1 Introduction
The discovery of new synthetic methodologies to improve the preparation of organic compounds is a pivotal point of research activity in the field of modern organic, bioorganic, and medicinal chemistry [1]. One approach to address this challenge involves the development of multicomponent reactions (MCR), in which three or more reactants are combined in a single reaction flask to generate a product, incorporating most of the atoms contained in the starting materials [2].
In recent years, the concept of “privileged medicinal scaffolds” [3] has emerged as one of the guiding principles of drug discovery process. It involves the utilization of molecular frameworks with inherent potential for biological activity. These privileged scaffolds commonly consist of a rigid hetero ring system that assigns well-defined orientation of appended functionalities for target recognition [4].
The chromene moiety often appears as an important structural element in both biologically active and natural compounds. It is widely performed in natural alkaloids, flavonoids, tocopherols, and anthocyanins [5]. Moreover, in recent years, functionalized chromenes have played an ever-increasing role in the synthetic approaches to promising compounds in the field of medicinal chemistry [6]. Among the different types of chromene systems, 2-amino-4H-chromenes (or 2-amino-4H-benzo[b]pyranes) are of particular utility as they belong to privileged medicinal scaffolds serving for the generation of small-molecule ligands with highly pronounced spasmolitic, diuretic, anticoagulant, and antianaphylactic activities [7]. The current interest in 2-amino-4H-chromene derivatives arises from their potential application in the treatment of human inflammatory TNFα-mediated diseases, such as rheumatoid and psoriatic arthritis, and in cancer therapy [8].
Among the nitrogen-containing heterocycles, functionally substituted 2-pyrazolin-5-ones have received considerable attention in the field of medicinal chemistry [9,10]. Thus, the N-methyl derivative of 3-methyl-1-phenyl-2-pyrazolin-5-one represents the first truly synthetic pain reliever, antipyrin, which is an approved non-steroidal anti-inflammatory drug (NSAID), possessing analgesic and antipyretic activities [11]. Different types of 4-substituted 3-methyl-2-pyrazolin-5-ones (or their hydroxy tautomers) have been reported as anticonvulsant [12], antidiabetic [13], neuroleptic [14], antihyperlipidemic [15], and gastric secretion stimulatory agents [16], as well as multidrug resistance modulators for cancer and antimicrobial therapy [17].
The modification of diverse biologically active 2-amino-4H-chromene systems with a pharmacologically active 3-methyl-2-pyrazolin-5-one fragment should combine the properties of both scaffolds and enhance their pharmacological activity.
There are three publications that deal with the synthesis of substituted 2-amino-4-(1H-pyrazol-4-yl)-4H-chromenes [18–20]. The first of them concerns electrocatalytic synthesis of the only 2-amino-4-(5-hydroxy-3-methyl-1H-pyrazol-4-yl)-4H-chromene-3-carbonitrile from salicylaldehyde, malononitrile, and 3-methyl-2-pyrazolin-5-one in an undivided cell in 86% yield (reaction time: 32 min) [18]. An analogous multicomponent indium trichloride-catalyzed process was reported later [19]. But, it is necessary to use sufficient quantities of expensive InCl3 (20 mol%), and the yields of substituted 2-amino-4-(1H-pyrazol-4-yl)-4H-chromene-3-carbonitriles are not very high, only in the range 79–85% (reaction time: 60–75 min). A four-component synthesis of substituted 2-amino-4-(1H-pyrazol-4-yl)-4H-chromene-3-carbonitriles from hydrazine hydrate, ethylacetoacetate, salicylaldehydes and malononitrile in water at ambient temperature under vigorous stirring (30 °C), in 74–92% yields, is also known [20]. But, when we repeated this four-component procedure, 2-amino-4-(5-hydroxy-3-methyl-1H-pyrazol-4-yl)-4H-chromene-3-carbonitrile was obtained in only 42% yield (NMR data). Even when we started from a three-component mixture of salicylaldehyde, malononitrile, and 3-methyl-2-pyrazolin-5-one, 2-amino-4-(5-hydroxy-3-methyl-1H-pyrazol-4-yl)-4H-chromene-3-carbonitrile was obtained in 63% yield (NMR data) and in 48% isolated yield.
Thus, all the known procedures for the synthesis of substituted 2-amino-4-(5-hydroxy-3-methyl-1H-pyrazol-4-yl)-4H-chromene-3-carbonitriles have their merits, but, the essence of a fast, facile, and convenient multicomponent solventless methodology is not yet known and remains to be developed.
Recently, we have accomplished a solvent-free cascade and multicomponent assembling of the 2-amino-4H-chromene scaffolds from salicylaldehydes and malononitrile [21], on the one hand, and from salicylaldehyde, malononitrile or cyanoacetate and nitroalkanes [22], on the other one.
Considering our preliminary results on the solvent-free transformation of C–H acids and salicylaldehydes as well as the certain biomedical application of 2-amino-4H-chromene derivatives mentioned above, we were prompted to design a convenient, fast and facile solvent-free methodology for the efficient multicomponent synthesis of functionalized 2-amino-4H-chromene system based on a multicomponent reaction of salicylaldehydes, malononitrile and 3-methyl-2-pyrazolin-5-one.
2 Results and discussion
As it follows from the introduction, we were prompted to design a fast, convenient, and facile solvent-free methodology for the efficient multicomponent synthesis of the functionalized 2-amino-4H-chromene systems based on a multicomponent reaction of salicylaldehydes, malononitrile and 3-methyl-2-pyrazolin-5-one.
Thus, in the present study, we report our results on the multicomponent transformation of salicylaldehydes 1a–h, malononitrile and 3-methyl-2-pyrazolin-5-one into substituted 2-amino-4-(1H-pyrazol-4-yl)-4H-chromenes 2a–h under solvent-free conditions by grinding in mortar (Scheme 1, Tables 1 and 2).
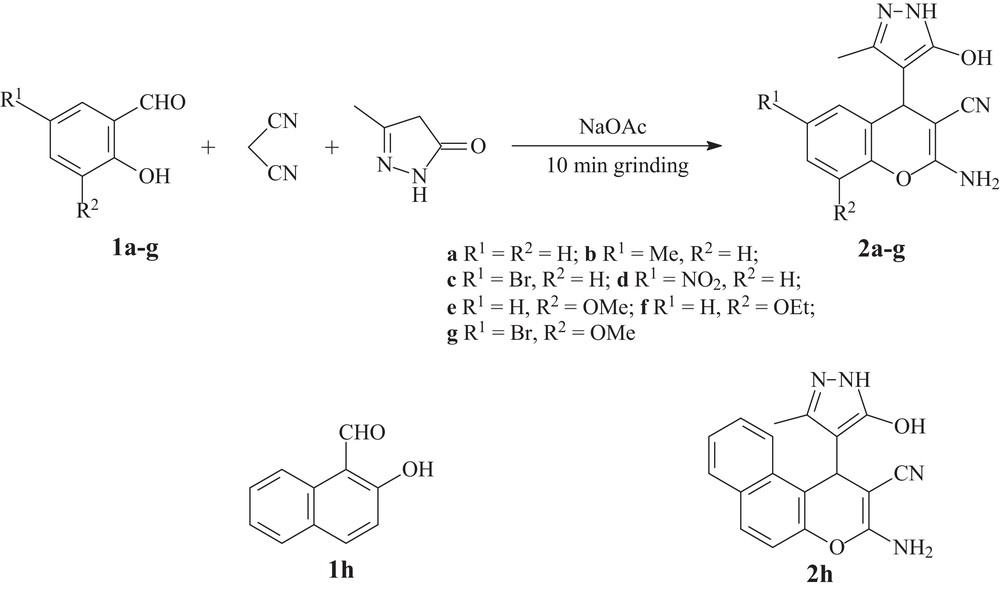
Multicomponent transformation of salicylaldehydes 1, malononitrile and 3-methyl-2-pyrazolin-5-ones into substituted 2-amino-4-(1H-pyrazol-4-yl)-4H-chromenes 2 by mortar grinding.
Multicomponent transformation of salycylaldehyde 1a, malononitrile and 3-methyl-2-pyrazolin-5-one into 2-amino-4-(1H-pyrazol-4-yl)-4H-chromene 2a by mortar grindinga.
| Entry | Aldehyde | Additive of water (mL) | Catalyst | Quantity of catalyst (mol%) | Reaction time (min) | Product | Yield (%)b |
| 1 | 1a | – | – | – | 10 | 2a | 21 |
| 2 | 1a | 1 | – | – | 10 | 2a | 43 |
| 3 | 1a | – | NaOAc | 5 | 10 | 2a | 55 |
| 4 | 1a | 1 | NaOAc | 5 | 10 | 2a | 81c |
| 5 | 1a | 1 | NaOAc | 10 | 10 | 2a | 95c |
| 6 | 1a | 1 | KF | 10 | 10 | 2a | 87c |
a 2 mmol of salycylaldehyde 1a, 2 mmol of malononitrile, 2 mmol of 3-methyl-2-pyrazolin-5-one.
b Yield by NMR data.
c Isolated yield.
Multicomponent transformation of salycylaldehydes 1a–h, malononitrile and 3-methyl-2-pyrazolin-5-one into 2-amino-4-(1H-pyrazol-4-yl)-4H-chromenes 2a–h by grinding in mortara.
| Entry | Aldehyde | Additive of water (mL) | Catalyst | Product | Yield (%)b |
| 1 | 1a | 1 | NaOAc | 2a | 95 |
| 2 | 1b | 1 | NaOAc | 2b | 91 |
| 3 | 1c | 1 | NaOAc | 2c | 90 |
| 4 | 1d | 1 | NaOAc | 2d | 92 |
| 5 | 1e | 1 | NaOAc | 2e | 93 |
| 6 | 1f | 1 | NaOAc | 2f | 96 |
| 7 | 1 g | 1 | NaOAc | 2g | 94 |
| 8 | 1 h | 1 | NaOAc | 2h | 91 |
a 2 mmol of salycylaldehyde 1a–h, 2 mmol of malononitrile, 2 mmol of 3-methyl-2-pyrazolin-5-one.
b Isolated yield.
The reaction of salicylaldehyde 1a, malononitrile and 3-methyl-2-pyrazolin-5-one without the catalyst by mortar grinding (entry 1, Table 1) resulted in the formation of 2-amino-4-(1H-pyrazol-4-yl)-4H-chromene 2a in 10 min, with only 21% yield.
Recently, we have found that the Knoevenagel condensation of isatins with malononitrile, which was performed by grinding at room temperature in the absence of any catalyst, but in the presence of 1–5 equivalents of water, resulted in formation of substituted (2-oxo-1,2-dihydro-3H-indol-3-ylidene)malononitriles in 89–99% yields as a result of the ‘on-water’ reaction [23].
‘On-water’ reactions are a group of organic reactions that take place as suspensions in water and that exhibit unusual reaction rate acceleration compared to the same reaction in an organic solvent or compared to the corresponding dry media reaction. This effect has been known for many years, but in 2005, the researchers in the group of Barry Sharpless presented a systematic study into this phenomenon [24].
Thus, the next experiment was carried out in the presence of 1 mL of water (entry 2, Table 1) and led to 2-amino-4H-chromene 2a formation in 43% yield.
In the presence of 5 mol% of NaOAc as a catalyst, 2-amino-4H-chromene 2a was obtained in 55% yield (Table 1, entry 3). Using 5 mol% of NaOAc and 1 mL of water led to 2a in good 79% yield (Table 1, entry 4). And at last, excellent 95% yield of 2a was obtained with 10 mol% of NaOAc and 1 mL of water in 10 min. KF was a slightly less effective catalyst in this multicomponent reaction compared to NaOAc.
Under the optimum conditions thus found (10 mol% of NaOAc as the catalyst, 1 mL of water, reaction time 10 min), substituted 2-amino-4-(1H-pyrazol-4-yl)-4H-chromenes 2a–h were obtained in excellent yields (90–96%) after a reaction time of only 10 min. As practically pure substituted 2-amino-4-(1H-pyrazol-4-yl)-4H-chromenes 2a–h were formed at the end of the reaction, the reaction mixture was only washed with water and dried under reduced pressure to isolate the pure 2-amino-4H-chromenes 2. Thus, in our solvent-free procedure, organic solvents were not used also on the isolation step in connection with current demands of “green chemistry”. So, the solvent-free procedure for the synthesis of substituted 2-amino-4-(1H-pyrazol-4-yl)-4H-chromenes 2a–h found by us is one step closer to the “ideal” synthesis [25].
With the above results taken into consideration and the mechanistic data on solvent-free cascade formation of the 2-amino-4H-chromene scaffold from salicylaldehydes and malononitrile [21] and also on ‘on-water’ Knoevenagel condensation of isatins with malononitrile [23], the following mechanism for the sodium acetate-catalyzed multicomponent transformation of salicylaldehydes 1, malononitrile and 3-methyl-2-pyrazolin-5-ones into substituted 2-amino-4-(1H-pyrazol-4-yl)-4H-chromenes 2 is proposed. The initiation step of the catalytic cycle begins with the deprotonation of a molecule of malononitrile by the action of sodium acetate, which leads to the formation of the anion of malononitrile A (Scheme 2).
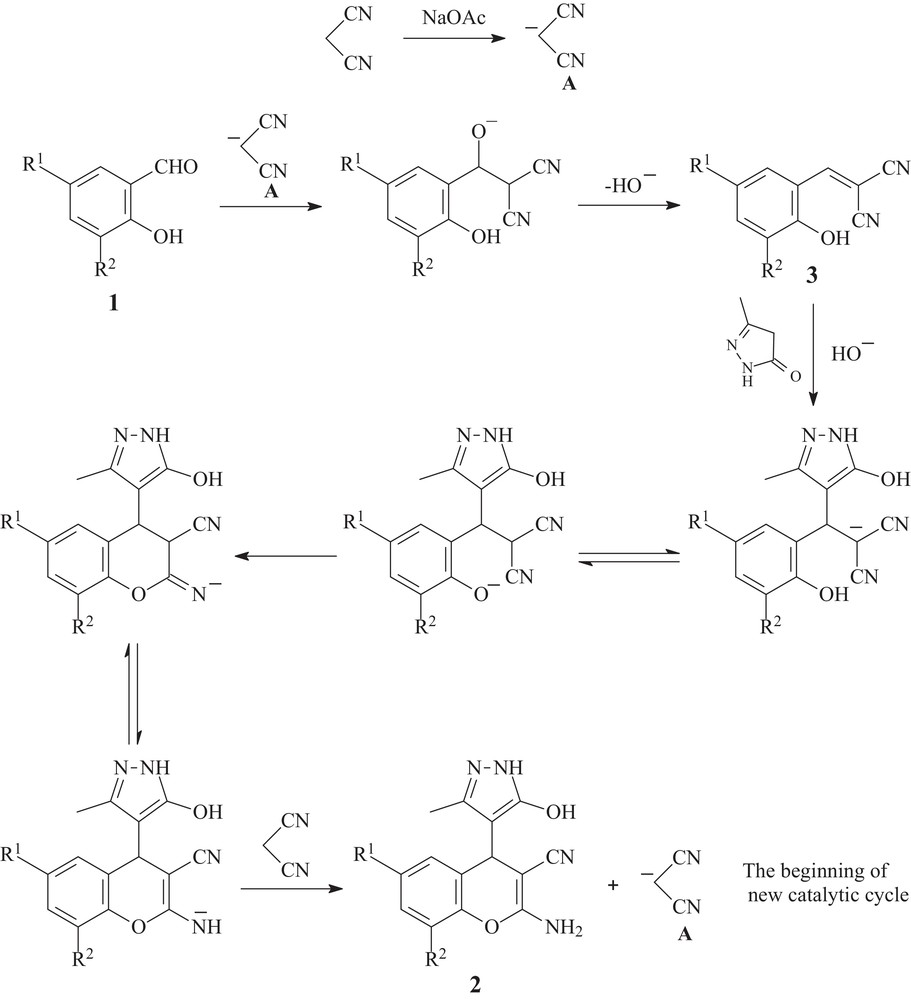
Mechanism of the multicomponent transformation of salicylaldehydes 1, malononitrile, and 3-methyl-2-pyrazolin-5-one into substituted 2-amino-4-(1H-pyrazol-4-yl)-4H- chromenes 2.
Then Knoevenagel condensation of the anion A with salicylaldehyde 1 takes place with the elimination of a hydroxide anion and the formation of a Knoevenagel adduct 3 [26]. The subsequent hydroxide-promoted Michael addition of 3-methyl-2-pyrazolin-5-one to the electron-deficient Knoevenagel adduct 3 leads to the corresponding 2-amino-4-(1H-pyrazol-4-yl)-4H-chromene 2 with the regeneration of the malononitrile anion A as the last step (Scheme 2).
The role of water in our multicomponent process is to accelerate all singular organic reactions. This effect of using water was known earlier [27]. Recently, it was shown that several uni- and bimolecular reactions are greatly accelerated when carried out in vigorously stirred aqueous suspensions [24]. The experiments were performed with one or two liquid, water-insoluble reaction partners or, occasionally, a mixture of one liquid and one solid. In spite of the absence of detailed kinetic experiments, the yields of the pure products after varying reaction times convincingly demonstrate that the rates are higher than those under solvent-free (“neat”) or homogeneous conditions [24].
3 Conclusions
Thus, sodium acetate as a catalyst can produce by mortar grinding, under mild conditions, in the presence of small quantities of water, a fast (10 min) and selective multicomponent transformation of salicylaldehydes, malononitrile and 3-methyl-2-pyrazolin-5-one into substituted 2-amino-4-(1H-pyrazol-4-yl)-4H-chromenes in excellent yields (90–96%). This new process opens an efficient and convenient multicomponent route to create substituted 2-amino-4-(1H-pyrazol-4-yl)-4H-chromenes – the promising compounds for the treatment of human inflammatory TNFα-mediated diseases and different biomedical applications. This catalytic procedure utilizes a simple equipment; it is easily carried out and is valuable from the viewpoint of environmentally benign diversity-oriented large-scale processes. This efficient and fast approach to substituted 2-amino-4-(1H-pyrazol-4-yl)-4H-chromenes represents a new synthetic concept for the multicomponent reactions that integrate solvent-free and ‘on-water’ reaction procedures, and allows combining the synthetic virtues of solvent-free MCR with the ecological benefits of ‘on-water’ reactions and convenience of the sodium acetate-catalyzed procedure.
4 Experimental
4.1 General remarks
All the melting points were measured with a Gallenkamp melting point apparatus and are uncorrected. 1H and 13C NMR were recorded with a Bruker AM300 at ambient temperature in DMSO-d6 solutions. Chemical shifts values are given in δ scale relative to Me4Si. IR spectra were registered with a Bruker ALPHA-T FT–IR spectrometer in KBr pellets. Mass-spectra (EI, 70 eV) were obtained directly using a Finningan MAT INCOS 50 spectrometer. HRMS (ESI) was measured on a Bruker micrOTOF II instrument; external or internal calibration was done with Electrospray Calibrant Solution (Fluka). All the starting materials were obtained from commercial sources and used without further purification.
4.2 General procedure
A mixture of salicylaldehyde (2 mmol), malononitrile (0.13 g, 2 mmol), 3-methyl-2-pyrazolin-5-one (0.20 g, 2 mmol), catalyst (10 mol%) and water (1 mL) was taken in a porcelain mortar (maximum diameter: 80 mm, minimum diameter: 40 mm) and mixed thoroughly with a porcelain pestle (diameter: 30 mm), followed by grinding for 10 min. The resulting mixture was air dried to cause crystallization of the product. Crude solid was then put on filter, rinsed with water (2 × 2 mL) and dried with water pump.
4.2.1 2-Amino-4-(5-hydroxy-3-methyl-1H-pyrazol-4-yl)-4H-chromene-3-carbonitrile (2a)
White solid (0.51 g, yield 95%); mp 218–219 °C (lit [18] mp 218–219 °C); 1H–NMR (DMSO-d6): 1.97 (s, 3H, CH3), 4.63 (s, 1H, CH), 6.67 (s, 2H, NH2), 6.95 (d, J = 8.1 Hz, 1H, Ar), 7.01–7.03 (m, 2H, Ar), 7.15–7.20 (m, 1H, Ar), 10.43 (br s, 2H, NH+OH) ppm.
4.2.2 2-Amino-4-(5-hydroxy-3-methyl-1H-pyrazol-4-yl)-6-methyl-4H-chromene-3-carbonitrile (2b)
White solid (0.51 g, yield 91%); mp 226–228 °C; 1H–NMR (DMSO-d6): 1.97 (s, 3H, CH3), 2.18 (s, 3H, CH3), 4.59 (s, 1H, CH), 6.64 (s, 2H, NH2), 6.81 (s, 1H, Ar), 6.85 (d, J = 8.3 Hz, 1H, Ar), 6.99 (d, J = 8.3 Hz, 1H, Ar), 10.47 (br s, 2H, NH+OH) ppm; 13C NMR (DMSO-d6): 9.9, 20.2, 28.7, 55.1, 105.1, 115.3, 120.9, 123.1, 128.1, 128.9, 133.2, 136.6, 146.4, 159.1, 160.2 ppm; IR (KBr): ν = 3464, 3428, 3359, 2187, 1658, 1610, 1589, 1534, 1495, 1403 cm−1; MS (m/z, relative intensity %): 282 ([M]+, 19), 267 (5), 184 (100), 157 (60), 140 (14), 129 (31), 115 (15), 102 (33), 98 (81), 77 (43); HRMS (ESI): 283.1190 [M+H]+, calcd for C15H15N4O2: 283.1195.
4.2.3 2-Amino-6-bromo-4-(5-hydroxy-3-methyl-1H-pyrazol-4-yl)-4H-chromene-3-carbonitrile (2c)
White solid (0.62 g, yield 90%); mp 234–236 °C; 1H–NMR (DMSO-d6): 2.00 (s, 3H, CH3), 4.63 (s, 1H, CH), 6.75 (s, 2H, NH2), 6.94 (d, J = 8.7 Hz, 1H, Ar), 7.13 (d, J = 2.3 Hz, 1H, Ar), 7.34 (dd, J1 = 8.7 Hz, J2 = 2.3 Hz, 1H, Ar), 10.63 (br s, 2H, NH+OH) ppm; 13C NMR (DMSO-d6): 9.8, 28.6, 54.8, 104.5, 115.6, 118.0, 120.5, 126.2, 130.4, 131.2, 136.7, 147.8, 159.0, 159.8 ppm; IR (KBr): ν = 3456, 3353, 2975, 2190, 1659, 1607, 1573, 1534, 1478, 1402 cm−1; MS (m/z, relative intensity %): 347 ([M]+, 8), 345 ([M]+, 7), 281 (2), 250 (75), 223 (54), 175 (9), 141 (17), 127 (4), 114 (100), 98 (63), 88 (32); HRMS (ESI): 347.0138 [M+H]+, calcd for C14H12BrN4O2: 347.0144.
4.2.4 2-Amino-4-(5-hydroxy-3-methyl-1H-pyrazol-4-yl)-6-nitro-4H-chromene-3-carbonitrile (2d)
Yellow solid (0.58 g, yield 92%); mp 245–247 °C; 1H–NMR (DMSO-d6): 2.08 (s, 3H, CH3), 4.77 (s, 1H, CH), 6.95 (s, 2H, NH2), 7.22 (d, J = 9.0 Hz, 1H, Ar), 7.87 (d, J = 2.7 Hz, 1H, Ar), 8.07 (dd, J1 = 9.0 Hz, J2 = 2.7 Hz, 1H, Ar), 10.61 (br s, 2H, NH+OH) ppm; 13C NMR (DMSO-d6): 9.8, 28.7, 55.1, 104.4, 117.1, 120.1, 123.4, 124.7, 125.0, 136.9, 143.5, 153.1, 159.1, 159.2 ppm; IR (KBr): ν = 3425, 3363, 2977, 2199, 1665, 1581, 1518, 1401, 1332, 1253 cm−1; MS (m/z, relative intensity %): 313 ([M]+, 2), 246 (3), 215 (39), 185 (7), 158 (8), 141 (7), 114 (43), 98 (56), 66 (38), 39 (100); HRMS (ESI): 314.0884 [M+H]+, calcd for C14H12N5O4: 314.0889.
4.2.5 2-Amino-4-(5-hydroxy-3-methyl-1H-pyrazol-4-yl)-8-methoxy-4H-chromene-3-carbonitrile (2e)
White solid (0.56 g, yield 93%); mp 219–221 °C; 1H–NMR (DMSO-d6): 1.95 (s, 3H, CH3), 3.79 (s, 3H, OCH3), 4.61 (s, 1H, CH), 6.57 (d, J = 7.5 Hz, 1H, Ar), 6.66 (s, 2H, NH2), 6.86 (d, J = 7.7 Hz, 1H, Ar), 6.93–6.98 (m, 1H, Ar), 10.49 (br s, 2H, NH+OH) ppm; 13C NMR (DMSO-d6): 10.0, 28.8, 55.1, 55.6, 104.8, 110.1, 120.1, 120.9, 123.9, 124.4, 136.9, 137.9, 146.7, 159.0, 160.1 ppm; IR (KBr): ν = 3469, 3383, 2976, 2185, 1651, 1580, 1536, 1481, 1410, 1277 cm−1; HRMS (ESI): 299.1140 [M+H]+, calcd for C15H15N4O3: 299.1144.
4.2.6 2-Amino-8-ethoxy-4-(5-hydroxy-3-methyl-1H-pyrazol-4-yl)-4H-chromene-3-carbonitrile (2f)
White solid (0.60 g, yield 96%); mp 226–228 °C; 1H–NMR (DMSO-d6): 1.35 (t, J = 6.9 Hz, 3H, CH3), 1.96 (s, 3H, CH3), 4.08 (q, J = 6.9 Hz, 2H, OCH2), 4.60 (s, 1H, CH), 6.56 (d, J = 7.5 Hz, 1H, Ar), 6.63 (s, 2H, NH2), 6.86 (d, J = 7.6 Hz, 1H, Ar), 6.91–6.96 (m, 1H, Ar), 10.51 (br s, 2H, NH+OH) ppm; 13C NMR (DMSO-d6): 9.9, 14.7, 28.9, 55.1, 64.1, 105.0, 111.5, 120.1, 120.9, 123.9, 124.4, 136.6, 138.2, 145.9, 159.1, 160.1 ppm; IR (KBr): ν = 3498, 3349, 2978, 2183, 1660, 1581, 1530, 1475, 1411, 1275 cm−1; MS (m/z, relative intensity %): 312 ([M]+, 11), 283 (4), 246 (22), 214 (80), 186 (100), 169 (16), 158 (45), 130 (13), 103 (12), 98 (20); HRMS (ESI): 313.1295 [M+H]+, calcd for C16H17N4O3: 313.1301.
4.2.7 2-Amino-6-bromo-4-(5-hydroxy-3-methyl-1H-pyrazol-4-yl)-8-methoxy-4H-chromene-3-carbonitrile (2g)
White solid (0.71 g, yield 94%); mp 229–232 °C; 1H–NMR (DMSO-d6): 2.01 (s, 3H, CH3), 3.83 (s, 3H, OCH3), 4.60 (s, 1H, CH), 6.71 (s, 1H, Ar), 6.77 (s, 2H, NH2), 7.07 (s, 1H, Ar), 10.68 (br s, 2H, NH+OH) ppm; 13C NMR (DMSO-d6): 9.9, 28.7, 54.8, 56.1, 104.4, 113.3, 115.2, 120.5, 122.2, 126.1, 136.8, 137.4, 147.8, 158.9, 159.7 ppm; IR (KBr): ν = 3473, 3416, 2973, 2188, 1652, 1613, 1595, 1528, 1486, 1415 cm−1; HRMS (ESI): 377.0244 [M+H]+, calcd for C15H14BrN4O3: 377.0249.
4.2.8 3-amino-1-(5-hydroxy-3-methyl-1H-pyrazol-4-yl)-1H-benzo[f]chromene-2-carbonitrile (2h)
White solid (0.58 g, yield 91%); mp 221–223 °C; 1H–NMR (DMSO-d6): 1.90 (s, 3H, CH3), 5.09 (s, 1H, CH), 6.75 (s, 2H, NH2), 7.22 (d, J = 8.9 Hz, 1H, Ar), 7.38–7.51 (m, 2H, Ar), 7.82–7.89 (m, 2H, Ar), 7.96 (d, J = 8.4 Hz, 1H, Ar), 10.57 (br s, 2H, NH+OH) ppm; 13C NMR (DMSO-d6): 9.7, 26.7, 56.5, 105.2, 115.2, 116.5, 120.9, 123.3, 124.7, 126.8, 128.4, 128.7, 130.5, 130.7, 136.7, 146.4, 158.6, 159.6 ppm; IR (KBr): ν = 3462, 3418, 3334, 2973, 2189, 1660, 1591, 1518, 1409, 1234 cm−1; MS (m/z, relative intensity %): 318 (2), 220 (85), 193 (94), 164 (74), 138 (34), 127 (16), 114 (15), 98 (91), 63 (41), 39 (100); HRMS (ESI): 319.1192 [M+H]+, calcd for C18H15N4O2 319.1195.
Acknowledgments
The authors gratefully acknowledge the financial support of the Russian Foundation for Basic Research (Project No. 13-03-00096а).


