1 Introduction
The transition metals in group five and six, such as V, Mo and W, are well known to form isopolyoxometalates (isopolyanions) by dehydrative condensation of the monooxoanions and heteropolyoxometalates (heteropolyanions) by involving a third element e.g. P or Si. These materials have attracted increasing attention worldwide because of their particularly interesting nanosized structures and their potential applications in many fields such as catalysis, pharmacology, medicine, nanotechnology, and molecular electronics [1–5].
Due to these reasons, many works containing the Anderson-Evans anion [TeMo6O24]6− (Fig. 1) have been devoted to the study of mixed molybdotellurate. However, the literature gives few works and partial results of compounds containing at the same time [TeMo6O24]6− cluster and (Te(OH)6) units: Rb6(TeMo6O24).Te(OH)6.6H2O [6], Cs6(TeMo6O24).2Te(OH)6.4H2O [7], (NH4)6(TeMo6O24).Te(OH)6.7H2O [8], Li6(TeMo6O24).Te(OH)6).18H2O [9]. As a continuation of these works, we report here, the results of a structure refinement of K5(NH4)(TeMo6O24)(Te(OH)6) .6(H2O). The compound is built up of a [TeMo6O24]6− cluster and a Te(OH)6 group linked to three potassium and ammonium cations which coordinated with six water molecules.

A polyhedral representation of the Anderson-Evans anion [TeMo6O24]6– in 1.
2 Experimental
2.1 Materials and measurements
KOH (98%), (NH4)6Mo7O24.4H2O (99%) and Te(OH)6 (99%) were purchased from Sigma-Aldrich and used without further purification. The infrared spectra were recorded as KBr pellets, in the 4000–400 cm-1 range on a Nicolet 470 FTIR spectrophotometer (resolution: 0.125 cm−1). The UV–visible absorption spectrum was recorded on a Perkin-Elmer Lambda 19 spectrophotometer. Cyclic voltammetry analyzes were carried out on a CHI 660B electrochemical station using a conventional three electrode single compartment cell. The working electrode was a glassy carbon, platinum gauze was used as counter electrode, and the Ag/AgCl (saturated KCl) was the reference electrode. The measurements were performed at room temperature and the cell was briefly deoxygenated with argon before each scan.
2.2 Synthesis
Single crystals of K5NH4[TeMo6O24].Te(OH)6.6H2O were obtained from an aqueous solution of KOH, (NH4)6Mo7O24.4H2O and Te(OH)6 with a K: Mo: Te molar ratio of 1:3:1. The resulting mixture was stirred and the pH was adjusted to 3.5 with acetic acid. The solution was kept for 2 weeks at ambient conditions, and then colorless block crystals suitable for X-ray crystallography were obtained. The semi-quantitative energy-dispersive spectroscopy (EDS) analysis of one of the colorless crystals obtained as mentioned above was performed with a JEOL-JSM 5400 scanning electron microscope; it revealed the presence of K, Te, Mo, N and O elements. Anal. calc. for K5NH4[TeMo6O24].Te(OH)6.6H2O (%): K, 11.93; Te, 15.57; Mo, 35.13 Found (%): K, 11.78; Te, 15.64; Mo, 35.21.
3 Results and discussion
3.1 X-ray data collection
A suitable crystal with dimensional (0.10 mm–0.10 mm–0.25 mm) was chosen for X-ray diffraction studies. The intensities of the diffraction data were measured using an Enraf-Nonius CAD-4 diffractometer with monochromated graphite Mo Ka radiation (λ = 0.71069Å) [10] at 293 K [10]. The numbers of collected and independent reflections were, respectively, 4430 and 3854. Unit cell dimensions were obtained by least-square refinement of the angular settings in the 2.16 < θ < 26.98°. The reflections were corrected for Lorentz and polarization effects; an empirical absorption correction was also applied using psi-scan data [11].
The structure was successfully developed in the centrosymmetric space group C2/c (No. 15), solved by the Patterson method using SHELXS-97 [12] and refined with anisotropic temperature factors for non-hydrogen atoms, by full matrix least-squares based on F2 using SHELXL-97 [13] program included in the WINGX software package [14]. Refinement of all atoms, except of nitrogen (N) led to R = 0.066 and RW= 0.167. Moreover, the displacement parameters led us to consider that the four K3 atoms of the cell are spread randomly over eight positions. At the stage, the difference synthesis map shows residual peaks at 0.5, –0.289, and 0.75 which are attributed to the nitrogen N. The refinement of the occupancy factors of the two N and K3 was then performed leading to 50% and 50% for N and K, respectively, and to an improvement of the agreement factors: R = 0.033 and RW = 0.087 for 3432 unique observed reflexions [I > 2σ(I)], the corresponding formula is thus K5NH4[TeMo6O24].Te(OH)6.6H2O.
Besides, the semi-quantitative energy-dispersive spectroscopy (EDS) analysis of one of colorless crystals obtained as mentioned above was performed with a JEOL-JSM 5400 scanning electron microscope; it revealed the presence of N, K, Te, Mo and O elements. The positions of the hydrogen atoms attached to oxygen water were determined from a difference Fourier map and were refined isotropically. The details of the data collection and structure refinements for K5NH4[TeMo6O24].Te(OH)6.6H2O are listed in Table 1. The final atomic coordinates are given in Table 2; for geometrical parameters see Table 3.
Crystal structure data for K5NH4[TeMo6O24].Te(OH)6.6H2O.
| Crystal data | |
| Compound | CSD-number |
| Formula/formula weight | K5NH4[TeMo6O24].Te(OH)6.6H2O/1638.53 |
| Space group | C2/c |
| Crystal system | Monoclinic |
| Z | 4 |
| Lattice parameters | a = 18.6841(1) Å |
| b = 10.0513(1) Å; β = 116.495(1)° | |
| c = 21.1065(1) Å | |
| Volume | 3547.49(4) Å3 |
| Calculated density (g/cm3) | 3.068 |
| Absorption coefficient, μ (mm−1) | 4.372 |
| F0 0 0 | 3072 |
| Crystal size (mm3)/color | 0.1 × 0.1 × 0.1/colorless |
| Intensity measurement | |
| Diffractometer | Enraf-Nonius CAD4 |
| Monochromator Graphite | Wavelength [Ka(Mo)] l = 0.71073 Å |
| Temperature | 293(2) K |
| Theta range | 2.16°/26.98° |
| h, k, l range | –23/1, –1/12, –24/26 |
| Number of measured reflexions | 4430 |
| Number of independent reflexions [Rint =] | 3854 [0,031] |
| Structure determination | |
| Unique reflexion included (I > 2σ(I)) | 3432 |
| Number of refined parameters | 249 |
| Absorption correction | Ψ-scan |
| Tmin, Tmax | 0.510, 0.945 |
| R, Rw[F2> 2σ(F2)] | 0.033, 0.088 |
| Weights | w = 1/[s2(Fo2) + (0.0398P)2 + 25.3973P] where P = (Fo2 + 2Fc2)/3 |
| Extinction coefficient | 0.00195(7) |
| Δρmin/Δρmax (e/Å3) | –1.177/1.047 |
| Largest shift/error | 0.001 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atomes | x | y | z | Occupancy | Uéq |
| Mo1 | 0.0471 (1) | 0.1048 (1) | 0.3750 (1) | 1 | 0.0217 (1) |
| Mo2 | 0.1844 (1) | 0.1002 (1) | 0.5406 (1) | 1 | 0.0206 (1) |
| Mo3 | 0.8624 (1) | 0.0046 (1) | 0.3325 (1) | 1 | 0.0220 (1) |
| Te1 | 0 | 0 | 0.5 | 1 | 0.0141 (1) |
| Te2 | 0.5 | 0 | 0.5 | 1 | 0.0291 (1) |
| K1 | 0.3661 (1) | –0.1379 (2) | 0.7245 (1) | 1 | 0.0542 (4) |
| K2 | 0.2687 (1) | 0.0039 (1) | 0.3713 (2) | 1 | 0.0663 (5) |
| K3 | 0.3419 (2) | 0.7755 (1) | 0.5312 (1) | 0.5 | 0.0686 (11) |
| N | 0.4746 (3) | –0.2882 (3) | 0.7371 (3) | 0.5 | 0.0493 (4) |
| O1 | 0.1177 (2) | 0.2066 (3) | 0.4588 (2) | 1 | 0.0228 (7) |
| O2 | 0.0815 (3) | –0.0363 (2) | 0.4695 (3) | 1 | 0.0183 (6) |
| O3 | –0.0419 (2) | 0.1264 (2) | 0.4227 (2) | 1 | 0.0178 (6) |
| O4 | –0.0770 (3) | –0.1237 (2) | 0.4338 (2) | 1 | 0.0185 (6) |
| O5 | –0.0371 (4) | –0.0269 (3) | 0.3273 (4) | 1 | 0.0259 (7) |
| O6 | 0.8013 (3) | 0.0313 (2) | 0.3853 (3) | 1 | 0.0253 (7) |
| O7 | 0.2367 (2) | 0.2277 (3) | 0.5954 (2) | 1 | 0.0335 (9) |
| O8 | 0.0076 (4) | 0.2413 (2) | 0.3232 (4) | 1 | 0.0343 (9) |
| O9 | 0.2467 (2) | 0.0242 (2) | 0.5123 (2) | 1 | 0.0343 (9) |
| O10 | 0.1149 (3) | 0.0403 (1) | 0.3504 (3) | 1 | 0.0381 (10) |
| O11 | 0.8107 (3) | –0.1277 (4) | 0.2803 (2) | 1 | 0.0363 (9) |
| O12 | 0.8284 (3) | 0.1418 (2) | 0.2794 (2) | 1 | 0.0349 (9) |
| O13 | 0.5930 (2) | –0.0449 (2) | 0.4884 (2) | 1 | 0.0557 (14) |
| H1 | 0.5920 | –0.1212 | 0.4591 | 1 | 0.2000 |
| O14 | 0.4312 (2) | –0.1104 (3) | 0.4228 (3) | 1 | 0.0524 (13) |
| H2 | 0.4380 | –0.0010 | 0.4211 | 1 | 0.2000 |
| O15 | 0.4816 (4) | 0.1389 (3) | 0.4310 (2) | 1 | 0.0512 (12) |
| H3 | 0.4572 | 0.2085 | 0.4415 | 1 | 0.2000 |
| OW1 | 0.3540 (4) | –0.0904 (3) | 0.7928 (3) | 1 | 0.0667 (16) |
| H11 | 0.4065 | –0.1244 | 0.8245 | 1 | 0.2000 |
| H12 | 0.3245 | –0.1591 | 0.7590 | 1 | 0.2000 |
| OW2 | 0.7064 (3) | 0.2522 (3) | 0.3642 (2) | 1 | 0.0898 (23) |
| H21 | 0.6613 | 0.2388 | 0.3182 | 1 | 0.2000 |
| H22 | 0.7467 | 0.1859 | 0.3702 | 1 | 0.2000 |
| OW3 | 0.4387 (3) | –0.0344 (2) | 0.6690 (2) | 1 | 0.1561 (5) |
| H31 | 0.4719 | –0.0932 | 0.7073 | 1 | 0.2000 |
| H32 | 0.4749 | 0.0191 | 0.6580 | 1 | 0.2000 |
Selected bond lengths (Å).
| Bond lengths | |||||
| Mo1-O1 | 1.697 (4) | Te1–O2ii | 1.933 (3) | K1–O12ix | 2.731 (5) |
| Mo1-O8 | 1.704 (4) | Te1–O2 | 1.933 (3) | K1–OW3 | 2.763 (1) |
| Mo1-O5 | 1.957 (4) | Te1–O3ii | 1.936 (3) | K1–OW1 | 2.773 (6) |
| Mo1-O1 | 1.958 (4) | Te1–O3 | 1.936 (3) | K1–O8viii | 2.808 (5) |
| Mo1-O2 | 2.292 (3) | Te1–O4 | 1.942 (3) | K1–O7 | 2.864 (5) |
| Mo1-O3 | 2.308 (3) | Te1–O4ii | 1.942 (3) | K1–O8i | 3.190 (5) |
| K1–O11iii | 3.262 (5) | ||||
| K1–O6iii | 3.403 (4) | ||||
| Mo2-O9 | 1.707 (4) | Te2–O14iii | 1.915 (5) | K2–O10 | 2.730 (4) |
| Mo2-O7 | 1.710 (4) | Te2–O14 | 1.915 (4) | K2–OW2iv | 2.760 (7) |
| Mo2-O1 | 1.939 (4) | Te2–O13iii | 1.914 (4) | K2–O7i | 2.800 (5) |
| Mo2-O6iii | 1.971 (4) | Te2–O13 | 1.914 (5) | K2–OW1xi | 2.897 (7) |
| Mo2-O2 | 2.294 (3) | Te2–O15iii | 1.933 (5) | K2–O13iii | 2.963 (6) |
| Mo2-O4ii | 2.309 (3) | Te2–O15 | 1.933 (5) | K2–O14 | 2.963 (5) |
| K2–O11v | 3.188 (5) | ||||
| K2–O9 | 3.188 (5) | ||||
| K2–O12v | 3.191 (5) | ||||
| Mo3-O12 | 1.711 (4) | K3–OW2x | 2.746 (1) | ||
| Mo3-O11 | 1.723 (4) | K3–O2i | 2.802 (5) | ||
| Mo3-O6 | 1.937 (4) | K3–O9vi | 2.989 (6) | ||
| Mo3-O5vii | 1.954 (4) | K3–O13x | 3.071 (7) | ||
| Mo3-O3vii | 2.297 (3) | K3–O15x | 3.146 (7) | ||
| Mo3-O4vii | 2.314 (3) | K3–OW3vi | 3.268 (13) | ||
| K3–OW2xii | 3.306 (9) | ||||
| K3–O9i | 3.363 (6) |
3.2 Structure description
Single-crystal X-ray diffraction analysis reveals that compound 1 crystallizes in the monoclinic space group C2/c. In the basic structural unit of 1, there is an Anderson anion clusters (TeMo6O24)6− and Te(OH)6 group linked to three potassium and ammonium cations which coordinated with six water molecules. Bond valence sum calculations indicate [15] that all the molybdenum atoms and tellurium atoms are hexavalent, all potassium atoms are monovalent, O13, O14 and O15 are OH groups and OW1-OW3 are water molecules. All other oxygen atoms have values close to 2. A projection of the structure, showing the displacement ellipsoids, is presented in Fig. 2.

Asymmetric unit of the title compound showing the 50% probability displacement ellipsoids.
The (TeMo6O24)6− cluster was characterized for the first time in 1948 [16], but its structure had been already predicted in 1937 [17]. The Anderson structure consists of a planar arrangement of six edge-sharing MoO6 polyhedra around the central tellurium atom. The six molybdenum atoms form a regular hexagon. The central TeVI atom is surrounded octahedrally by six oxygen atoms. The Te-O bond lengths and O-Te-O bond angles differ only slightly in the compounds and are in good agreement with those bond lengths and angles found in other compounds containing the (TeMo6O24)6− anion [18,19].
The oxygen atoms of the anion can be divided into three groups: terminal oxygen atoms (Ot) with short Mo-O-bond lengths, molybdenum-bridging oxygen atoms (Ob) with middle Mo-O bond lengths and oxygen atoms bonded to one tellurium and two molybdenum atoms (Oc) bearing long Mo-O bond lengths. As expected, the terminal oxygen atoms show the shortest Mo-O bond lengths between 1.697 (3) and 1.722 (4) Å. The intermediate Mo-O bonds exhibit Mo-O distances between 1.937 (1) and 1.971 (1) Å. The two long Mo-O bonds are located trans to the short Mo-O bonds and show Mo-O distances between 2.292 (3) and 2.3147 (3) Å. The Mo–Mo distances lie in the 3.266 (2) - 3.353 (1) Å range and the Te–Mo distances are in the 3.279 (1) - 3.347 (3) Å range. Each (TeMo6O24)6− cluster is joined to the telluric acid molecule Te(OH)6 through hydrogen bonds forming infinite chains and all oxygen atoms of the Te(OH)6 molecule are involved in the coordination of potassium cations which is similar to the situation observed in Li6(TeMo6O24)(Te(OH)6).18H2O. However, the difference exists in the connectivity fashions between the anion (TeMo6O24)6− and K+ on the one hand, and between the anion and Li+ on the other hand. In 1 the K+ ions occupy a three distinct sites and shares all the O atoms of the anion (TeMo6O24)6−, except O1, O3, O4 and O5. Whereas, in Li6(TeMo6O24)(Te(OH)6.18H2O, only three oxygen atoms: (O(13), O(18) and O(23)) of the anion are involved in the coordination of the six independent Li+ ions (Fig. 3).
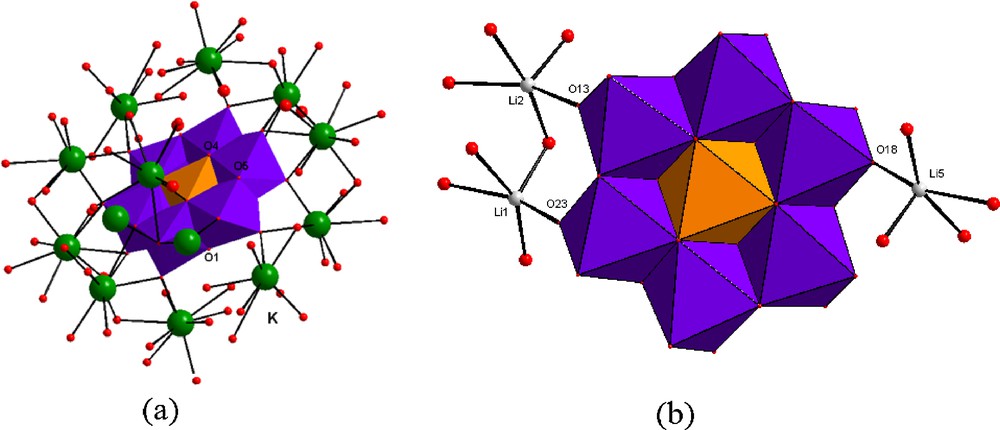
The (TeMo6O24)6– anion environment in 1(a) and in Li6(TeMo6O24)(Te(OH)6).18H2O (b).
In the present structure, the telluric acid molecule is a nearly regular octahedron, with an average Te2–OH bond length of 1.921 (3) Å and an average O-Te2-O angles of 83.333 (1)°. These distances and angles are of the same magnitude in accordance with previous observations of Te(OH)6 involved in other molybdotellurates: Rb6TeMo6O24.Te(OH)6.6H2O, Cs6TeMo6O24.2Te(OH)64H2O, (NH4)6TeMo6O24Te(OH)6.7H2O, Li6(TeMo6O24)Te(OH)6).18 H2O.
The Anderson (TeMo6O24)6− polyanions are assembled in a 1-D chain through the coordination of the terminal oxygen atoms and the potassium K1 cations. Then, each chain is connected to two other parallel chains through K(2) and K(3) to yield a 2D layer (Fig. 4). The neighboring layers are further held together by extensive hydrogen-bonding and telluric acid molecule Te(OH)6 forming a 3D open framework structure (Figs. 5 and 6). The K+ ions occupy three distinct sites. Its environment is consisted of eight O atoms for K1, K3 and nine O atoms for K2, with K-O distances ranging from 2.731 (5) to 3.403 (4) Å, 2.730 (4) to 3.191 (1) Å and 2.746 (1) to 3.363 (6) Å for K1, K2 and K3, respectively.

A view showing the association between [TeMo6O24]6– and the polyhedral KOn.

A view showing the hydrogen-bonding interactions between water molecules, Te(OH)6 groups and terminal oxygen atoms of polyoxoanions.

The three-dimensional supramolecular framework of 1. Water molecules atoms are omitted for clarity.
As listed in Table 4, OW–H…O, OW–H…OW and OW–H…N hydrogen bonds between the solvent water molecules, clusters and Te(OH)6 have interatomic OW…O distances ranging from 0.914 Å to 1.119 Å and hydrogen-bond angles from 110.78° to 163.18°. These hydrogen bonds hold the components together into a three-dimensional network and make the crystal structure of the compound more stable.
Hydrogen-bonding geometry (Å,°).
| D-H…A | D-H | H…A | D…A | D-H…A |
| O13-H1…O1xiii | 0.980 | 1.796 | 2.664 | 145.71 |
| O14-H2…OW1xi | 1.119 | 2.612 | 3.186 | 110.78 |
| O15-H3…O4xiv | 0.914 | 1.785 | 2.637 | 153.94 |
| OW1-H11…O15xvi | 0.970 | 2.057 | 2.868 | 139.90 |
| OW1-H12…O11 ix | 0.970 | 2.227 | 2.925 | 127.98 |
| OW1-H12…OW2iii | 0.970 | 2.575 | 3.402 | 143.21 |
| OW1-H12…O12iii | 0.970 | 2.606 | 3.096 | 111.46 |
| OW2-H21…Niii | 0.970 | 2.327 | 3.114 | 137.71 |
| OW2-H22…O6 | 0.970 | 1.807 | 2.750 | 163.18 |
| OW3-H31…N | 0.970 | 2.052 | 2.858 | 139.18 |
| OW3-H31…Nxv | 0.970 | 2.273 | 3.192 | 157.74 |
| OW3-H31…OW3xv | 0.970 | 2.461 | 3.153 | 128.09 |
| OW3-H32…O8i | 0.970 | 2.437 | 3.092 | 124.49 |
3.3 IR absorption spectroscopy
For a MoO6 vibration ion having Oh symmetry, there are six fundamental vibrations, symmetric stretching mode υ1, asymmetric stretching modes υ2 and υ3, asymmetric bending mode υ4, symmetric bending mode υ5, and inactive mode υ6.
In the crystal, the MoO6 octahedral ions lose their Oh symmetry by the sharing oxygen atoms with TeO6 group. Since there are three different Mo-O distances and different O-Mo-O angles in the crystal, the spectrum is expected to be very complex with separate frequencies for the individual Mo-O distances and O-Mo-O angles.
As shown in Fig. 7, the spectra can be divided into the following typical regions: 3600 and 2800 cm−1 (O-H and N-H stretchings), 1650 and 1400 cm−1 (O-H and N-H bendings). The low-wavenumber characteristic peaks are attributed to the Anderson anion [TeMo6O24]6− [20]: l000 and 800 cm−1 (Mo-Ot vibrations), 750 and 550 cm−1 (fundamentally Mo-Ob modes) and below 450 cm−1 (Mo-Oc, and some other modes). Between 550 and 500 cm−1, it is possible to observe some bands attributable to water liberations.
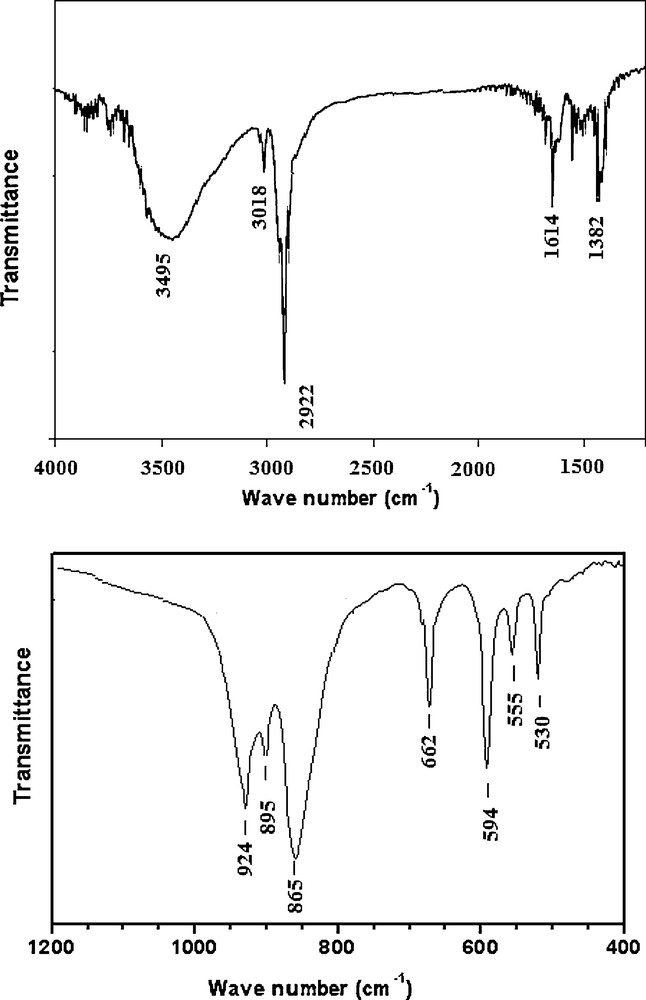
IR spectrum of K5NH4[ToMo6O24].Te(OH)6.6H2O.
3.4 UV–visible spectra
When water was used as solvent, the UV spectrum of compound 1 (Fig. 8) exhibits two peaks at 202 and 244 nm ascribed to the ligand-to-metal charge transfers (LMCT) of Ot→Mo and Ob→ Mo, respectively, where electrons are promoted from the low-energy electronic states, mainly comprised of oxygen 2p orbitals, to the high-energy states, which are mainly comprised of metal d orbitals [21–23].

UV-visible absorption spectrum of the compound K5(NH4)(TeMo6O24)(Te(OH)6).6 (H2O).
3.5 Thermal analysis
Thermogravimetric and differential thermal analyses (TG–DTA) curves of the compound 1 are shown in Fig. 9. The TGA curve of the structure shows four steps of decomposition. The first corresponds to the elimination of tow water molecules per formula unit (weight loss observed 2.23%) in the temperature range 100–223 °C. This dehydration is related to the first endothermic peak on the DTA curve with a maximum elimination at 145 °C. The second process starts at 223 °C and is complete at 282 °C, which corresponds to the remaining four crystallization water molecules (weight loss observed 6.68%). The third and the fourth effect at 318 °C and 347 °C, respectively correspond probably to the removal of NH4+ cation and to the decomposition of the compound. Further heating shows no noticeable loss in between 400 °C and 700 °C.
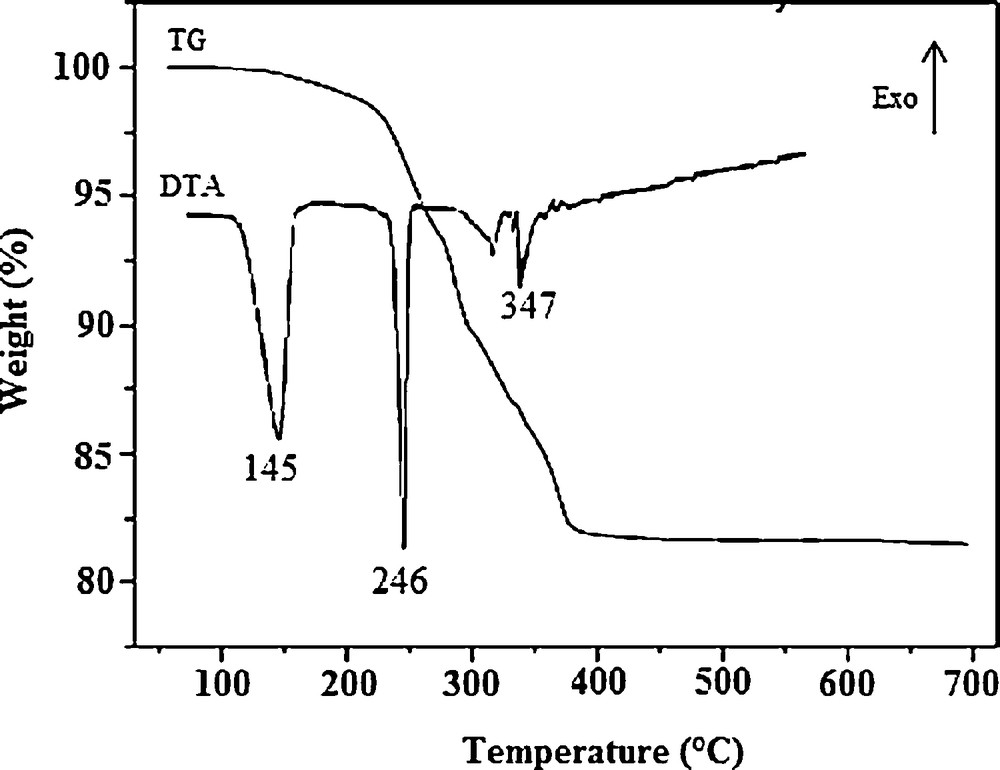
TG–DTA thermograms of K5NH4[ToMo6O24].Te(OH)6.6H2O.
3.6 Electrochemical behavior
The Anderson structure contains not only MoO6 octahedra with single terminal oxygen but also octahedra containing cis terminal oxygen; so it is electrochemically active and can be reduced. Cyclic voltammetry on the compound 1 was carried out in 1 mol L−1 H2SO4 aqueous solution in the potential range from –600 to 400 mV. Fig. 10 shows the typical cyclic voltammetric behaviors of the compound at scan rate 10 mV s−1. It can be seen that two redox peaks appear and the mean peak potential E1/2= (Ep + Epc)/2 are -440 mV (I–I’) and -533 mV (II–II’) (vsAg/AgCl), respectively. The two peaks I–I’ and II–II’ may be attributed to the redox of the MoVI/V in the polyanion framework.
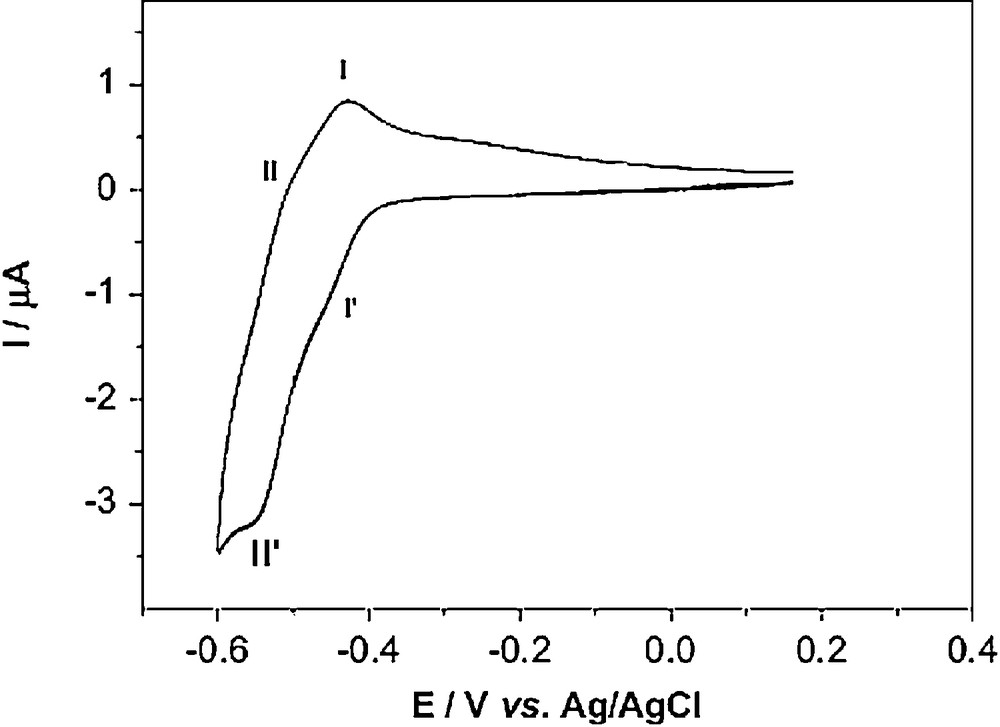
CV of compound 1 in 1 mol L–1 H2SO4 solution at a scan rate of 100 mV s–1.
4 Conclusion
In summary, new compounds have been isolated under conventional solution method by controlling the pH value of the reactive system. The crystal structure of the title compound has been elucidated by X-ray crystallography and confirmed by EDS, IR, UV and TG-DTA. The main geometrical feature of this structure is the existence of (TeMo6O24)6− clusters. These clusters are connected to the Te(OH)6 groups through hydrogen bonds and potassium ions to form three-dimensional framework.
Acknowledgment
I thank Dr. Ahmed Driss laboratoire de matériaux et Cristallochimie, faculté des sciences de Tunis, 2092 El Manar II, Tunis, Tunisie.


