1 Introduction
The genus Phlomis L. (Lamiaceae) is represented overall by 34 species and 52 taxa, in which 34 are endemic (including 12 natural hybrids) in Turkey [1–3]. It is documented that some Phlomis species are used as a tonic and stimulant in Anatolian folk medicine [4].
Previous phytochemical investigations on Phlomis lunariifolia, Phlomis monocephala and Phlomis sieheana were reported, where an aliphatic alcohol glycoside, a phenylethanoid glycoside, and a flavone glycoside were isolated from the aerial parts of P. lunariifolia, in addition to 15 known glycosides, by Calis and Kirmizibekmez [5]. Also from the overground parts of P. monocephala, two iridoid glycosides, three phenylethanoid glycosides, and one lignan glucoside were isolated by Yalcin and co-workers [6]. From the aerial parts of P. sieheana, an iridoid glucoside, six phenylethanoid glycosides, and a monoterpene glycoside were isolated and characterized by Ersoz et al. [7]. Very recently a pimarane-type diterpene was reported from Phlomis amanica [8].
As part of our ongoing research into essential oil of Phlomis species grown in Turkey, we report here the essential oil composition of five species, P. lunariifolia Sm., P. amanica Vierh., P. monocephala P.H. Davis, P. sieheana Rech. fil, and Pholmis armeniaca Willd., both by gas chromatography (GC) and gas chromatography–mass spectrometry (GC–MS). To the best of our knowledge, this is the first report on the essential oil chemistry of these five Phlomis species. Also the isolation of a diterpene and the identification of an iridoid within the essential oil are presented. In addition, antifungal Candida TLC bioautographic assay using Phlomis essential oils was applied initially to screen for bioactive constituents within the oils. Microbroth dilution assay was also used to determine the inhibitory activity against various human pathogenic Gram-positive and Gram-negative bacteria as well as against the yeast Candida albicans and Candida tropicalis.
2 Materials and methods
2.1 General
If not indicated otherwise, all chemicals, solvents, media and standards were purchased from Sigma/Aldrich at high purity (>99%).
Optical rotation was measured on a JASCO DIP-1000 polarimeter. IR spectra were measured using a Shimadzu FTIR-8400S. 1H and 13C NMR spectra were recorded on a Varian Unity 600 system at 600 and 150 MHz, respectively. 1D and 2D NMR data were also obtained using the same system, and spectra were measured and reported in ppm. Tetramethylsilane (TMS) at 0.0 ppm or the solvent CDCl3 was used as reference internal standard. HRMS analyses were obtained on a Jeol JMS-AX 500 system. GC and GC–MS analyses were carried out by Hewlett Packard 6890 and Hewlett–Packard GCD systems using a Innowax FSC column (60 m × 0.25 mm ∅, with 0.25 μm film thickness), respectively (see for analytical details as in Demirci et al. [9]).
2.2 Plant material
The aerial parts of the plants were collected from different regions and identified by one of us. Voucher specimens were deposited at the Herbarium of Erciyes University, Faculty of Science and Letters. Detailed information on the plant materials used is given in Table 1.
Information on the plant material and essential oils
| Phlomis ssp. | Collection site | Altitude (m) | Date | Oil yielda (%) | MYDb |
| P. lunariifolia (PL) | Icel; Aydincik–Gulnar 13th km | 50 | 03.07.2003 | tr | 1671 |
| P. amanica (PAm) | Hatay; Arsuz, Haymaseki village | 250–300 | 22.05.2004 | 0.14 | 1723 |
| P. monocephala (PM) | Icel; Aydincik–Gulnar 13th km | 50 | 03.07.2003 | 0.20 | 1669 |
| P. sieheana (PS) | Kayseri; Gesi, Ildem Koop | 1150 | 19.06.2003 | 0.04 | 1643 |
| P. armeniaca(PAr) | Kayseri; Kayakevi–Develi road 13th km | 1700 | 24.07.2002 | 0.10 | 1698 |
a Essential oil yields are given on moisture free basis.
b MYD: herbarium code of the collector.
2.3 Isolation of the essential oils
The plant materials were dried in the shade at room temperature and were subjected to hydrodistillation for 3 h using a Clevenger-type apparatus [10] to produce essential oils. The percentage yields were calculated on a dry weight basis as given in Table 2. The oils were obtained neat, dried over anhydrous sodium sulfate to remove the water and stored at +4 °C until analyzed and tested further.
The essential oil composition of Phlomis species
| RRI | Compound | PL | PAm | PM | PS | PAr |
| 1032 | α-Pinene | tr | 2.1 | 4.9 | tr | tr |
| 1035 | α-Thujene | – | 0.1 | 0.3 | – | – |
| 1118 | β-Pinene | tr | 0.1 | 0.3 | 0.1 | tr |
| 1146 | δ-2-Carene | tr | – | tr | 0.1 | tr |
| 1174 | Myrcene | – | – | 0.3 | – | – |
| 1203 | Limonene | 0.1 | 0.1 | 3.9 | 0.2 | tr |
| 1213 | 1,8-Cineole | tr | – | 0.1 | tr | 0.1 |
| 1280 | p-Cymene | 0.1 | – | 0.5 | tr | tr |
| 1400 | Nonanal | 0.2 | 0.2 | 0.2 | – | 0.1 |
| 1400 | Tetradecane | – | – | – | – | tr |
| 1443 | Dimethyl tetradecanea | 0.1 | – | – | 0.5 | 0.6 |
| 1452 | α,p-Dimethylstyrene | – | – | 0.1 | – | – |
| 1452 | 1-Octen-3-ol | – | 0.1 | 0.1 | – | – |
| 1466 | α-Cubebene | 2.0 | 0.1 | 0.7 | – | 0.1 |
| 1477 | 4,8-Epoxyterpinolene | – | – | 0.1 | – | – |
| 1493 | α-Ylangene | 0.3 | 0.1 | 0.1 | – | tr |
| 1495 | Bicycloelemene | 0.1 | – | 0.2 | 0.4 | 0.4 |
| 1497 | α-Copaene | 1.5 | 0.5 | 0.7 | 0.1 | 0.5 |
| 1506 | Decanal | 0.2 | tr | 0.1 | tr | 0.1 |
| 1528 | α-Bourbonene | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| 1535 | β-Bourbonene | 0.2 | 0.7 | 0.6 | 1.5 | 1.1 |
| 1544 | α-Gurjunene | 0.5 | – | tr | – | tr |
| 1549 | β-Cubebene | 1.0 | 0.1 | 0.3 | 0.2 | 0.2 |
| 1553 | Linalool | 0.2 | 0.1 | 0.1 | 0.7 | 0.9 |
| 1577 | α-Cedrene | 1.0 | – | – | – | – |
| 1589 | Isocaryophyllene | 0.5 | 0.2 | – | – | – |
| 1589 | β-Ylangene | 0.4 | 0.2 | – | 0.7 | 0.8 |
| 1594 | trans-β-Bergamotene | 0.1 | – | – | – | – |
| 1597 | β-Copaene | – | 0.2 | 0.4 | 0.3 | 0.5 |
| 1600 | β-Elemene | – | – | 0.6 | 0.9 | 0.9 |
| 1604 | 2-Undecanone | – | – | – | 0.2 | – |
| 1612 | β-Caryophyllene | 9.0 | 0.9 | 5.1 | 1.1 | – |
| 1628 | Aromadendrene | 0.1 | 0.2 | 0.1 | – | 0.1 |
| 1638 | β-Cyclocitral | – | – | 0.1 | – | 0.1 |
| 1650 | γ-Elemene | – | – | 0.2 | 1.4 | 0.5 |
| 1661 | Alloaromadendrene | 0.2 | – | 0.4 | – | – |
| 1668 | (Z)-β-Farnesene | 6.5 | 8.3 | 3.1 | 11.7 | 6.2 |
| 1659 | γ-Gurjunene | – | – | 0.1 | 0.2 | tr |
| 1669 | Sesquisabinene | 0.2 | – | – | – | – |
| 1674 | Muurola-4,11-diene | 0.3 | – | – | – | – |
| 1687 | α-Humulene | 0.9 | – | 0.4 | 0.3 | 0.2 |
| 1700 | p-Mentha-1,8-dien-4-ol (=limonen-4-ol) | – | – | 0.1 | – | – |
| 1704 | γ-Muurolene | – | 0.3 | tr | – | 0.5 |
| 1688 | Selina-4,11-diene (=4,11-eudesmadiene) | 2.0 | 0.5 | 2.9 | 0.7 | – |
| 1695 | (E)-β-Farnesene | – | – | – | 1.6 | 1.2 |
| 1700 | Heptadecane | – | – | – | 0.2 | – |
| 1708 | Ledene | – | 0.2 | 0.2 | – | – |
| 1726 | Germacrene-D | 7.7 | 14.7 | 6.0 | 16.6 | 23.4 |
| 1740 | α-Muurolene | 0.4 | 0.3 | 0.4 | – | – |
| 1742 | β-Selinene | – | – | – | 6.7 | 2.6 |
| 1744 | α-Selinene | – | – | 0.1 | 1.5 | tr |
| 1754 | (Z)-γ-Bisabolene | 0.5 | – | – | – | – |
| 1755 | Bicyclogermacrene | 2.6 | 10.7 | 1.5 | 1.6 | 2.3 |
| 1763 | Naphthalene | – | – | 0.1 | – | – |
| 1764 | (E)-2-Undecenal | – | – | – | 0.1 | 0.1 |
| 1765 | (E)-γ-Bisabolene | 0.1 | – | – | – | – |
| 1773 | δ-Cadinene | 1.7 | 0.8 | 0.4 | 0.6 | 1.0 |
| 1776 | γ-Cadinene | 0.2 | 0.3 | 0.3 | 0.2 | 0.3 |
| 1779 | (E,Z)-2,4-Decadienal | – | – | – | 0.5 | – |
| 1785 | 7-epi-α-Selinene | 0.4 | 0.2 | 0.5 | 0.1 | 0.2 |
| 1786 | ar-Curcumene | 1.8 | – | – | – | – |
| 1798 | Methyl salicylate | – | – | 0.2 | – | 0.2 |
| 1800 | Drimenenea | – | – | 0.1 | – | – |
| 1808 | Nerol | – | – | – | 0.1 | |
| 1827 | (E,E)-2,4-Decadienal | 0.1 | 0.1 | 0.1 | 0.4 | 0.2 |
| 1834 | Ethyl salicylate | – | – | tr | – | – |
| 1838 | (E)-β-Damascenone | 0.2 | – | 0.1 | 0.3 | 0.1 |
| 1849 | Calamenene | 0.3 | 0.1 | 0.3 | – | – |
| 1854 | Germacrene-B | – | – | – | 0.9 | 0.3 |
| 1857 | Geraniol | – | – | – | – | 0.4 |
| 1864 | p-Cymen-8-ol | 0.1 | – | 0.2 | – | – |
| 1868 | (E)-Geranyl acetone | 0.1 | – | 0.1 | 0.5 | 0.2 |
| 1884 | 1-Methyl naphthalene | – | 0.1 | – | – | – |
| 1900 | Nonadecane | – | – | – | – | 0.1 |
| 1900 | epi-Cubebol | 0.3 | – | 0.2 | 0.1 | 0.1 |
| 1941 | α-Calacorene | – | 0.2 | – | – | – |
| 1945 | 1,5-Epoxy-salvial(4)14-ene | – | – | – | 0.6 | 0.4 |
| 1953 | Palustrol | – | 0.1 | – | – | – |
| 1957 | Cubebol | 0.8 | 0.1 | 0.3 | – | – |
| 1958 | (E)-β-Ionone | – | 0.2 | – | 0.3 | 0.2 |
| 1969 | cis-Jasmone | – | – | – | – | 0.1 |
| 1972 | 1-Ethyl naphthalene | – | 0.1 | – | – | – |
| 1984 | γ-Calacorene | – | – | – | – | 0.1 |
| 2001 | Isocaryophyllene oxide | 0.3 | – | 0.3 | – | 0.1 |
| 2008 | Caryophyllene oxide | 1.2 | 0.1 | 1.2 | 0.7 | 0.7 |
| 2033 | epi-Globulol | – | 0.2 | – | – | – |
| 2036 | 2-Pentadecanone | – | – | – | – | 0.3 |
| 2037 | Salvial-4(14)-en-1-one | – | – | 0.1 | 0.5 | 0.2 |
| 2046 | Norbourbonone | – | – | 0.5 | 0.1 | |
| 2050 | (E)-Nerolidol | 0.1 | – | 0.1 | 0.2 | 0.3 |
| 2057 | Ledol | – | 0.1 | – | – | – |
| 2069 | Germacrene-D-4β-ol | – | – | – | – | 0.1 |
| 2071 | Humulene epoxide-II | – | – | 0.2 | – | – |
| 2080 | Cubenol | 0.3 | 0.2 | – | – | |
| 2084 | Octanoic acid | – | – | – | – | 0.3 |
| 2088 | 1-epi-Cubenol | 0.6 | 0.3 | 0.4 | – | 0.1 |
| 2095 | Hexyl benzoate | – | – | – | – | 0.3 |
| 2098 | Globulol | 0.8 | 1.5 | 0.6 | 0.5 | – |
| 2104 | Viridiflorol | 0.4 | 1.1 | 0.5 | – | 0.3 |
| 2144 | Rosifoliol | 0.3 | 0.2 | – | 0.1 | |
| 2131 | Hexahydrofarnesyl acetone | 1.0 | 0.7 | 0.5 | 1.9 | 2.3 |
| 2144 | Spathulenol | 3.9 | 6.3 | 3.8 | 3.0 | 1.9 |
| 2148 | (Z)-3-Hexen-1-yl benzoate | 0.2 | – | – | 0.4 | 0.2 |
| 2174 | Fokienol | – | – | – | – | 0.2 |
| 2179 | 3,4-Dimethyl-5-pentylidene-2(5H)-furanone | – | – | – | 0.8 | 0.2 |
| 2179 | Nor-Copaonone | – | – | – | 0.4 | – |
| 2187 | T-Cadinol | 0.4 | 0.6 | 0.4 | 0.3 | 0.5 |
| 2192 | Nonanoic acid | – | – | – | – | 0.6 |
| 2200 | Docosane | – | – | – | 0.4 | 0.1 |
| 2209 | T-Muurolol | 0.9 | 0.7 | 0.5 | 0.5 | 0.6 |
| 2219 | δ-Cadinol (=α-muurolol) | 0.3 | 0.2 | 0.6 | 0.2 | 0.2 |
| 2239 | Carvacrol | – | – | – | – | 0.3 |
| 2240 | 1-Methyl ethyl hexadecanoate | – | – | – | – | 0.2 |
| 2247 | trans-α-Bergamotol | 0.4 | 0.2 | 0.5 | 0.5 | 0.3 |
| 2255 | α-Cadinol | 1.2 | 1.4 | 1.2 | 1.4 | 1.2 |
| 2264 | Intermedeol | 0.4 | – | 0.3 | – | – |
| 2269 | Guaia-6,10(14)-dien-4β-ol | 0.4 | – | 0.4 | – | – |
| 2273 | Selin-11-en-4α-ol | – | 0.2 | – | – | – |
| 2278 | Torilenol | – | – | 0.5 | – | |
| 2287 | 8,13-Epoxy-15,16-dinor-labd-12-ene | – | 0.5 | 0.8 | – | – |
| 2298 | Decanoic acid | – | – | – | 0.5 | 1.0 |
| 2357 | Sandracopimaradiene | – | 0.4 | 1.1 | – | – |
| 2300 | Tricosane | – | 0.7 | – | 1.1 | 0.7 |
| 2357 | Octadecanal | – | – | – | 0.5 | – |
| 2369 | Eudesma-4(15),7-dien-1β-ol | – | 0.5 | – | – | 0.2 |
| 2376 | Manoyl oxide | 0.8 | – | 6.1 | – | – |
| 2380 | 8α,13-Oxy-14-en-epilabdane(=epi-manoyl oxide) | 0.2 | – | – | – | – |
| 2384 | Farnesyl acetone | – | – | – | 0.7 | – |
| 2384 | Hexadecanol | – | – | – | 0.4 | – |
| 2392 | Caryophylla-2(12),6-dien-5β-ol (=caryophyllenol II) | 0.1 | 0.6 | – | – | – |
| 2396 | γ-Dodecalactone | – | – | – | 0.2 | 0.2 |
| 2400 | Tetracosane | – | – | – | 0.5 | 0.3 |
| 2485 | 4-Methoxycarbonyl-7-methyl cyclopenta[c]pyrane (2) | – | – | – | 0.2 | 1.4 |
| 2500 | Pentacosane | – | – | – | 1.1 | 2.5 |
| 2503 | Dodecanoic acid | – | 0.7 | – | 1.8 | 1.4 |
| 2600 | Hexacosane | – | – | – | 0.2 | 0.1 |
| 2607 | Octadecanol | – | – | – | 0.7 | 0.6 |
| 2622 | Phytol | – | – | – | 0.5 | 0.4 |
| 2655 | Benzyl benzoate | – | – | – | – | 0.2 |
| 2670 | Tetradecanoic acid | – | – | 0.3 | 0.3 | 0.4 |
| 2700 | Heptacosane | – | – | – | 1.2 | 2.3 |
| 2900 | Nonacosane | – | – | – | 0.9 | 1.2 |
| 2917 | 8(14),15-Isopimaradien-11α-ol (1) | 5.5 | 22.8 | 12.7 | – | – |
| 2931 | Hexadecanoic acid | 9.7 | – | 1.7 | 2.1 | 4.9 |
| Total | 74.2 | 83.7 | 72.8 | 79.8 | 77.1 | |
| Identified compounds | 68 | 60 | 78 | 76 | 93 |
a Correct isomer not identified.
2.4 Isolation of (−)-8(14),15-isopimaradiene-11α-ol (1)
Compound 1 was isolated initially from P. monocephala (PM) essential oil (65.8 mg) by column chromatography. Silica Gel 60 G (ca. 3 g, Merck 7734) was used as a packing material, and filled with wet n-hexane (column size: 10 × 500 mm). n-Hexane:diethyl ether (100 → 0) was used as an eluant in a gradient system. The essential oil was applied and eluted with n-hexane:diethyl ether (90:10) to yield 1 (3.2 mg) as a colorless oily material. [α]D −17.0° (CHCl3; c 0.38), EI-MS m/z (rel. int.): 288 [M]+ (5), 273(4), 270(3), 255(8), 137(100), 123(28), 95(31), 81(35), 69(28). HRMS: 288.2458 [M]+ calcd for C20H32O, 220.2453. FT-IR (liquid film: CHCl3) νmax cm−1: 3546 (–OH), 1635, 1602, 1458, 1436, 1367, 1020, 1004 (1H, 13C and 2D NMR data are given in Table 3).
1H and 13C NMR data for compound 1
| 1H | 13C | |||
| No | Assignment | 1H–13C correlations | NOE correlations | Assignment |
| 1 | 1.34 (ddd, 4,13,13) ax. | C-20 | H-5,9 | 40.2 |
| 1.85 (br d, 13) eq. | H-11 | |||
| 2 | 1.51(m) ax. | 19.1 | ||
| 1.56(m) eq. | ||||
| 3 | 1.21 (ddd, 4,13,13) ax. | C-19 | 42.0 | |
| 1.42 (br d, 13) eq. | ||||
| 4 | – | 33.4 | ||
| 5 | 1.12 (dd, 3,13) | C-4,6,10,19,20 | H-1ax,9,7ax,18 | 54.8 |
| 6 | 1.30 (dddd, 5,13,13,13) ax. | C-5,7 | H-19,20 | 23.0 |
| 1.64 (m) eq. | C-8 | |||
| 7 | 2.05 (br ddd, 5,13,13) ax. | C-5,6,8,14 | H-5 | 36.3 |
| 2.32 (ddd, 2,5,14) eq. | C-5,8,9,14 | H-14 | ||
| 8 | – | 136.4 | ||
| 9 | 1.75 (br d, 5) | C-8,10,11,12,14,20 | H-5,1ax | 59.9 |
| 10 | – | 39.1 | ||
| 11 | 4.03 (ddd, 5,7,7) | C-10,12,13 | H-1eq,17, 20 | 66.2 |
| 12 | 1.65 (d, 7) 2H | C-9,11,13,14,15,17 | 43.4 | |
| 13 | – | 37.5 | ||
| 14 | 5.30 (t,2) | C-7,9,12,13,15 | H-7eq,17 | 127.4 |
| 15 | 5.88 (dd, 10,17) | C-12,13,17 | H-17 | 149.1 |
| 16 | 5.01 (dd, 1,17) trans | C-13,15 | H-17 | 110.6 |
| 4.92 (dd, 1,10) cis | C-13 | |||
| 17-CH3 | 1.05 | C-12,13,14,15 | H-16trans,11,20,15,14 | 27.0 |
| 18-CH3 | 0.89 | C-3,4,5,19 | H-4 | 33.8 |
| 19-CH3 | 0.85 | C-3,4,5,18 | H-6ax | 22.1 |
| 20-CH3 | 0.83 | C-1,5,9,10 | H-6ax,11,17 | 15.9 |
2.5 Preparation of 4-methoxycarbonyl-7-methyl cyclopenta[c]pyrane (2)
Ipolamiide (5 mg) was treated with 0.1 N HCl at pH 5 and stirred vigorously at approx. 100 °C. After 1 h all ipolamiides were converted into its aglycone. The solution extracted with Et2O and evaporated under nitrogen gave a red-orange residue (2 mg). The sample was analyzed by GC–MS. EI-MS m/z (rel. int.): 190[M]+ (100), 189(69), 175(61), 131(15), 129(23), 103(10), 102(15), 77(21), 51(13). MS data were in agreement with those reported by Bianco et al. [11].
2.6 Bioassays
2.6.1 Microorganisms
All microorganisms were obtained from various culture collections (ATCC, NRRL) and were maintained in 15% glycerol as cryoprotectant at −85 °C. Prior to use in the assays, bacteria were inoculated in Mueller–Hinton agar (MHA, Merck, Germany), and fungi in Sabouraud dextrose agar (SDA, Acumedia, MD) in Petri dishes until sufficient growth under optimum conditions for purity check. Microorganisms were then transferred to Mueller–Hinton broth (MHB, Merck, Germany) and incubated at 37 °C for 24 h.
2.6.2 Antimicrobial bioassay
A microdilution broth susceptibility assay was used [12,13]. Stock solutions of the test samples were prepared in 25% (v/v) dimethylsulfoxide (DMSO, Carlo Erba). Serial dilutions of samples were prepared up to 1.95 μg/ml using sterile distilled water in a 96-well microtiter plate. Microbial suspensions previously grown as described above were standardized to 1 × 108 CFU/ml (McFarland No: 0.5) in double strength Mueller–Hinton broth (MHB, Merck, Germany). One hundred microliters of each microbial suspension was then added to the appropriate well. The last row, which contained only the serial dilutions of the essential oil without microorganism, was used as a negative control. ‘Only DMSO’ dilutions were considered as another control to eliminate solvent effects. After incubation at 37 °C for 24 h the first well without turbidity was determined as the minimum inhibition concentration (MIC, μg/ml). Chloramphenicol and Ampicillin were used as standard antibacterials, whereas Ketoconazole and Clotrimazole were used antifungal positive controls. All experiments were repeated in triplicate. Average MIC results are given in Table 4.
Minimum inhibitory concentrations (MICs, μg/ml) for Phlomis essential oils
| Microorganisms | PL | PAm | PM | PS | PAr | St1 | St4 | St3 | St4 |
| Escherichia coli NRRL B-3008 | 500 | 500 | 500 | 500 | 500 | 7.8 | 7.8 | nt | nt |
| Staphylococcus aureus ATCC 6538 | 1000 | 1000 | 1000 | 1000 | >1000 | 1.9 | 1.9 | nt | nt |
| Pseudomonas aeruginosa ATCC 27853 | >1000 | >1000 | >1000 | >1000 | >1000 | 125 | 125 | nt | nt |
| Enterobacter aerogenes NRRL 3567 | 1000 | 1000 | 1000 | 1000 | 1000 | 3.9 | 3.9 | nt | nt |
| Proteus vulgaris NRRL B-123 | 500 | 500 | 500 | 500 | 500 | 15.6 | 15.6 | nt | nt |
| Salmonella typhimurium ATCC 13311 | >1000 | 1000 | 1000 | >1000 | 500 | 3.9 | 3.9 | nt | nt |
| Bacillus cereus NRRL B-3711 | 500 | 250 | 125 | 250 | 500 | 31.25 | 31.25 | nt | nt |
| Candida tropicalis NRRL Y-12968 | 1000 | 500 | 500 | 0.25 | 500 | nt | nt | 250 | 15.6 |
| Candida albicans NRRL 27077 | nt | 62.5 | 125 | nt | nt | nt | nt | 7.8 | 3.9 |
2.6.3 Bioautography method
For determining the active constituent a bioautography technique was used [12,14]. First the samples were subjected to TLC; following the separation, the inoculated medium was applied onto the developed TLC plate as described below to identify the active principle(s).
2.6.3.1 Thin layer chromatography
Precoated Silica Gel 60 GF 254 (0.2 mm) (Merck) plates, cut to appropriate size, on aluminum support were used. Essential oils (2 mg/ml; 2 × 1 μl) and Ketoconazole and Clotrimazole (2 mg/ml; conc., 2 μl) were applied using Drummond micro-capillaries onto the TLC plates and developed (8:2, v:v, n-hexane:ethyl acetate). Separated compounds were visualized with UV light (365 and 254 nm) and developed with anisaldehyde/H2SO4 spray reagent followed by heating to 110 °C, for the duplicate which served as reference. The other plate was utilized for the assay after complete solvent evaporation.
2.6.3.2 Agar overlay bioautographic assay
Nutrient Agar (15 ml, Difco) was poured onto the Petri plate (12 cm diameter) for the formation of an agar base. One of the developed and untreated TLC plates was carefully placed on the agar base under aseptic conditions. C. albicans as well as C. tropicalis were previously grown and incubated as described above. MHB with an Agar (7.5%, Acumedia, MD) was used in molten form and kept at 45 °C after sterilization, where the pre-grown Candida suspensions were transferred and adjusted to McFarland No: 0.5 each. Immediately, the Candida inoculated media were poured onto the TLC plates to form a thin layer and incubated at 37 °C for 24–36 h, separately. After sufficient growth, the Petri plates were sprayed using a 1% (w/v, EtOH) Tetrazolium Violet (2,5-diphenly-3-[α-naphthyl] tetrazolium chloride, Sigma) reagent and further incubated at 37 °C for 1 h. Inhibition zones were visualized against the red colored background. Ketoconazole and Clotrimazole were used as standard antifungal agents for comparison.
3 Results and discussion
3.1 Determination of essential oil components
Except for P. lunariifolia all other investigated species are endemic plants of Turkey [1]. The essential oils were obtained by hydrodistillation from the aerial parts of P. lunariifolia Sm., P. amanica Vierh., P. monocephala P.H. Davis, P. sieheana Rech. fil, P. armeniaca Willd., respectively. The oils were subsequently analyzed using both GC and GC–MS systems with which the individual components were identified according to their relative retention indices (RRI) and their relative percentages (see Table 2).
A sum of 68 individual compounds were identified in the oil of P. lunariifolia (PL), representing 74.2% of the total essential oil. This oil was characterized by a high content of hexadecanoic acid (9.7%), β-caryophyllene (9.0%), germacrene-D (7.7%), and (Z)-β-farnesene (6.5%). Overall, the oil was found to be rich in sesquiterpene hydrocarbons.
In the oil of P. amanica (PAm), 60 components were identified representing 83.7% of the total oil. This oil was characterized by germacrene-D (14.7%), bicyclogermacrene (10.7%), (Z)-β-farnesene (8.3%) and spathulenol (6.3%).
Analysis of P. monocephala (PM) oil revealed 78 components, representing 72.8% of the total oil. An unknown compound was detected as the major compound (22.8% and 12.7%) in both the essential oils of PAm and PM, respectively.
A total of 76 and 93 compounds were characterized in P. sieheana (PS) and Phlomis armenica (PAr) essential oils, representing 79.8% and 77.1% of the total oils, respectively. Germacrene-D (16.6% and 23.4%) and (Z)-β-farnesene (11.7% and 6.2%) were identified as the major constituents of PS and PAr essential oils, respectively.
Altogether a 143 volatile compounds were identified within the investigated Phlomis species in this study. To the best of our knowledge, this is the first report on the essential oil chemistry of these species. GC–MS analyses showed two interesting compounds to be present in some of the investigated Phlomis essential oils, a diterpene (1) and an iridoid derivative (2).
3.2 Structure elucidation
The unknown compound (1) was isolated from PM essential oil by column chromatography. The IR spectrum of 1 showed the absorption band characteristic for a hydroxyl group at 3546 cm−1. Its EI-mass spectrum gave a molecular ion peak at m/z 288, and a fragment peak at m/z 273 [M+ − CH3] among others. The HR-EI-MS analysis of the molecular ion peak at m/z 288.2458 suggested a molecular formula of C20H32O, confirming 5° of unsaturation for 1. The 1H NMR spectrum (data given in Table 3) of 1 showed the presence of four tertiary methyl groups at δ 0.83, 0.85, 0.89 and 1.05, an oxygenated methine group at δ 4.03 (ddd, J = 5, 7, 7 Hz) and an sp2 methine proton at δ 5.30 (br t, J = 2 Hz), and vinyl group protons, which were mutually spin coupled at δ 4.92 (dd, J = 1, 10 Hz), 5.01 (dd, J = 1, 17 Hz) and 5.88 (dd, J = 10, 17 Hz). The 13C NMR spectrum of 1 indicated the presence of 20 carbons, including 4 sp2 carbons and the oxygenated methine group at δ 66.2. Conclusively, the spectral data suggested that 1 was a tricyclic compound.
Analysis of the HMQC and HMBC spectra supported the structural assignment (details in Table 3). The long range 1H–13C correlation of two tertiary methyls at δH 0.85 and 0.89 (H3-19, 18) with a methylene carbon at δC 42.0 (C-3), a quaternary carbon at δ 33.4 (C-4) and a methine carbon at δ 54.8 (C-5), and mutual 1H–13C correlation between the both methyl groups, supported that the two methyls were gem-dimethyl structure in compound 1. Further correlation between the methyl proton signal at δH 0.83, the methine carbon at δC 54.8, and δC 59.9 was observed, respectively. The signal at δC 59.9 showed 1J-direct coupling with a broad doublet signal at δH 1.75 in the HMQC spectrum. The methine proton at δH 1.75 showed spin–spin coupling with the oxygenated methine proton at δH 4.03 in the 1H–1H COSY spectrum of 1. The remaining methyl group at δH 1.05 correlated with sp2 carbon at δC 149.1 which was attached by the vinyl proton at δH 5.88, and methylene carbon at δC 43.4 whose proton showed spin–spin coupling with the oxygenated methine proton.
Consideration of these spectral data and further 1H–13C correlations (summarized in Table 2) led to the conclusion that the structure of 1 was 8(14),15-isopimaradiene-11α-ol.
Extensive NOESY (also summarized in Table 3) spectral analysis allowed us to define the stereochemistry of the hydroxyl group at C-11. Since NOE correlation (as shown in Fig. 2) between C-11 proton and H3-17, 20 and H-1eq protons was observed, the hydroxyl group at C-11 of compound 1 should have α-equatorial orientation. Further NOE correlation (summarized in Table 3) between H-5 proton and H-1ax and H-9 protons was in particular important for the confirmation of the stereochemistry of the ring junction of 1 which was assigned in trans position. Accordingly, the structure of compound 1 was elucidated as 8(14),15-isopimaradiene-11α-ol (=11α-hydroxy-sandaracopimara-8(14),15-diene). To the best of our knowledge this is the first report on this compound isolated from essential oils. However, a very recent report coincides with the same structure; also isolated from a Turkish endemic P. amanica where all the spectroscopic data are in agreement with amanicadol [8].
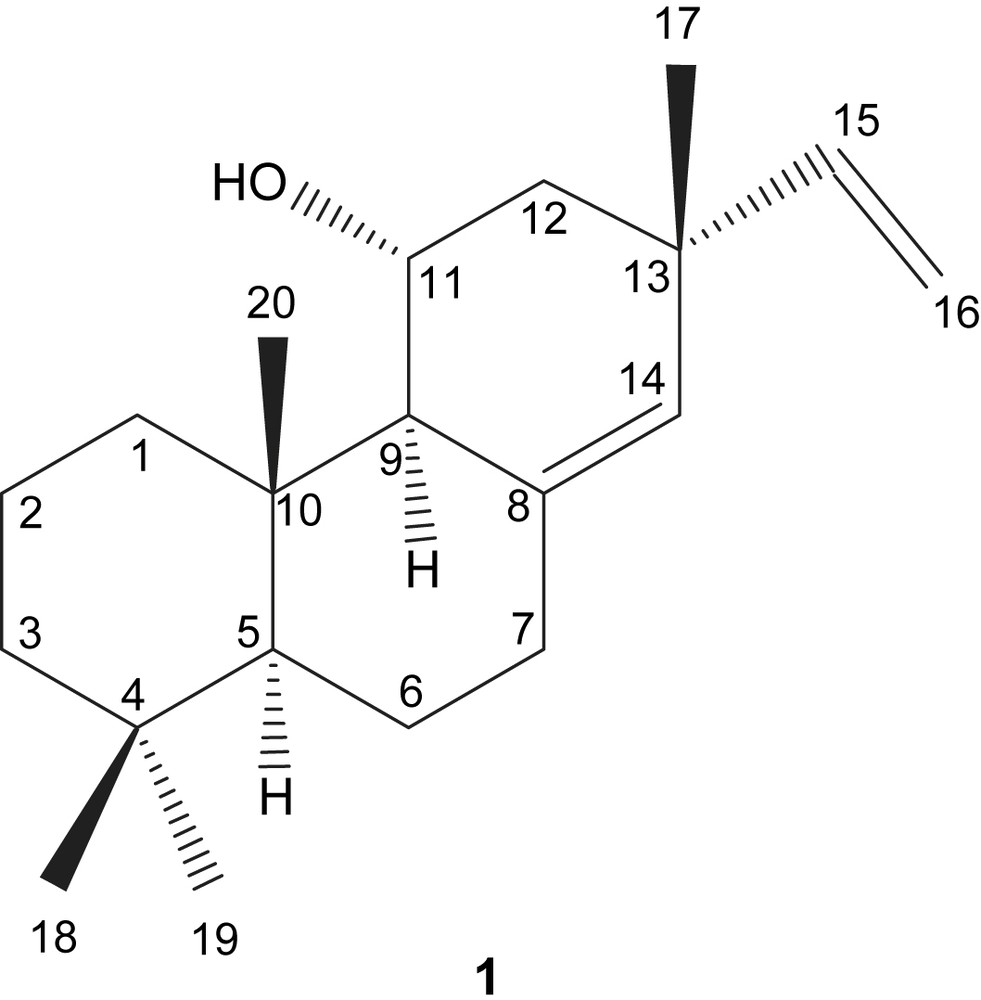
Assignment of (−)-8(14),15-isopimaradiene-11α-ol (1).

NOE correlations for 1.
3.3 Determination of fulvoiridoid
GC–MS analyses of the essential oils showed an unknown minor compound with a molecular weight of 190 corresponding to C11H10O3 from the computer peak matching against the commercial Wiley GC–MS Library [15]. As the relative contents of 2 were in minor amounts within the oils, chemical derivatization aided in the structure elucidation. The suggested iridoid agylcone (2) was obtained from the iridoid (3) frequently occurring in Phlomis species [5–8] including the investigated species in this study.
For the identification of compound 2, the iridoid ipolamiide (3) was subjected to acid hydrolysis. The resulting compound 4-methoxycarbonyl-7-methyl cyclopenta[c]pyrane (2) was characterized by co-injection of the Phlomis essential oils to confirm its presence in the oils of PAr (1.4%) and PS (0.2%). This compound was previously also reported as a transformation product of ipolamiide after acid hydrolysis, and was classified as a fulvoiridoid (or pseudoazulene) [11]. To the best of our knowledge, this is the first report of the rare compound 2 in an essential oil as a natural product. The presence of 3 in Phlomis species supports the hydrolytic formation of compound 2 from hydrodistillations possibly as an artifact as shown in Scheme 1.
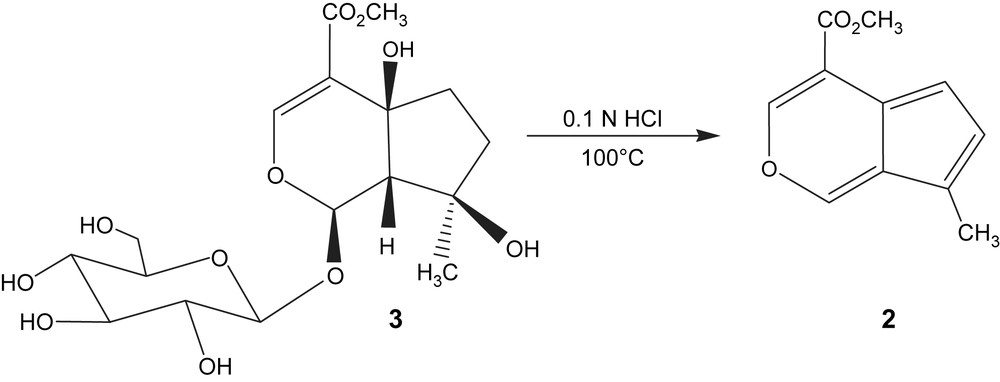
Acid hydrolysis of ipolamiide (3).
3.4 Antimicrobial activity
Phlomis essential oils were investigated first for their antifungal properties using a TLC bioautographic assay where the diterpene (1) was shown as the active principle against C. albicans and C. tropicalis when compared to the standard antifungal agent. A strong inhibitory zone was observed in the bioautographic assay when compared with the antifungal standards, especially PAm essential oil was followed by PM and PL oils. Minimum inhibitory concentrations (MICs) against various human pathogenic bacteria and C. albicans were determined using a microdilution assay. As seen in Table 4, weak to moderate antibacterial activity of the tested essential oils was observed (from MICs 500 to >1000 μg/ml) with exception against the pathogen Bacillus cereus (125–500 μg/ml). PM oil showed relatively good inhibition against B. cereus. The activity observed in the bioautographic assay against Candida sp. was repeated with a strong inhibitory effect of the PAm essential oil with decreasing action of PM and PL, respectively (62.5–1000 μg/ml). The relative percentage amount of the diterpene 8(14),15-isopimaradiene-11α-ol (1) showed correlation with the anticandidal effect in a dose response fashion.
In conclusion, the essential oils of Phlomis species are a natural source for antimicrobial and in particular antifungal applications. It is worthwhile to investigate further parts and species from this widespread genus.
Acknowledgements
The authors would like to thank Prof. Dr. Ihsan Calis (Hacettepe University, Faculty of Pharmacy, Ankara, Turkey) for supplying the ipolamiide used in the experiments for authentification and comparison.


