plot for 70 at.% Cu-containing sample. The upper trace shows the observed data as dots, while the calculated pattern is represented by the solid line. The lower trace is a plot of the difference between observed and calculated data. The vertical markers show the positions calculated for Bragg reflections for both zinc and copper pyrovanadate.

SEM micrographs of (a) 30 at.% Cu-containing sample (b) 50 at.% Cu-containing sample.
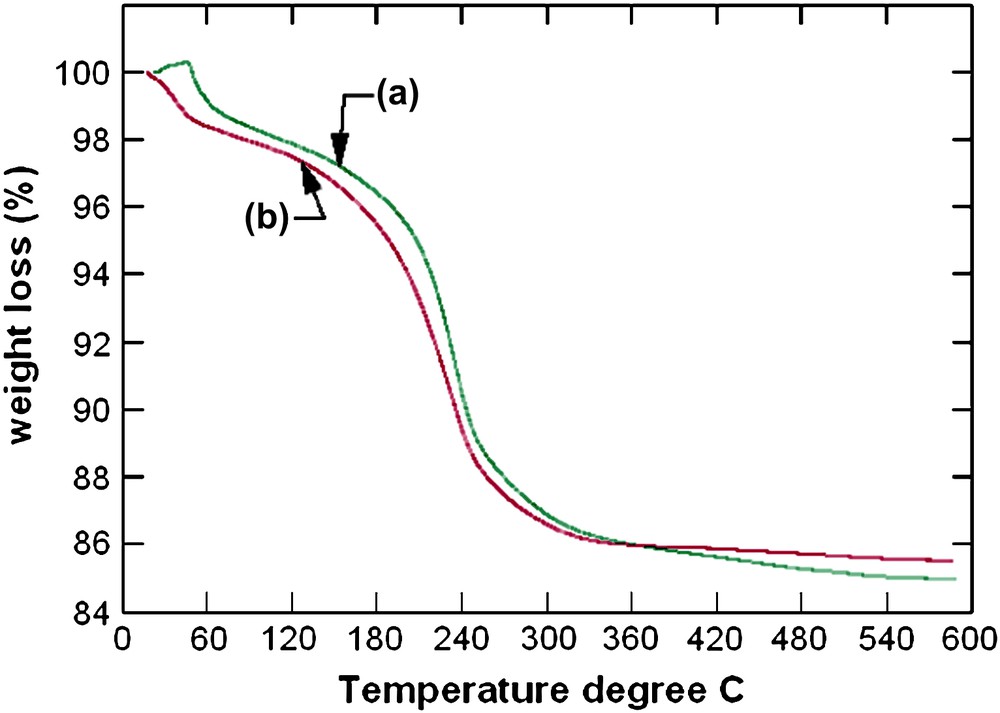
TGA curves (a) 30 at.% Cu-containing sample (b) 50 at.% Cu-containing sample.
Details of Rietveld refinements of 30 and 50 at.% Cu-containing sample
| 30 at.% Cu | 50 at.% Cu | |
| Wavelength (Å) | 1.54056 | 1.54056 |
| Step length | 0.02 | 0.02 |
| 2θ range (°) | 9.0–120.0 | 9.0–120.0 |
| Symmetry | Trigonal | Trigonal |
| Lattice parameters | ||
| a (Å) | 6.0318(3) | 6.0422(4) |
| c (Å) | 7.1730(4) | 7.1936(7) |
| Volume (Å3) | 226.01(2) | 227.44(3) |
| Space group | P-3m1 (No. 164) | P-3m1 (No. 164) |
| Z | 1 | 1 |
| No. of reflections | 168 | 179 |
| No. of structural parameters | 17 | 17 |
| No. of profile parameters | 10 | 10 |
| Background information | Linear interpolation | Linear interpolation |
| Rp | 0.076 | 0.073 |
| Rwp | 0.086 | 0.071 |
| Rexp | 0.062 | 0.088 |
| Rb | 0.015 | 0.014 |
| Rf | 0.018 | 0.025 |
Final Rietveld plot for 30 at.% Cu-containing sample. The upper trace shows the observed data as dots, while the calculated pattern is represented by solid line. The lower trace is a plot of the difference between observed and calculated. The vertical markers show the positions calculated for Bragg reflections.
Final Rietveld plot for 50 at.% Cu-containing sample. The upper trace shows the observed data as dots, while the calculated pattern is represented by solid line. The lower trace is a plot of the difference between observed and calculated. The vertical markers show the positions calculated for Bragg reflections.
Fractional atomic coordinates and isotropic atomic displacement parameters of 30 and 50 at.% Cu-containing samples
| Atom | Site | X (30) | X (50) | Y (30) | Y (50) | Z (30) | Z (50) | Biso (Å2) (30) | Biso (Å2) (50) | Occu. (30) | Occu. (50) |
| Zn | 3e | 0.5 | 0.5 | 0 | 0 | 0 | 0 | 2.66(99) | 3.00(99) | 0.167 | 0.125 |
| Cu | 3e | 0.5 | 0.5 | 0 | 0 | 0 | 0 | 2.66(99) | 3.00(99) | 0.083 | 0.125 |
| V | 2c | 0 | 0 | 0 | 0 | 0.2539(3) | 0.2556(3) | 0.80(99) | 0.36(99) | 0.167 | 0.167 |
| O1 | 2d | 0.6667 | 0.6667 | 0.3333 | 0.3333 | 0.8829(9) | 0.9138(13) | 4.88(99) | 6.70(99) | 0.5 | 0.5 |
| O2 | 6i | 0.1434(5) | 0.1404(5) | 0.2867(11) | 0.2808(11) | 0.8173(4) | 0.8223(5) | 4.88(99) | 6.70(99) | 0.083 | 0.083 |
| O3 | 1b | 0 | 0 | 0 | 0 | 0.5 | 0.5 | 4.88(99) | 6.70(99) | 0.167 | 0.167 |
| Ow | 2d | 0.6667 | 0.6667 | 0.3333 | 0.3333 | 0.4901(29) | 0.4642(22) | 4.88(99) | 6.70(99) | 0.167 | 0.167 |
Selected bond distances (Å) and angles (°) with their standard deviations of 30, 50 at.% Cu-containing samples and pure zinc pyrovanadate (0 at.% Cu) [1]
| 0 at.% Cu | 30 at.% Cu | 50 at.% Cu | |||
| Tetrahedron: VO4 | |||||
| V–O2: 3 × 1.695(2) | O2–V–O2: 3 × 110.52(12) | V–O2: 3 × 1.582(3) | O2–V–O2: 3 × 110.1(2) | V–O2: 3 × 1.573(3) | O2–V–O2: 3 × 108.0(2) |
| V–O3: 1.781(1) | O2–V–O3: 3 × 108.40(9) | V–O3: 1.765(2) | O2–V–O3: 3 × 108.8(2) | V–O3: 1.758(2) | O2–V–O3: 3 × 110.9(2) |
| Octahedron MO6 (M = Zn or Cu) | |||||
| Zn–O1: 2 × 1.941(2) | O1–Zn–O1: 179.98(13) | M–O1: 2 × 1.933(3) | O1–M–O1: 180.0(3) | M–O1: 2 × 1.851(3) | O1–M–O1: 180.0(3) |
| Zn–O2: 4 × 2.226(2) | O2–Zn–O2: 2 × 179.99(8) | M–O2: 4 × 2.287(3) | O2–M–O2: 2 × 180.0(3) | M–O2: 4 × 2.287(3) | O2–M–O2: 2 × 180.0(3) |
| O1–Zn–O2: 2 × 94.24 (11) | O1–M–O2: 2 × 92.6(1) | O1–M–O2: 2 × 96.6(2) | |||
| O1–Zn–O2: 2 × 85.76(11) | O1–M–O2: 2 × 87.4(2) | O1–M–O2: 2 × 83.4(3) | |||
| O2–Zn–O2: 2 × 94.37(8) | O2–M–O2: 2 × 97.4(2) | O2–M–O2: 2 × 99.7(2) | |||
| O2–Zn–O2: 2 × 85.63(8) | O2–M–O2: 2 × 82.6(2) | O2–M–O2: 2 × 80.3(2) |
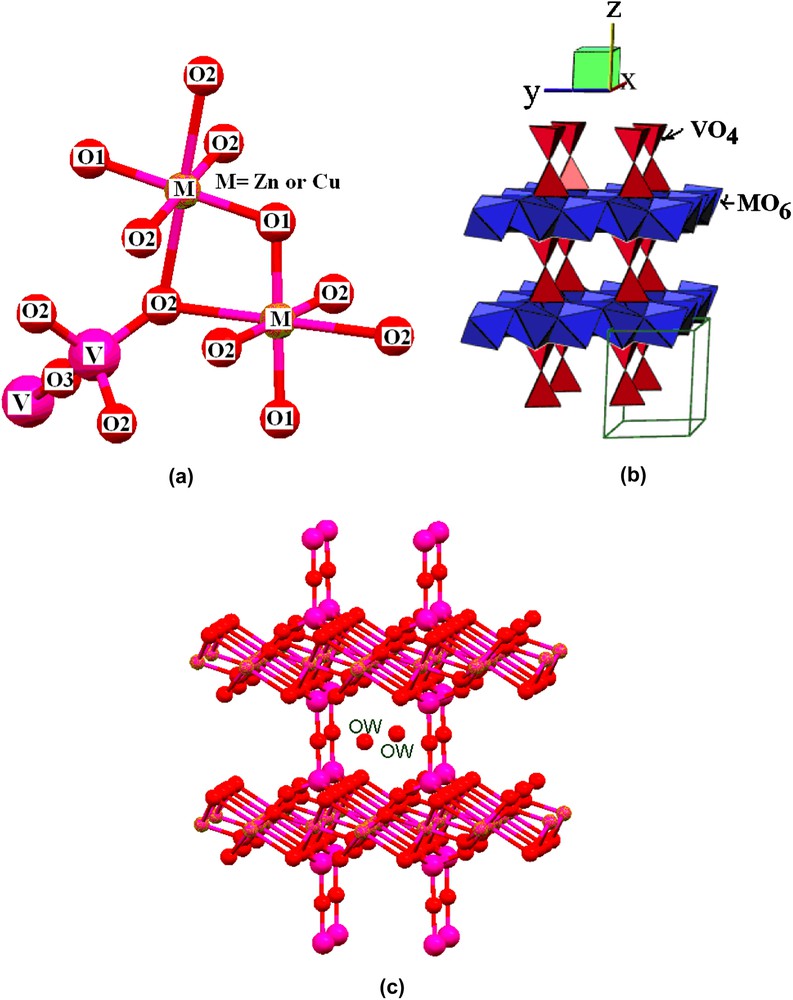
View of the structure (figures displayed by mercury [10] and balls and stick [11]).
4. Conclusions
New copper zinc pyrovanadate compounds are obtained by using a simple, room pressure, chimie douce method. The amount of copper incorporated in the zinc pyrovanadate lattice is limited by the distortion caused, which is mainly due to the Jahn–Teller effect. The simplicity and the high product yield make the present method suitable for preparing transition metal oxides, especially oxides based on vanadium, which are potential candidates for many industrial applications.
Acknowledgement
This work was financially supported by the Sultan Qaboos University. The author would like to thank Mr. Issa Al-Amri for SEM and EDAX measurements and Mr. Nasser Al-Mandhary for TGA measurements.


