1 Introduction
Perilla frutescens (L.) Britt. (Lamiaceae) is an edible plant frequently used in some Asian countries such as China, Korea and Japan. Two major varieties (var.) are generally grown because they are traditionally used by local people: var. frutescens, and var. crispa (Thunb.) Deane. Seeds of P. frutescens var. frutescens were also considered as an interesting oil source for non-food uses and this variety has become widely cultivated in China and Korea. The seeds are also used to flavor traditional foods in Japan, Korea, China and Nepal because they contain aromatic essential oil [1]. Leaves of var. frutescens are used as a fresh vegetable and to process pickles, whereas, P. frutescens var. crispa is more often used in China for its medicinal properties and people from the Far East use it as a fresh vegetable.
Chemotypes can be found within the two Perilla varieties. External aspects differentiate them by different leaf and stem colors, varying from green to red, even to purple, indicating that these plants contain a great range of anthocyanins and flavonoids. The anthocyanin-rich chemotype is generally used as a food colorant in Japan and China, because of its bright red color. It has been shown that this red color was given by the presence of a major anthocyanin, malonylshisonin, 3-O-(6-O-(E)-p-coumaryl-β-d-glucopyranosyl)-5-O-(6-O-malonyl-β-d-glucopyranosyl)-cyanidin [2], and other related anthocyanin compounds that accumulate in the epidermal cells of leaves and stems of the red-leaf chemotype [3]. The green-leaf chemotypes show only trace amount of anthocyanin type compounds among all polyphenol compounds encountered. They are not only used as food ingredients but also for skin cream, soap, and medicinal preparations, because of their recognized bioactivities, such as antioxidant [4], anti-allergic [5], anti-inflammatory [6], and antibacterial activities [7]. They are also claimed having a potential inhibition effect on the production of a tumor necrosis factor (TNF-α) [8]. Phenolic compounds found in these plant extracts, such as rosmarinic acid, luteolin structure-like compounds, and essential oils are said to be responsible for these bioactivities [9]. It was also reported that rosmarinic acid content was generally found higher in green type than in red type of P. frutescens [10].
Manufacturing of concentrated polyphenol water-extracts from P. frutescens var. frutescens was shown to be possible at a semi-industrial scale in a previous work [11]. The raw material processed was a red-green cvs, obtained from the Guangdong area (China). To enlarge the supply in raw material, we studied the potentialities of other sources of cvs grown in China. In this study, we compared water-extracts of various P. frutescens cvs, to highlight the influence of plant variety and geographical plant origin on the polyphenolic composition, especially in anthocyanins, flavonoids and cinnamic derivatives. Among the eight cvs compared, seven were harvested in different locations in China (Table 1) during the same season (2005), and one sample was collected from Japan (cvs locally called “Shiso”) (Fig. 1). This comparative study was undertaken to identify the most interesting P. frutescens cv to be processed at a pilot plant scale (i.e. most colored cvs, rich in polyphenolic components), in view to set up low scale and a low cost semi-industrial manufactures of P. frutescens polyphenol concentrated extracts, with effective functional and active properties, to provide new marketable bioproducts.
Harvesting locations and time of the eight P. frutescens (Pf) cultivar samples
| Sample identification | Harvesting area (town) | Fresh leaf color | P. frutescens variety (var.) | Harvesting time (2005) |
| Pf 1 | Guangdong (Yangjiang) | Red-green | frutescens | July |
| Pf 2 | Guangdong (Guangzhou) | Red-green | frutescens | June |
| Pf 3 | Liaoning (Dandong) | Red | crispa | September |
| Pf 4 | Japan | Red | crispa | July |
| Pf 5 | Shanghai | Green | frutescens | August |
| Pf 6 | Yunnan | Green | frutescens | July |
| Pf 7 | Yunnan | Green | frutescens | July |
| Pf 8 | Fujian (Zhangzhou) | Green | frutescens | July |
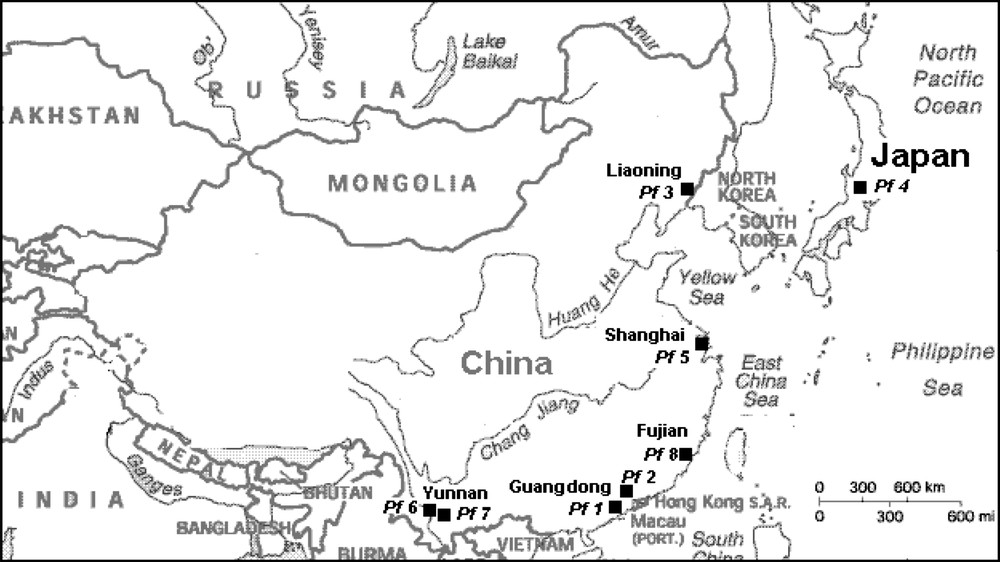
Harvesting locations of samples (■, Pf1–Pf8) of P. frutescens cultivars investigated.
2 Materials and methods
2.1 Plant material
Eight cvs of P. frutescens (L.) Britt. were collected during the 2005 harvesting season from six growing areas, five areas in China and one in Japan, as shown in Table 1 and Fig. 1. After harvesting, the fresh material samples, Pf1, Pf2 and Pf5, were sent to the South China Agricultural University (SCAU) to be stabilized by air-drying at 60 °C. The other samples were sent, already sun-dried by the farmers on the area of production. All samples were packed in sealed plastic bags to be sent to CIRAD laboratory in France where they were extracted at a laboratory scale. Water extracted polyphenolics were analyzed.
2.2 Extraction and fractionation of polyphenolic compounds
For each of the eight cvs studied (Table 1), a dried leaf sample (1 g) was extracted by diffusion, at room temperature for 4 h, in 100 ml deionized water, acidified with 0.01 M H2SO4, with constant light stirring. The extracts were paper-filtered and stored at 4 °C. To avoid compound degradation, DAD-HPLC analysis was performed on the extraction, the following day. Pf2 sample was the only one obtained using similar extraction method with conditions scaled up from laboratory to pilot plant level. These conditions were extraction of 0.6 kg of dry leaves with 300 L of acidified deionized water, kept in contact overnight at room temperature. Clarification and concentration of the polyphenol water-extract was made using a semi-industrial coupled-membrane technology including a cross-flow microfiltration (CFM) and a reverse osmosis (RO) pilot plant unit, as previously described [11]. An aliquot of the concentrated extract was evaporated until dryness at 60 °C using a laboratory rotating evaporator. Because a sufficient quantity of Pf2 was obtained this way, this extract was used to fractionate the extracted polyphenol bulk. A sample (2 g) of the dry powder obtained was dissolved in 50 mL deionized water. The aqueous solution obtained was extracted with 50 mL butanol three times, using a separatory funnel. The three alcoholic phases were combined. They contained all the polyphenol compounds extracted from the Pf2 sample. The butanol extract was then brought to dryness using a rotating evaporator at 35 °C. The dry matter obtained was dissolved in 50 mL deionized water. This solution was again extracted with 30 mL ethyl acetate, three times. The organic phases were joined. They contained the phenolic acids, while the remaining aqueous phase contained anthocyanins and flavones. The ethyl acetate phase was dried under vacuum. The residue obtained was dissolved in a minimum volume of MeOH before compound fractionation, using an open-column (16 mm × 220 mm) filled with 14 g of silica gel 100-RP 18 (particle size = 0.040–0.063 mm, Sigma). The mobile phase was a gradient of sequential mixtures of acetonitrile/water (v/v), percolated in the following order: 20 mL 1/9, 20 mL 2/8, 20 mL 3/7, etc. Every 1 mL of the output mobile phase was collected in different glass tubes. Presence of polyphenolics was checked in every tube by UV–vis spectrophometry at 530 and 325 nm. The phenolic acid content of the dried ethyl acetate phase was separated using the same open-column chromatography. Two fractions were obtained and were evaporated to dryness. One aliquot of dried residue was checked by HPLC for compound purity. Another aliquot was dissolved in CD3OD and analyzed by NMR for chemical structure determination of each phenol acid.
2.3 DAD-HPLC analysis
Anthocyanins and flavones were analyzed by HPLC equipped with a diode array detector – DAD (Agilent Technologies, France). The detection was made at 530 nm for anthocyanins and at 325 nm for flavones and other phenolic compounds. The separation column was a 250 mm × 4.6 mm i.d., 5 μm, RP 18-Satisfaction (Cil Cluzeau, France). The binary elution system was composed of 10:90 (v/v) formic acid/water (solvent A) and 10:90 (v/v) formic acid/acetonitrile (solvent B). The 55 min linear elution gradient started with an initial mobile phase of 94% A and 6% B and ended with a mobile phase of 76% A and 24% B. The column washing cycle used a mixture of 70% A and 30% B percolated for 10 min. The flow rate for both analysis and washing cycles was 0.8 mL/min. Three calibration curves were obtained, using commercial standards cyanidin, luteolin 7-O-glucoside and rosmarinic acid (Extrasynthese, France). Each calibration curve was a set of 7 points representing standard concentrations ranging from 2.5 μg/mL up to 250 μg/mL. Three determinations were done for each extract giving a CV = ±10%.
2.4 HPLC–MS analysis
The HPLC–MS was used to identify chemical structures of anthocyanin and other phenolic molecules extracted from the different cvs. The HPLC was a Waters-Alliance 2690 DAD detector equipped with a 250 × 2 mm i.d., 5 μm, Merck Lichrospher 100-RP 18 column, coupled with an LCQ-Advantage ion trap mass spectrometer (Thermo Electron S.A., Courtaboeuf, France). The HPLC mobile phases were (A) water/formic acid (98:2, v/v) and (B) water/acetonitrile/formic acid (18:80:2, v/v). The elution linear gradient started at 0.25 mL/min from 94% to 50% (A) within 55 min. The DAD was set at 530 nm to detect anthocyanin compounds and at 325 nm for other polyphenol compounds. The MS heated capillary was maintained at 175 °C and the voltage was set at 2 kV. The full-mass spectrum was a scan from m/z 100 to 1000. All mass spectrometry data were acquired with a positive and a negative ionization mode.
2.5 Nuclear magnetic resonance spectroscopy
1H and 13C NMR spectra were recorded on a Bruker AMX-300 spectrometer in CD3OD solutions. 13C resonance multiplicities were established via the acquisition of distorsionless enhancement by polarization transfer (DEPT) spectra.
3 Results and discussion
3.1 Identification of phenolic compounds water extracted from P. frutescens samples
The concentrated water-extract of P. frutescens var. frutescens Pf2 sample, collected from Guangdong (Guangzhou) in China, was analyzed by DAD-HPLC at 530 and 325 nm simultaneously. A typical DAD-HPLC chromatogram of anthocyanins of red and half-red P. frutescens leaves at 530 nm is given in Fig. 2. Peaks of phenolic compounds were first tentatively identified on the basis of their retention times. Peaks obtained by HPLC–MS chromatography were matched with those obtained by DAD-HPLC. Among them, 17 major peaks were selected and their chemical structures were tentatively determined, using UV–vis absorption spectrum and mass fragmentation (Fig. 3). Retention times, molecular mass of parent peak, mass fragmentation peaks, λmax adsorption, UV–vis spectra curves obtained from HPLC–MS analysis were compared with literature data to determine the chemical structure of the 17 selected compounds (Table 2). As it was the richest sample in phenol acid content, Pf1 sample was used for chemical structure determination of the extracted polyphenolics. Phenol acids were specifically extracted by ethyl acetate from the bulk concentrated polyphenol extract and were separated using open-column chromatography. Two purified compounds were identified by matching peak retention times with co-injected commercial standards of phenol acids as caffeic acid (peak 2) and rosmarinic acid (peak 15), as shown in Fig. 4. NMR analysis confirmed the structure of these 2 compounds.
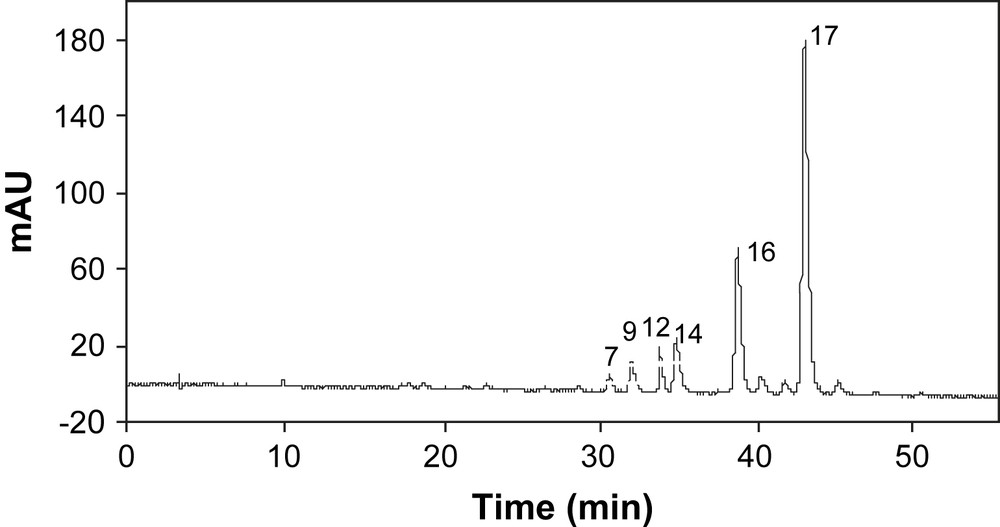
Typical DAD-HPLC chromatogram at 530 nm of anthocyanins of red and half-red P. frutescens.

Chemical structures of major polyphenolic compounds in P. frutescens leaves.
Identification of phenolic compounds of P. frutescens samples by HPLC–MS
| Peak no.a | RTb | DAD-HPLC λmax (shoulder) | Mass mode + | Mass mode − | Compound names | Ref. |
| 1 | 13.9 | 314.9 | 295 | Coumaroyl tartaric acid | ||
| 2 | 15.29 | 324.5 | 179 | Caffeic acid | [9] | |
| 3 | 17.61 | 310.2 | ||||
| 4 | 20.86 | 329–(334) | 595 | 593 | Apigenin 7-O-caffeoylglucoside or apigenin-6,8-diglucosidef | [3] |
| [13] | ||||||
| 5 | 22.98 | 334 | 639–287 | 637 | Scutellarein 7-O-diglucuronide | [3] |
| 6 | 23.84 | 348.3 | 639–287 | 637 | Luteolin 7-O-diglucuronide | [3] |
| 7 | 26.17 | 506–(285, 319) | Pelargonidin typef | |||
| 8 | 26.89 | 338.8 | 623–271 | 621 | Apigenin 7-O-diglucuronide | [3] |
| 9 | 27.88 | 506–(285, 319) | Pelargonidin typef | |||
| 10 | 28.86 | 343 | 463–287 | 461 | Luteolin 7-O-glucuronide | [3] |
| 11 | 29.25 | 334 | 463–287 | 461 | Scutellarein 7-O-glucuronide | [3] |
| 12 | 30.7 | 520.8 | 757–287 | Cyanidin 3-(Z)-p-coumaroylglucoside-5-glucosidec | [14] | |
| 13 | 30.90 | 295.9 | Unidentified | |||
| 14 | 32.10 | 525.6 | 859–287 | Cyanidin 3-O-(E)-caffeoylglucoside-5-O-malonylglucoside | [14] | |
| 15 | 32.34 | 329 | 359 | Rosmarinic acid | [9] | |
| 16 | 34.05 | 520.8 | 757–287 | Cyanidin 3-(E)-p-coumaroylglucoside-5-glucoside d | [15] | |
| 17 | 35.77 | 525.6 | 843–287 | Cyanidin 3-(E)-p-coumaroylglucoside-5-malonylglucoside e | [2] |
a See Figs. 2 and 4 for typical chromatograms and peak identifications.
b For HPLC conditions, see Section 2.
c cis-Shisonin.
d Shisonin.
e Malonylshisonin.
f Compound not yet characterized with enough accuracy.

DAD-HPLC chromatograms at 325 nm of flavonoids of P. frutescens samples investigated.
3.2 Polyphenolic composition of water extracted P. frutescens samples
Eight samples representing the P. frutescens cvs (Pf1–Pf8) were collected from China (7 samples) and from Japan (1 sample). P. frutescens cvs showed different degrees of red coloration that may change with the period of the year they are harvested. Pf1 and Pf2 cvs generally showed leaves with a green upper side and a red backside, Pf3 and Pf4 were both side red leaves, Pf5–Pf8 showed leaves with uniform green color. Water-soluble polyphenolics were extracted from the dried leaves and analyzed by DAD-HPLC. The red type samples, Pf1–Pf4, gave similar DAD-HPLC chromatograms (Fig. 2), showing 10 peaks at 530 nm. Among them, 6 peaks have been identified as anthocyanins (peaks 7, 9, 12, 14, 16, and 17). The other minor peaks, detected by DAD-HPLC, were not detected by HPLC–MS and their structure could not be identified. Semi-preparative HPLC chromatography is underway to purify and concentrate these minor and HPLC-detectable compounds for further structure identification.
3.3 Anthocyanin composition
The relative proportion of the 6 anthocyanins detected in the extracts showed only slight differences among samples (Fig. 4, Table 3). The total anthocyanin content varied from 0.7 to 1.8 mg/g (dry matter). Malonylshisonin (peak 17) was the major anthocyanin in all P. frutescens samples. It represented 55–66% of the total anthocyanin content. Shisonin (peak 16) was the second major anthocyanin and represented 17–26% of the total anthocyanin content. Water-extracts of Pf1, Pf2 and Pf4 samples showed 1.6, 1.8, 1.6 mg/g dry Perilla, as cyanidin equivalent, of total anthocyanin content, respectively. These contents were two times higher than that of Pf3 sample (0.7 mg/g dry Perilla). No anthocyanin compound was detected by DAD-HPLC in Pf5–Pf8 samples. Different levels of structural anthocyanin gene expression found in P. frutescens var. crispa (such as Pf3 and Pf4 samples) may explain this difference between found anthocyanin content and leaf external aspect. The literature states that a protein complex, including MYC-F3G1, MYC-RP, Myb-P1 and PFWD genes, is involved in the regulation of the expression of anthocyanin biosynthetic genes in P. frutescens. Gong et al. [12] also reported that the expression of Myb-P1 was increased 10-fold in the red type P. frutescens cvs with respect to the green ones. This is in agreement with our findings, based on anthocyanin content, but the increase in water extracted anthocyanin content was less than 10-fold. These genes are coordinately regulated by light in a form-specific manner in aerial vegetative tissues. Pf3 cv, generally red during summer time in China, turned partly green in autumn season, as sunlight duration declined. Pf3 sample collected for this study was harvested in September and, therefore, it seems logical that it showed less anthocyanin content than Pf4 sample from Japan, even if they were of the same botanic family P. frutescens var. crispa.
Anthocyanin composition of red type P. frutescens samples
| Peak no.a | Compounds detected by DAD-HPLC at 530 nm | P. frutescens samples (%) | |||
| Pf1 | Pf2 | Pf3 | Pf4 | ||
| 7 | Unidentified anthocyanin | 2 | 2 | 2 | 3 |
| 9 | Unidentified anthocyanin | 4 | 3 | 2 | 4 |
| 12 | cis-Shisonin | 5 | 7 | 7 | 6 |
| 14 | Cyanidin 3-O-caffeoylglucoside-5-O-malonylglucoside | 8 | 7 | 5 | 6 |
| 16 | Shisonin | 22 | 19 | 18 | 26 |
| 17 | Malonylshisonin | 59 | 62 | 66 | 55 |
| Total anthocyaninb | 1.6 | 1. 8 | 0.7 | 1.6 |
3.4 Phenol acid composition
Water-extract samples quantitatively analyzed by DAD-HPLC at 325 nm, showed 9 compounds, including 3 phenolic acids and 6 flavones. We observed that the number of phenolic compounds, other than anthocyanins, detected in both red-green and red type P. frutescens cvs was higher than in the green type ones (Fig. 4).
Total cinnamic derivative content, including the 2 major phenol acids, varied from 0.1 to 11 mg/g and total flavone content varied from 3.5 to 18.5 mg/g among cultivars (Table 4). Red type samples (Pf1–Pf4) were globally richer in phenolic acids than the green type ones (Pf5–Pf8), except for Pf3 in which rosmarinic acid was not detected. This result was in contradiction with results published by Okuda et al. [10], a long time ago. Rosmarinic acid was the most important cinnamic acid derivative in plant water-extracts. It accounted for more than 80% of total phenolic acids in Pf1, Pf2 and Pf4, and for more than 67% in Pf6. It was not detected in other water-extracts. Caffeic acid was generally lower than rosmarinic acid content and its variation among extracts did not follow those of rosmarinic acid.
Cinnamic acid derivatives and flavone compositions of P. frutescens cultivars
| Peak no.a | Compounds detected by DAD-HPLC at 325 nm | P. frutescens samples (mg/g dry matter) | |||||||
| Pf 1 | Pf 2 | Pf 3 | Pf 4 | Pf 5 | Pf 6 | Pf 7 | Pf 8 | ||
| 1 | Coumaroyl tartaric acidb | 0.8 | 0.3 | n.d. | n.d. | 0.05 | 0.4 | n.d.c | n.d. |
| 2 | Caffeic acid | 0.8 | 0.3 | 0.3 | 0.7 | 0.05 | 1.2 | 0.1 | 0.1 |
| 15 | Rosmarinic acid | 8.4 | 4.4 | n.d. | 10 | n.d. | 3.4 | n.d. | n.d. |
| Total cinnamic derivatives | 10 | 5 | 0.3 | 11 | 0.1 | 5 | 0.1 | 0.1 | |
| 4 | Apigenin 7-O-caffeoylglucoside | 1.2d | 0.5 | n.d. | 0.2 | 0.6 | 0.6 | 0.2 | 0.3 |
| 5 | Scutellarein 7-O-diglucuronide | 0.5 | 0.6 | 0.7 | 1.8 | 0.7 | 0.7 | 0.1 | 0.3 |
| 6 | Luteolin 7-O-diglucuronide | 4.2 | 6.6 | 4.9 | 7.4 | 0.5 | 1.0 | 0.4 | 0.3 |
| 8 | Apigenin 7-O-diglucuronide | 4.0 | 8.7 | 4.2 | 4.0 | 2.2 | 4.5 | 4.4 | 2.6 |
| 10 | Luteolin 7-O-glucuronide | 0.4 | 0.3 | n.d. | 0.5 | n.d. | 0.3 | n.d. | n.d. |
| 11 | Scutellarein 7-O-glucuronide | 1.7 | 1.8 | 0.2 | 0.6 | 0.2 | 1.6 | 0.3 | n.d. |
| Total flavones | 12.0 | 18.5 | 10.0 | 14.5 | 4.2 | 8.7 | 5.4 | 3.5 |
3.5 Flavone composition
The red and red-green type cvs (Pf1–Pf4) were richer in total flavone content than the green ones (Pf5–Pf8). Pf2 and Pf4 showed the highest total flavone content (18.5 and 14.5 mg/g, respectively). The green type cvs showed only 3–9 mg/g total flavone content. Apigenin 7-O-diglucuronide (2–9 mg/g dry matter) was one of the 2 major flavones. This compound was detected in all water-extracts and accounted for 27–78% of total flavones. It was the major component in the green type samples Pf7 and Pf8, and represented more than 70% of total flavone. The second important flavone was luteolin 7-O-diglucuronide (4.2–7.4 mg/g dry matter) that was found essentially in the red and red-green type samples Pf1–Pf4. It represented 35–51% of total flavone. This flavone content was less concentrated (0.3–1.0 mg/g) in the green type (Pf5–Pf8) than in the other samples. The two red-green type cvs (Pf1 and Pf2), harvested in the same area (Guangdong province, China), contained all the 9 phenol compounds. The other samples lacked in some of them: the red type Pf3 only contained 5 polyphenolics of which 2 major flavones accounted for more than 90% of the total flavone content. This chemotype did not show any rosmarinic acid as it was in high amount in the other red-green and red type chemotype. The red type Pf4, harvested in Japan, was the sample richest in rosmarinic acid (10.7 mg/g) and contained also appreciable flavone amounts. It showed only trace amount of coumaroyl tartaric acid and apigenin 7-O-caffeoylglucoside which are readily present in the red-green type cvs Pf1 and Pf2. The green type cvs (Pf5–Pf8) showed only one major flavone, apigenin 7-O-diglucuronide (2.2–4.5 mg/g), which was also found in similar amounts in all other P. frutescens cvs, including the red and red-green types (4.0–8.7 mg/g). Pf6 show a noticeable rosmarinic acid content (3.4 mg/g) compared to the other green type cvs, which lacked this phenol acid. Regarding the polyphenol composition within cvs, the red-green type cvs were the most interesting cvs for natural polyphenolic extraction from P. frutescens, in tropical and subtropical countries, using the semi-industrial scale process based on water extraction, including various membrane technologies, as published earlier [11]. Considering the different phenolic family compositions found in these P. frutescens water-extracts (Table 5), it appeared that they could be classified into 4 chemovars. The first chemovar is composed of samples containing anthocyanin (1.6–1.8 mg/g), with high total phenolics (23–27 mg/g). It includes Pf1, Pf2 and Pf4 cvs. The second group represented by Pf3 contained less amount of anthocyanin (0.7 mg/g) and was not rich in total phenolics (11 mg/g). The third group (Pf6) was characterized by an average amount of total phenolics (14 mg/g), but did not show any anthocyanins. The fourth group (Pf5, PF7 and Pf8 cvs) was characterized by the poorest total phenolics (4–6 mg/g), essentially composed of flavones (∼97%). No evident relationship appeared between the botanical classification and either the growing location or the harvesting area of the P. frutescens cvs studied. Both Pf3 and Pf4 cvs are of var. crispa, and were grown at the same latitude (Japan and north of China), but their polyphenolic compositions were different, particularly in phenolic acid content. It was found higher in Pf4 sample, especially in rosmarinic acid (10.7 mg/g) than in Pf3 that only showed trace amount. The other cultivars were of var. frutescens and showed also some composition differences. Even they were grown in the same location in China (Yunnan), Pf6 and Pf7 cvs showed different phenolic compositions. Pf1 and Pf2 cvs were harvested in close locations: Yangjiang is only 250 km away from Guangzhou area. Their water-extract contents showed the same polyphenolics, but Pf1 was richer in cinnamic acid derivatives and Pf2 was richer in flavones. Pf5, Pf7 and Pf8 cvs were classified as the same chemovar, on the basis of their similar chemical composition, but they were harvested in locations geographically far from each other, with different climates (Shanghai, Yunnan and Fujian area).
Composition in phenolic families of P. frutescens cultivars (%)
| Compound families | Pf 1a | Pf 2 | Pf 3 | Pf 4 | Pf 5 | Pf 6 | Pf 7 | Pf 8 |
| Phenolic acids | 43 | 20 | 3 | 41 | 2 | 37 | 2 | 4 |
| Flavones | 51 | 73 | 90 | 53 | 98 | 63 | 98 | 96 |
| Anthocyanins | 6 | 7 | 7 | 6 | n.d. | n.d. | n.d. | n.d. |
| Total phenolics (mg/g dry matter) | 24 | 25 | 11 | 27 | 4 | 14 | 6 | 4 |
a See Table 1 for cultivar identification.
Coumaroyl tartaric acid was generally detected in all P. frutescens water-extracts at different concentrations, but still low compared to those found for the other phenol components. It was never reported in the literature before as a P. frutescens water extracted compound. This compound was not generally detected in freshly extracted dry leaf samples. It was only detected by DAD-HPLC, both in green or red type cvs after only one-day storage. Such a compound was also formed as a whitish precipitate during concentration of filtrated P. frutescens water-extracts or during its overnight cold storage at 4 °C. We presumed that in fresh P. frutescens extracts, free tartaric acid was extracted from the leaf samples with the polyphenolics, along with some endogenous mineral ions (K+ or Ca2+). These compounds gave coumaroyl tartaric acid and tartrate through an in situ chemical reaction. We have shown the presence of a large amount of tartrate in P. frutescens water-extracts obtained during pilot plant trials, including cross-flow microfiltration at 0.2 μm. The tartrate precipitate was formed indeed after the microfiltration step. Its chemical structure was previously determined by different analytical techniques [11].
4 Conclusions
Water-soluble phenolic compounds extracted from P. frutescens, showed 4 anthocyanins, 6 flavones and 3 phenolic acids that were identified by various analytical means. Coumaroyl tartaric acid was characterized for the first time in water-extract of the various P. frutescens cvs studied in this work. This component was not reported before in published works undertaken on this medicinal plant. The phenol composition of 6 P. frutescens var. frutescens cvs grown in China in Guangdong, Shanghai, Yunnan and Fujian areas (2 of red-green type, 4 of green type), and 2 P. frutescens var. crispa grown in Liaoning area of China and in Japan, (red type) were compared. The 8 cvs were divided into 4 chemovars, considering their anthocyanin, phenolic acid and flavone contents. Among them, 3 red or red-green cvs, Pf1, Pf2 (var. frutescens) and Pf4 (var. crispa), represented an anthocyanin-rich chemovar. The red type cvs (var. crispa) from Liaoning area (Pf3) was an anthocyanin-contained and not phenolic-rich chemovar. The green type cvs (Pf6) from Yunnan (var. frutescens) was anthocyanin-lacking and phenolic-rich chemovar. The 3 green type cvs (var. frutescens) from different Chinese provinces were phenolic-poor chemovar (Pf5, Pf7 and Pf8). There is no obvious relationship between the phenolic composition and the botanical origin of P. frutescens, which may also be largely influenced by the agro-climatic growing conditions. These extracts could be used for colored water beverage or various antioxidant pharmaceutical/biomedical applications.
Acknowledgements
The authors wish to thank the French Embassy in China for providing a Ph.D. thesis grant for LM and CIRAD for its financial support. The authors thank the Research Polyphenols Platform (INRA-Montpellier) for some LC–MS analyses.


