1 Introduction
The ‘greening’ of global chemical processes has become a major issue in the chemical industry [1]. Organic reactions in water without using harmful organic solvents have attracted a great deal of interest in both academic and industrial research because, in addition to environmental concerns, there are beneficial effects of aqueous solvents on rates and selectivities of important organic transformations [2].
In many cases, significant beneficial effects, in terms of reaction rates and selectivity, have been observed for reactions between reagents that are insoluble in aqueous media and therefore take place under suspension in water; the term “on water” was coined by Sharpless et al. to refer to this type of reaction [3]. When the rate acceleration is negligible, the use of water as the only supporting medium has other advantages including ease of product isolation, safety, thanks to its high heat capacity and unique redox stability. The “on water” effect has been observed in different types of reactions, such as cycloadditions, pericyclic reactions, nucleophilic substitutions, and in particular, multicomponent reactions (MCR) [4].
MCR are very efficient synthetic methods. The strategy offers significant advantages over classical stepwise approaches allowing the formation of several bonds and the construction of complex molecular architectures from simple precursors in a single synthetic operation without the need for isolation of intermediates [5]. MCRs, particularly those performed in aqueous media, have become increasingly useful tools for the synthesis of chemically and biologically important compounds because of their environmentally friendly atom economy and green characteristics [6].
Pyrazolones and pyrazoles are an important class of bio-active drug targets in the pharmaceutical industry that exhibit a wide range of biological activities [7]. For example, pyrazole derivatives such as 4,4′-(arylmethylene)bis(3-methyl-1H-pyrazol-5-ols) have a broad spectrum of approved biological activity, being used as anti-inflammatory [8], antipyretic [9], gastric secretion stimulatory [10], antidepressant [11], antibacterial [12], and antifilarial agents [13]. Also these derivatives are applied as fungicides [14], pesticides [15], insecticides [16], and dyestuffs [17], and as the chelating and extracting reagents for different metal ions [18].
The conventional chemical approach to 4,4′-(arylmethylene)bis(3-methyl-1H-pyrazol-5-ols) involves the successive Knoevenagel synthesis of the corresponding arylidenepyrazolones and its base-promoted Michael reaction, and also one-pot tandem Knoevenagel–Michael reaction of arylaldehydes with 2 equiv of 3-methyl-1H-pyrazol-5-ol performed under a variety of reaction conditions [19]. However, most of the methods have one or more limitations that may include moderate yields, long reaction times, harsh reaction conditions, or tedious workup procedures. Also one limitation special for this method is two components of reaction; first 3-methyl-1H-pyrazol-5-ol should be synthesised from condensation hydrazine hydrate, ethyl acetoacetate and then condensation with aldehyde afforded 4,4′-(arylmethylene)bis(3-methyl-1H-pyrazol-5-ols).
Herein, we aimed to modify the reaction to make it more convenient and efficient. Since hydrazine hydrate and ethyl acetoacetate could be used instead of 3-methyl-1H-pyrazol-5-ol in above reaction, theoretically using these substrates could allow instead 3-methyl-1H-pyrazol-5-ol.
Therefore, we examined the reaction between ethyl acetoacetate 1, hydrazine hydrate 2, and aldehydes 3 in water using pyridine trifluoroacetate or acetic acid at 70 °C. Gratifyingly, this reaction worked well leading to the expected product 4 (Scheme 1).
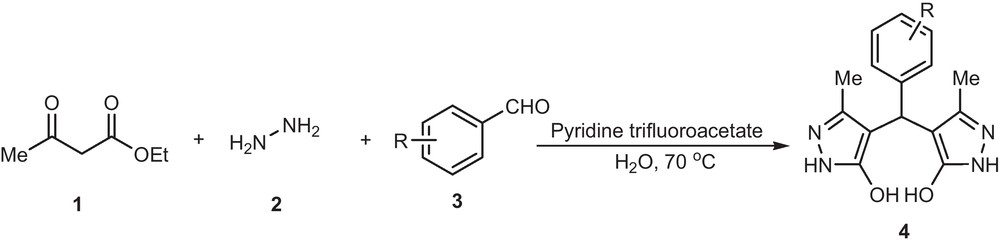
Synthesis of 4,4′-(arylmethylene)bis(3-methyl-1H-pyrazol-5-ols) derivatives.
2 Results and discussion
As a model reaction we first investigated the condensation of ethyl acetoacetate 1 (2 equiv) and hydrazine hydrate 2 (2 equiv) with 3-nitrobenzaldehyde 3 (1 equiv) under various conditions (Table 1). We first investigated the model reaction rate catalyzed by pyridine trifluoroacetate (20 mol%) in different solvents by measuring the isolated yield using identical amounts of reactants for a fixed reaction time of 5 h at reflux temperature of solvents. The desired product obtained in protic solvents such as water, ethanol, and methanol but water can afford the product in good yield even better than ethanol and methanol (Table 1, entries 1, 2 and 3). The desired product was scarcely obtained in aprotic or non-polar solvent as dichloromethane, benzene, and ethyl acetate, 1,4-dioxane and acetonitrile (Table 1, entries 4, 5, 6, 7 and 8). This effect can be explained by a simple acid-catalysis mechanism facilitated by the strong hydrogen bond interaction at the organic-water interface which stabilizes the reaction intermediate [20]. Also to evaluate the effect of catalyst concentration, the model reaction was carried out in the presence of different amounts of catalyst (10, 15, 20 and 25 mol%) at 70 °C. The result showed that 20 mol% of catalyst was sufficient to achieve a fairly high yield (Table 1, entry 1). It is worth mentioning here that during the optimization of this reaction we used acetic acid as acid catalyst and result was similar but pyridine trifluoroacetate was a little better (yield of reaction in pyridine trifluoroacetate was 1–3% more than when acetic acid was used). Therefore it was decided to employ this reagent for the synthesis of 4,4′-(arylmethylene)bis(3-methyl-1H-pyrazol-5-ols) derivatives 4.
Optimization of the reaction.
| Entry | Solvent | Temperature (°C) | Pyridine trifluoroacetate (mol%) | Yield (%) |
| 1 | Water | 70 | 20 | 95 |
| 2 | Ethanol | Reflux | 20 | 85 |
| 3 | Methanol | Reflux | 20 | 75 |
| 4 | Ethyl acetate | Reflux | 20 | 65 |
| 5 | Acetonitrile | Reflux | 20 | 60 |
| 6 | Benzene | Reflux | 20 | 70 |
| 7 | 1,4-dioxane | Reflux | 20 | 40 |
| 8 | Dichloromethane | Reflux | 20 | 20 |
| 9 | Water | 70 | 10 | 85 |
| 10 | Water | 70 | 15 | 90 |
| 11 | Water | 70 | 25 | 96 |
To explore the substrate scope, we examined a range various aromatic aldehydes under the optimized reaction conditions. The results are summarized in Table 2. The structures of the products were established by spectroscopic methods. In all cases, good yields were obtained, regardless of the nature of the substituent present on the aldehyde (electron-donating or electron-withdrawing). The independency of the reaction outcome to the electronic nature of aromatic aldehyde could be rationalized by high reactivity of pyrazolone (5) (for more details see Scheme 2). This confirms the reliability of the synthetic method.
Synthesis of 4,4′-(arylmethylene)bis(3-methyl-1H-pyrazol-5-ols) derivatives from condensation hydrazine hydrate, ethyl acetoacetate and aldehydes.
| Entry | Aldehyde | Time (h) | Product | Yield (%) |
| 1 | 12 | 85 | ||
| 2 | 12 | 90 | ||
| 3 | 13 | 75 | ||
| 4 | 5 | 87 | ||
| 5 | 11 | 75 | ||
| 6 | 10 | 85 | ||
| 7 | 12 | 95 | ||
| 8 | 15 | 94 | ||
| 9 | 15 | 95 | ||
| 10 | 13 | 80 |
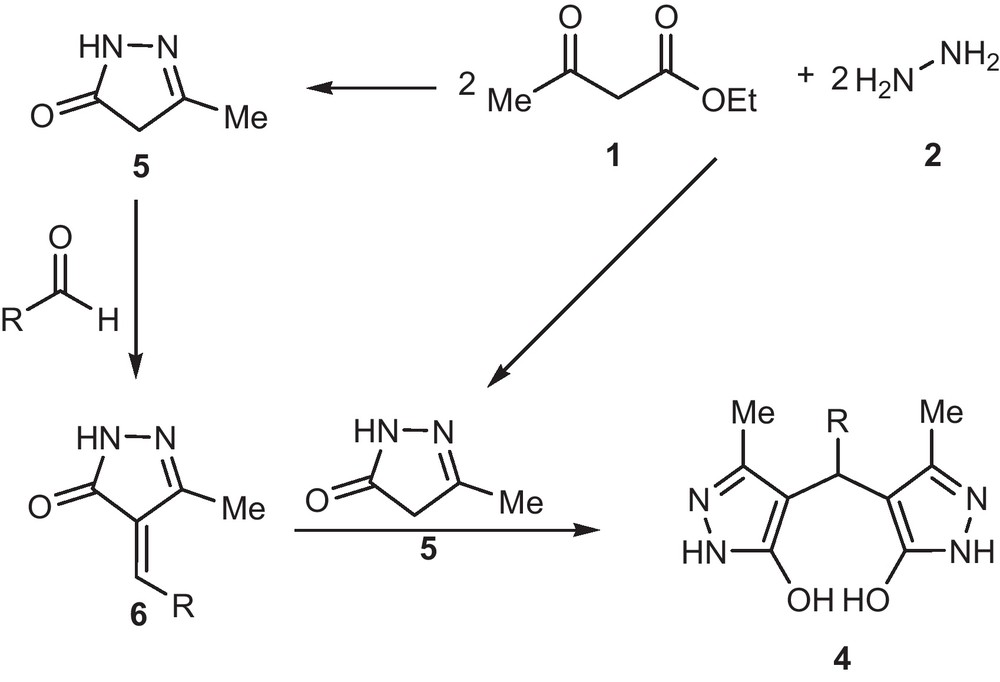
Proposed mechanism reaction.
Mechanistically, it is conceivable that the reaction involves the initial formation pyrazolone (5) by reaction between ethyl acetoacetate (2 equiv) and hydrazine hydrate (2 equiv). Afterwards the intermediate 5 undergoes Knoevenagel condensation with aldehyde 3 to afford activated alkene 6. Michael addition reaction activated alkene 6 with pyrazolone 5 to give 4,4′-(arylmethylene)bis(3-methyl-1H-pyrazol-5-ols) derivatives as shown in Scheme 2.
3 Conclusion
In conclusion, the efficient synthesis of 4,4′-(arylmethylene)bis(3-methyl-1H-pyrazol-5-ols) derivatives in water, in the presence of pyridine trifluoroacetate as catalyst has been described. A pseudo five-component reaction involving ethyl acetoacetate (2 equiv) and hydrazine hydrate (2 equiv), and an aromatic aldehyde (1 equiv) afforded the desired products with good yields. A wide range of aromatic aldehydes were tested, bearing either electron-donating or electron-withdrawing substituent's, suggesting that this method can be used to synthesize a large variety of compounds. It has therefore been demonstrated that using water as the solvent and is ideally suited for the synthesis of 4,4′-(arylmethylene)bis(3-methyl-1H-pyrazol-5-ols) derivatives.
4 Experimental
4.1 Materials and techniques
Melting points were taken on an Electrothermal 9100 apparatus and left uncorrected. IR spectra were obtained on a Shimadzu IR-470 spectrometer. Elemental analyses were performed using a Heraeus CHN elemental analyzer. 1H and 13C NMR spectra were recorded on a Bruker DRX-200 Avance spectrometer at 300 and 75 MHz. NMR spectra were obtained on solutions in DMSO using TMS as internal standard. All of the chemicals were purchased from Fluka, Merck and Aldrich and used without purification.
4.2 General procedure for the synthesis of pyrazole compounds 4
A solution of hydrazine hydrate (2 mmol), ethyl acetoacetate (2 mmol), and pyridine trifluoroacetate (0.2 mmol) in water (10 mL) was stirred at 70 °C. After 15 min, aldehyde (1 mmol) was added and the mixture stirred at 70 °C for appropriate time indicated in Table 1. After completion of the reaction, as indicated by TLC, the precipitated solid was filtered and washed with mixture water/ethanol (1:1) and products obtained as pure.
4.3 4-((5-Hydroxy-3-methyl-1H-pyrazol-4-yl)(4-nitrophenyl)methyl)-3-methyl-1H-pyrazol-5-ol (4a)
White powder (0.28 g, yield 85%). mp 275–278 °C. IR (KBr) (νmax/cm−1): 3405, 2922, 1706, 1598, 1482. MS, (m/z): 330 (M + 1), 241, 232, 135, 97. 1H NMR (300 MHz, DMSO-d6): δH (ppm) 2.12 (6H, s, 2CH3), 3.54 (2NH and 2OH exchanged with water of DMSO-d6), 5.00 (1H, s, CH), 7.40 (2H, d, 3JHH = 8.8 Hz, H-Ar), 8.13 (2H, d, 3JHH = 8.8 Hz, H-Ar). 13C NMR (75 MHz, DMSO-d6): δC (ppm) 10.3 (CH3), 33.0 (CH), 103.3, 123.0, 128.8, 140.0, 145.6, 151.7, 160.9 (C-Ar). Anal. Calcd for C15H15N5O4: C, 54.71; H, 4.59; N, 21.27. Found: C, 54.77; H, 4.49; N, 21.33.
4.4 4-((5-Hydroxy-3-methyl-1H-pyrazol-4-yl)(4-hydroxyphenyl)methyl)-3-methyl-1H-pyrazol-5-ol (4b)
White powder (0.27 g, yield 90%). mp 267–270 °C. IR (KBr) (νmax/cm−1): 3403, 2924, 1709, 1595, 1483. MS, (m/z): 301 (M + 1), 299, 267, 241, 203, 186, 115. 1H NMR (300 MHz, DMSO-d6): δH (ppm) 2.12 (6H, s, 2CH3), 4.78 (1H, s, CH), 5.54 (2NH and 3OH exchanged with water of DMSO-d6), 6.67 (2H, d, 3JHH = 8.4 Hz, H-Ar), 7.00 (2H, d, 3JHH = 8.4 Hz, H-Ar). 13C NMR (75 MHz, DMSO-d6): δC (ppm) 10.3 (CH3), 31.9 (CH), 104.8, 114.6, 128.3, 133.3, 140.1, 155.1, 161.1 (C-Ar). Anal. Calcd for C15H16N4O3: C, 59.99; H, 5.37; N, 18.66. Found: C, 59.96; H, 5.33; N, 18.71.
4.5 4-((5-Hydroxy-3-methyl-1H-pyrazol-4-yl)(4-methoxyphenyl)methyl)-3-methyl-1H-pyrazol-5-ol (4c)
White powder (0.23 g, yield 75%). mp 200–203 °C. IR (KBr) (νmax/cm−1): 3231, 2930, 2827, 1607, 1508. MS, (m/z): 315 (M + 1), 283, 241, 217, 97. 1H NMR (300 MHz, DMSO-d6): δH (ppm) 2.11 (6H, s, 2CH3), 3.72 (3H, s, OCH3), 4.79 (1H, s, CH), 6.80 (2H, d, 3JHH = 8.4 Hz, H-Ar), 7.05 (2H, d, 3JHH = 8.4 Hz, H-Ar), 11.82 (4H, brs, 2OH, 2NH). 13C NMR (75 MHz, DMSO-d6): δC (ppm) 10.3 (CH3), 31.7 (CH), 55.0 (OCH3), 104.6, 113.4, 128.3, 134.9, 140.1, 157.3, 169.9 (C-Ar). Anal. Calcd for C16H18N4O3: C, 61.13; H, 5.77; N, 17.82. Found: C, 61.17; H, 5.75; N, 17.87.
4.6 4-((5-Hydroxy-3-methyl-1H-pyrazol-4-yl)(3-nitrophenyl)methyl)-3-methyl-1H-pyrazol-5-ol (4d)
White powder (0.29 g, yield 87%). mp 288–291 °C. IR (KBr) (νmax/cm−1): 3400, 2920, 1700, 1598. MS, (m/z): 330 (M + 1), 241, 232, 135, 97. 1H NMR (300 MHz, DMSO-d6): δH (ppm) 2.16 (6H, s, 2CH3), 5.02 (1H, s, CH), 5.27 (2NH and 2OH exchanged with water of DMSO-d6), 7.58–8.02 (4H, m, H-Ar). 13C NMR (75 MHz, DMSO-d6): δC (ppm) 10.2 (CH3), 32.5 (CH), 103.4, 120.8, 121.9, 129.3, 134.6, 140.1, 145.6, 147.6, 160.9 (C-Ar). Anal. Calcd for C15H15N5O4: C, 54.71; H, 4.59; N, 21.27. Found: C, 54.73; H, 4.47; N, 21.31.
4.7 4-((5-Hydroxy-3-methyl-1H-pyrazol-4-yl)(3-hydroxyphenyl)methyl)-3-methyl-1H-pyrazol-5-ol (4e)
White powder (0.22 g, yield 75%). mp 209–211 °C. IR (KBr) (νmax/cm−1): 3404, 2923, 1706, 1598, 1483. MS, (m/z): 301 (M + 1), 299, 267, 241, 203, 186, 115. 1H NMR (300 MHz, DMSO-d6): δH (ppm) 2.09 (6H, s, 2CH3), 3.92 (2NH and 2OH exchanged with water of DMSO-d6), 4.74 (1H, s, CH), 6.56–7.02 (4H, m, H-Ar), 9.20 (H, brs, OH). 13C NMR (75 MHz, DMSO-d6): δC (ppm) 10.3 (CH3), 32.5 (CH), 104.3, 112.4, 114.5, 118.2, 138.5, 140.1, 144.7, 156.9, 161.1 (C-Ar). Anal. Calcd for C15H16N4O3: C, 59.99; H, 5.37; N, 18.66. Found: C, 59.93; H, 5.35; N, 18.65.
4.8 4-((5-Hydroxy-3-methyl-1H-pyrazol-4-yl)(2-nitrophenyl)methyl)-3-methyl-1H-pyrazol-5-ol (4f)
White powder (0.28 g, yield 85%). mp 237–240 °C. IR (KBr) (νmax/cm−1): 3403, 2923, 1706, 1593, 1483. MS, (m/z): 330 (M + 1), 241, 232, 135, 97. 1H NMR (300 MHz, DMSO-d6): δH (ppm) 2.07 (6H, s, 2CH3), 3.55 (2NH and 2OH exchanged with water of DMSO-d6), 5.44 (1H, s, CH), 7.40–7.66 (4H, m, H-Ar). Anal. Calcd for C15H15N5O4: C, 54.71; H, 4.59; N, 21.27. Found: C, 54.79; H, 4.46; N, 21.31.
4.9 4-((5-Hydroxy-3-methyl-1H-pyrazol-4-yl)(2-hydroxyphenyl)methyl)-3-methyl-1H-pyrazol-5-ol (4g)
White powder (0.28 g, yield 95%). mp 242–245 °C. IR (KBr) (νmax/cm−1): 3410, 2987, 1593, 1527. MS, (m/z): 301 (M + 1), 299, 267, 241, 203, 186, 115. 1H NMR (300 MHz, DMSO-d6): δH (ppm) 2.07 (6H, s, 2CH3), 3.43 (2OH exchanged with water of DMSO-d6), 5.08 (1H, s, CH), 6.63–7.50 (4H, m, H-Ar), 11.50 (H, brs, 2NH). 13C NMR (75 MHz, DMSO-d6): δC (ppm) 10.5 (CH3), 26.8 (CH), 104.2, 112.5, 118.2, 126.3, 129.3, 130.4, 140.0, 153.8, 161.5 (C-Ar). Anal. Calcd for C15H16N4O3: C, 59.99; H, 5.37; N, 18.66. Found: C, 59.91; H, 5.39; N, 18.66.
4.10 4-((2,4-dichlorophenyl)(5-hydroxy-3-methyl-1H-pyrazol-4-yl)methyl)-3-methyl-1H-pyrazol-5-ol (4h)
White powder (0.33 g, yield 94%). mp 267–270 °C. IR (KBr) (νmax/cm−1): 3423, 3109, 1607, 1521, 793. MS, (m/z): 355 (M + 2), 353 (M+), 278, 255, 241, 219, 175, 158, 112. 1H NMR (300 MHz, DMSO-d6): δH (ppm) 1.96 (6H, s, 2CH3), 5.01 (1H, s, CH), 7.32–7.53 (3H, m, H-Ar), 10.97 (4H, brs, 2NH, 2OH). 13C NMR (75 MHz, DMSO-d6): δC (ppm) 10.4 (CH3), 31.1 (CH), 101.8, 126.5, 128.3, 130.9, 131.9, 133.1, 138.6, 140.0, 160.5 (C-Ar). Anal. Calcd for C15H14Cl2N4O2: C, 51.01; H, 4.00; N, 15.86. Found: C, 51.11; H, 4.01; N, 15.75.
4.11 4-((5-hydroxy-3-methyl-1H-pyrazol-4-yl)(2-hydroxy-5-nitrophenyl)methyl)-3-methyl-1H-pyrazol-5-ol (4i)
White powder (0.33 g, yield 95%). mp 269–272 °C. IR (KBr) (νmax/cm−1): 3400, 2921, 1704, 1598. MS, (m/z): 347 (M + 1), 299, 248, 241, 217, 97. 1H NMR (300 MHz, DMSO-d6): δH (ppm) 2.11 (6H, s, 2CH3), 3.65 (2OH and 2NH exchanged with water of DMSO-d6), 5.04 (1H, s, CH), 6.99-7.41 (3H, m, H-Ar). 13C NMR (75 MHz, DMSO-d6): δC (ppm) 10.4 (CH3), 27.3 (CH), 102.8, 114.9, 123.5, 125.3, 131.2, 140.0, 141.0, 160.0, 161.0 (C-Ar). Anal. Calcd for C15H15N5O5: C, 52.17; H, 4.38; N, 20.28. Found: C, 52.20; H, 4.33; N, 20.29.
4.12 4-((furan-2-yl)(5-hydroxy-3-methyl-1H-pyrazol-4-yl)methyl)-3-methyl-1H-pyrazol-5-ol (4j)
White powder (0.22 g, yield 80%). mp 270–272 °C. IR (KBr) (νmax/cm−1): 3429, 1600, 1515. MS, (m/z): 275 (M + 2), 241, 178, 177, 175, 149. 1H NMR (300 MHz, DMSO-d6): δH (ppm) 2.05 (6H, s, 2CH3), 4.00 (2OH and 2NH exchanged with water of DMSO-d6), 4.84 (1H, s, CH), 5.95–8.66 (3H, m, H-Ar). 13C NMR (75 MHz, DMSO-d6): δC (ppm) 10.0 (CH3), 27.8 (CH), 102.4, 105.9, 110.2, 139.2, 141.0, 155.6, 160.6 (C-Ar). Anal. Calcd for C13H14N4O3: C, 56.93; H, 5.14; N, 20.43. Found: C, 56.96; H, 5.17; N, 20.38.
Acknowledgements
We gratefully acknowledge financial support from the Iran National Science Foundation (INSF) and Research Council of Razi University.


