1 Introduction
Imidazo[1,2-a]quinoline is a synthetically designed scaffold with a broad spectrum of biological activities. Its derivatives have been claimed to be contraceptive [1a], hypotensive [1b], anti-allergic and anti-asthmatic agents [1c]. Moreover, several prospective non-sedative anxiolytic agents with the imidazoquinoline structure have been discovered [2a], and one of them, referred to as Ru 31719, is now under clinical evaluation. Its affinity to GABA receptors in the brain is being studied intensively in order to evidence its action mechanism [2b–d]. In addition, imidazoquinolines have been explored as novel immunostimulant drugs [3]. The known syntheses of imidazo[1,2-a]quinolines usually utilize various quinoline derivatives as the starting materials. Among them, 2-aminoquinolines are the most popular. Thus, their reactions with α-halo carbonyl compounds [2a,4], and related 1,2-bielectrophiles [5] lead to the target system. Furthermore, 2-aminoquinolines have been employed in Strecker–Ugi reactions, also, resulting in imidazoquinolines [6,7]. As for other quinoline precursors, the transformation of 1-phenacylquinolinium salts into the title system upon treatment with primary amines [8a], hydroxylamines [8b] and hydrazine derivatives [8c], as well as the condensation of 2-chloro- or 2-alkoxyquinolines with α-amino carbonyl compounds [9], should be mentioned. Finally, a few less general approaches to imidazoquinolines have been reported [10]. However, many of these procedures are not fully satisfactory with regard to operational simplicity, cost of the reagent, drastic reaction conditions, and they afford the products in relatively low yields. Therefore, a simple, general, and efficient procedure is still in demand for the preparation of these important heterocyclic compounds and research is still in progress to find out a better and improved methodology.
2 Results and discussion
Continuing our research work on MCR of aminals and enamines [11] with a view to the synthesis of fused polycyclic N-heterocycles, we evidence in this paper a very efficient and environmentally benign strategy for the synthesis of new imidazo[1,2-a]quinolin-6-one derivatives by a one-pot, four-component reaction of aromatic aldehyde, cyclic 1,3-dione, diamine, and nitro ketene dithioacetal under melt conditions with excellent yields (Scheme 1).
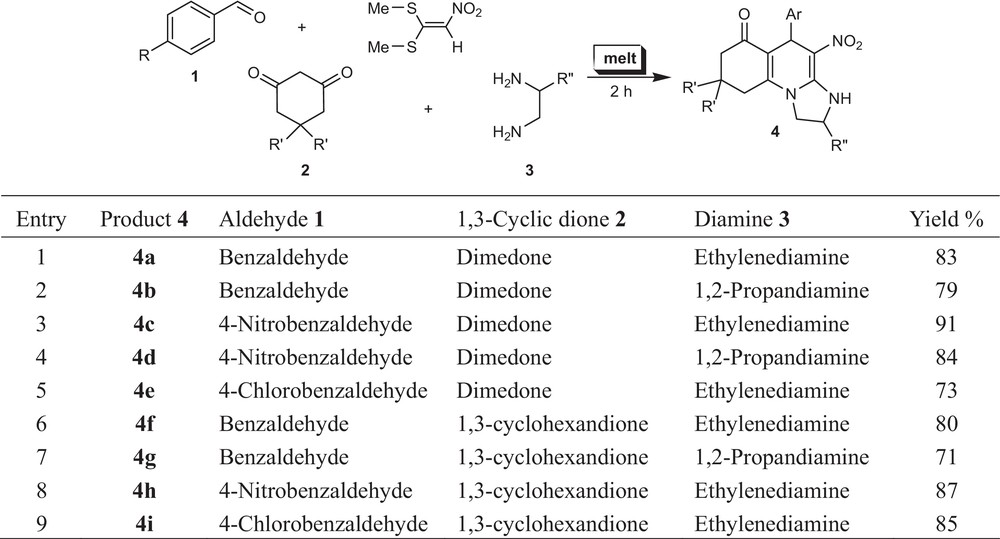
Synthesis of imidazo[1,2-a]quinolin-6-one derivatives 4.
The reactions between aromatic aldehydes and cyclic 1,3-diones are too slow at room temperature and require a basic or acidic catalyst; on the other hand, according to our previous experiments, the formation of ketene aminal from diamine and nitro ketene dithioacetal requires reflux at high temperature. Further investigation led us to the conclusion that both solvent and catalyst can be avoided. To avoid disadvantages such as volatility and toxicity inherent to many organic solvents, we applied the melt method to the four-component reaction as a green medium. Initially, the four-component reaction of 4-nitrobenzaldehyde, dimedone, ethylenediamine and nitro ketene dithioacetal as a simple model substrate was investigated to establish the feasibility of the strategy and to optimize the reaction conditions.
The effects of solvents and reaction temperature were evaluated for this model reaction, and the results are summarized in Table 1.
Optimization of reaction conditions.
| Entry | Reaction conditions | Catalyst | T (°C) | Time (h) | Yield (%) |
| 1 | EtOH | TsOH | RT | 28 | 31 |
| 2 | CH3CN | Et3N | RT | 24 | 37 |
| 3 | EtOH | TsOH | 80 | 14 | 55 |
| 4 | THF | Et3N | 60 | 20 | 32 |
| 5 | CH3CN | TsOH | 70 | 16 | 44 |
| 6 | EtOH | Et3N | 80 | 15 | 59 |
| 7 | Melt | – | 100 | 3 | 87 |
| 8 | Melt | – | 110 | 2 | 91 |
The melt method provided higher yields and shorter reaction times than those using organic solvents. The optimized reaction conditions were then tested for library construction with nine derivatives. The corresponding octahydro-imidazo[1,2-a]quinoline derivatives 4 were obtained in good yields at 110 °C in melt conditions without any catalyst.
The structures of compounds 4a–i were deduced from their elemental analysis, IR, and high-field 1H, and 13C NMR spectra. The mass spectrum of 4a displayed the molecular ion peak at m/z 339, which is in agreement with the proposed structure. The IR spectrum of this compound showed an absorption band due to NH stretching at 3336 cm−1. Absorption bands at 1641, 1532 and 1350 cm−1 are due to the CO and C–NO2 groups. The 1H NMR spectrum of 4a showed two singlets for CH3 groups (δ = 0.83, 1.03 ppm), four doublets for the two CH2 groups because these H-atoms are diastereotopic (δ = 1.98 ppm, 2JHH = 16.10 Hz, δ = 2.18 ppm, 2JHH = 16.05 Hz, δ = 2.50 ppm, 2JHH = 17.55 Hz, δ = 2.59 ppm, 2JHH = 14.30 Hz), three multiplets for the CH2 groups of the imidazole ring (δ = 3.80–3.83, 3.98–4.04, 4.15–4.20 ppm), two singlets for the CH and NH groups (δ = 5.06 and 9.38 ppm), and the phenyl moiety gave rise to characteristic signals in the aromatic region of the spectrum. The 1H-decoupled 13C NMR spectrum of 4a showed 17 distinct resonances, in agreement with the suggested structure.
A possible mechanism route for the described four-component reaction is outlined in Scheme 2.
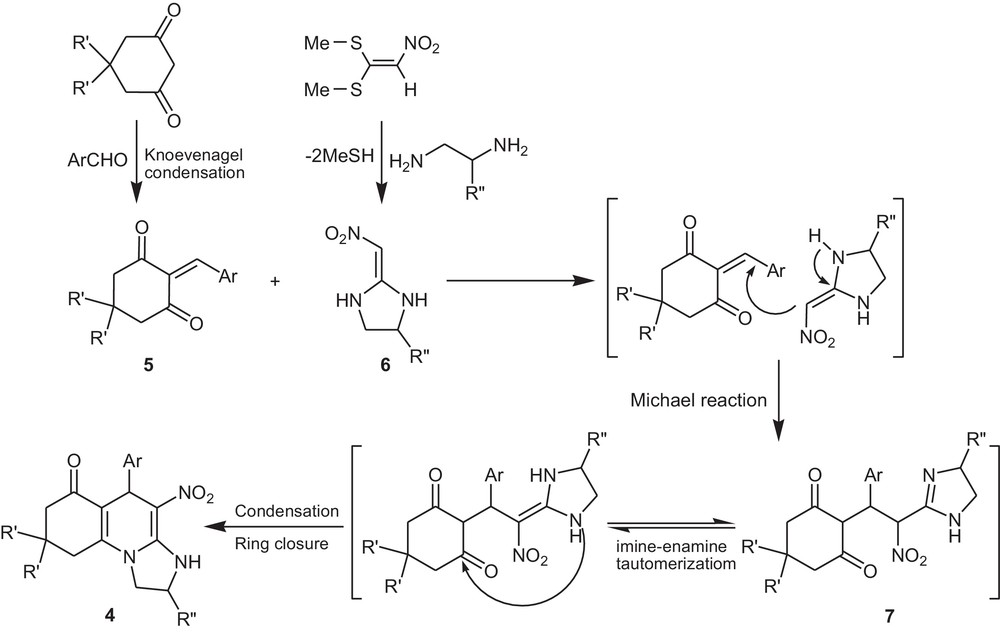
Proposed mechanism for the formation of octahydro-imidazo[1,2-a]quinolin-6-ones.
Firstly, we assume that the initial step is a Knoevenagel condensation between the aldehyde and the cyclic 1,3-dione, resulting in the adduct 5, then the reaction between intermediate 5 and the ketene aminal 6 (which is derived from the addition of diamine to nitro ketene dithioacetal) gives the Michael adduct 7. The Michael adduct 7 undergoes a cyclocondensation reaction through amino and carbonyl to afford compound 4.
Two products for propane-1,2-diamine were expected, but when 1,n-diamine was applied in the reaction, only one of the two possible products was obtained stereospecifically.
3 Conclusions
In conclusion, we have recently disclosed a novel and convenient one-pot synthesis of multisubstituted octahydro-imidazo[1,2-a]quinolin-6-ones via four-component reactions. This catalyst-free sequential reaction proceeded smoothly in excellent yields and offered several other advantages, including short reaction time, simple experimental workup procedures, and no toxic byproducts. This approach to octahydro-imidazo[1,2-a]quinolin systems presented herein avoids the use of a catalyst, toxic organic solvents, and anhydrous conditions. This protocol represents a promising green route to the class of compounds. In addition, all of the cyclo-adducts were quite stable and easy to handle under standard conditions.
4 Experimental
4.1 Materials and techniques
The aldehydes, cyclic 1,3-diones, diamines and nitro ketene dithioacetal were obtained from Merck (Germany) and Fluka (Switzerland) and were used without further purification. Melting points were measured on an Electrothermal 9100 apparatus. Elemental analyses for C, H and N were performed using a Heraeus CHN–O–Rapid analyzer. Mass spectra were recorded on a FINNIGAN-MATT 8430 mass spectrometer operating at an ionization potential of 70 eV. 1H and 13C NMR spectra were measured (CDCl3 solution) with a Bruker DRX-500 AVANCE spectrometer at 500.13 and 125.8 MHz, respectively. IR spectra were recorded on a Shimadzu IR-460 spectrometer; absorbencies are reported in cm−1.
4.2 General procedure for the preparation of compounds 4a–i, exemplified on 4a
The mixtures of benzaldehyde (0.106 g, 1 mmol), dimedone (0.140 g, 1 mmol), were melted under vacuum in a 50-mL flask in a drying oven at 110 °C for 1 h. Then ethylenediamine (0.60 g, 1 mmol) and nitro ketene dithioacetal (0.165 g, 1 mmol) were added. After completion, the mixture was dried at 110 °C under a vacuum and the precipitate was washed with ethanol to afford the pure product 4a.
4.3 Spectral data
4.3.1 8,8-Dimethyl-4-nitro-5-phenyl-1,2,3,5,6,7,8,9-octahydro-imidazo[1,2-a]quinoline-6-one (4a)
Yellow powder (yield 83%); mp: 270 °C (decomp); IR (KBr) (νmax, cm−1): 3336 (NH), 1641 (CO), 1541 and 1350 (C–NO2). 1H NMR (500.1 MHz, CDCl3): δH (ppm) 0.83 (3 H, s, CH3), 1.03 (3 H, s, CH3), 1.98 (1 H, d, 2JH,H = 16.1 Hz, CH2), 2.18 (1 H, d, 2JH,H = 16.0 Hz, CH2), 2.50 (1 H, d, 2JH,H = 17.5 Hz, CH2), 2.59 (1 H, d, 2JH,H = 14.3 Hz, CH2), 3.80–3.83 (2 H, m, CH2NH), 3.98–4.04 (1 H, m, CH2N), 4.15–4.20 (1 H, m, CH2N), 5.06 (1 H, s, CH), 7.08–7.19 (5 H, m, 5 CH of Ph), 9.38 (1 H, s, NH). 13C NMR (125.7 MHz, CDCl3): δC (ppm) 29.80 (2 CH3), 32.22(C), 37.54 (CH2), 38.69 (CH), 43.83 (CH2NH), 45.22 (CH2N), 49.89 (CH2), 107.74 (CCO), 114.18 (C–NO2), 126.45 (CH of Ph), 128.03 (2 CH of Ph), 128.23 (2 CH of Ph), 144.72 (Cipso of Ph), 149.41 (NCN), 152.12 (C–N), 193.63 (CO). MS: m/z (%) = 339 (M+, 8), 293 (36), 262 (100), 216 (36), 132 (33), 77 (77), 51 (46). Anal. calcd for C19H21N3O3 (339.39): C, 67.24; H, 6.24; N, 12.38%. Found: C, 67.19; H, 6.22; N, 12.41%.
4.3.2 1,8,8-Trimethyl-4-nitro-5-phenyl-1,2,3,5,6,7,8,9-octahydro-imidazo[1,2-a]quinoline-6-one (4b)
Yellow powder (yield 79%); mp: 260 °C (decomp); IR (KBr) (νmax, cm−1): 3349 (NH), 1646 (CO), 1522 and 1351 (C–NO2). 1H NMR (500.1 MHz, CDCl3): δH (ppm) 0.81 (3 H, s, CH3), 1.01 (3 H, s, CH3), 1.32 (3 H, br, CH3), 1.96 (1 H, d, 2JH,H = 16.0 Hz, CH2), 2.16 (1 H, d, 2JH,H = 16.0 Hz, CH2), 2.47–2.56(2 H, m, CH2), 3.32–3.57 (2 H, m, CH2N), 4.11–4.26 (1 H, m, CHNH), 5.04 (1 H, s, CH), 7.08–7.17 (5 H, m, 5 CH of Ph), 9.51 (1 H, s, NH). 13C NMR (125.7 MHz, CDCl3): δC (ppm) 21.13 (CH3), 28.65 (2 CH3), 31.11 (C), 33.56 (CH2), 39.42 (CH), 49.96 (CH2N), 52.75 (CH2), 53.00 (CH), 107.98 (CCO), 114.17 (C–NO2), 127.33 (CH of Ph), 128.56 (2 CH of Ph), 128.97 (2 CH of Ph), 144.22 (Cipso of Ph), 149.16 (NCN), 151.39 (C–N), 193.42 (CO). MS: m/z (%) = 353 (M+, 2), 348 (4), 262 (100), 216 (22), 137 (18), 77 (67), 51 (53). Anal. calcd for C20H23N3O3 (353.419): C, 67.97; H, 6.56; N, 11.89%. Found: C, 67.98; H, 6.54; N, 11.90%.
4.3.3 8,8-Dimethyl-4-nitro-5-(4-nitrophenyl)-1,2,3,5,6,7,8,9-octahydro-imidazo[1,2-a]quinoline-6-one (4c)
Yellow powder (yield 91%); mp: 265 °C (decomp); IR (KBr) (νmax, cm−1): 3344 (NH), 1626 (CO), 1523 and 1349 (C–NO2). 1H NMR (500.1 MHz, CDCl3): δH (ppm) 0.82 (3 H, s, CH3), 1.03 (3 H, s, CH3), 1.96–1.96 (1 H, m, CH2), 2.13–2.19 (1 H, m, CH2), 2.43–2.61 (2 H, m, CH2), 3.84 (2 H, br, CH2NH), 4.02–4.04 (1 H, m, CH2N), 4.11–4.17 (1 H, m, CH2N), 5.14 (1 H, s, CH), 7.48 (2 H, d, 2JH,H = 8.3 Hz, 2 CH of Ar), 8.05 (2 H, d, 2JH,H = 8.3 Hz, 2 CH of Ar), 9.48 (1 H, s, NH). 13C NMR (125.7 MHz, CDCl3): δC (ppm) 26.65 (2 CH3), 29.65 (C), 32.23 (CH2), 39.50 (CH), 43.96 (CH2NH), 45.25 (CH2N), 49.76 (CH2), 107.23 (CCO), 113.41 (C–NO2), 123.27 (2 CH of Ar), 129.65 (2 CH of Ar), 146.30 (NCN), 150.14 (Cipso of Ar), 151.85 (Cipso of Ar), 152.37 (C–N), 193.65 (CO). MS: m/z (%) = 384 (M+, 3), 339 (14), 262 (100), 235 (42), 111(39), 55 (24). Anal. calcd for C19H20N4O5 (384.38): C, 59.37; H, 5.24; N, 14.58%. Found: C, 59.33; H, 5.23; N, 14.61%.
4.3.4 1,8,8-Trimethyl-4-nitro-5-(4-nitrophenyl)-1,2,3,5,6,7,8,9-octahydro-imidazo[1,2-a]quinoline-6-one (4d)
Yellow powder (yield 84%); mp: 273 °C (decomp); IR (KBr) (νmax, cm−1): 3359 (NH), 1632 (CO), 1518 and 1353 (C–NO2). 1H NMR (500.1 MHz, CDCl3): δH (ppm) 1.01 (3 H, s, CH3), 1.11 (3 H, s, CH3), 1.34 (3 H, d, 2JH,H = 10.0 Hz, CH3), 1.96 (1 H, d, 2JH,H = 16.2 Hz, CH2), 2.18 (1 H, d, 2JH,H = 16.2 Hz, CH2), 2.48–2.54 (2 H, m, CH2), 3.62–3.78 (1 H, m, CH2N), 4.11–4.22 (2 H, m, CH2N and CHNH), 5.13 (1 H, s, CH), 7.46 (2 H, d, 2JH,H = 14.0 Hz, 2 CH of Ar), 8.05 (2 H, d, 2JH,H = 13.52 Hz, 2 CH of Ar), 9.68 (1 H, s, NH). 13C NMR (125.7 MHz, CDCl3): δC (ppm) 21.13 (CH3), 26.66 (2 CH3), 29.77 (C), 32.25 (CH2), 38.67 (CH), 49.74 (CH2N), 51.74 (CH2), 51.95 (CH), 106.52 (CCO), 112.73 (C–NO2), 123.42 (2 CH of Ar), 129.66 (2 CH of Ar), 146.30 (NCN), 150.26 (Cipso of Ar), 151.14 (Cipso of Ar), 152.37 (C–N), 193.74 (CO). MS: m/z (%) = 339 (M+, 8), 293 (36), 262 (100), 216 (36), 132 (33), 77 (77), 51 (46). Anal. calcd for C20H22N4O5 (398.41): C, 60.29; H, 5.57; N, 14.06%. Found: C, 60.28; H, 5.55; N, 14.08%.
4.3.5 5-(4-Chlorophenyl)-8,8-dimethyl-4-nitro-1,2,3,5,6,7,8,9-octahydro-imidazo[1,2-a]quinoline-6-one (4e)
Yellow powder (yield 73%); mp: 254 °C (decomp); IR (KBr) (νmax, cm−1): 3351 (NH), 1641 (CO), 1553 and 1350 (C–NO2). 1H NMR (500.1 MHz, CDCl3): δH (ppm) 0.82 (3 H, s, CH3), 1.02 (3 H, s, CH3), 1.96–2.08 (2 H, m, CH2), 2.16–2.26 (1 H, m, CH2), 2.48–2.60 (1 H, m, CH2), 3.81 (2H, br, CH2NH), 3.98–4.01 (1 H, m, CH2N), 4.16–4.17 (1 H, m, CH2N), 5.02 (1 H, s, CH), 7.15–7.26 (4 H, m, 5 CH of Ar), 9.42 (1 H, s, NH). 13C NMR (125.7 MHz, CDCl3): δC (ppm) 26.94 (2 CH3), 31.37 (C), 37.35 (CH2), 39.48 (CH), 43.86 (CH2NH), 45.22 (CH2N), 49.89 (CH2), 107.38 (CCO), 113.62 (C–NO2), 127.95 (CH of Ar), 130.15 (2 CH of Ar), 130.96 (Cipso of Ar), 143.71 (Cipso of Ar), 149.61 (NCN), 163.49 (C–N), 193.65 (CO). MS: m/z (%) = 373 (M+, 1), 349 (74), 273 (100), 217 (70), 161 (41), 111 (25). Anal. calcd for C19H20ClN3O3 (373.83): C, 61.05; H, 5.39; N, 9.48%. Found: C, 67.19; H, 6.22; N, 12.41%.
4.3.6 4-Nitro-5-phenyl-1,2,3,5,6,7,8,9-octahydro-imidazo[1,2-a]quinoline-6-one (4f)
Yellow powder (yield 80%); mp: 248 °C (decomp); IR (KBr) (νmax, cm−1): 3159 (NH), 1649 (CO), 1582 and 1392 (C–NO2). 1H NMR (500.1 MHz, CDCl3): δH (ppm) 1.83–1.94 (2 H, m, CH2), 2.27–2.49 (2 H, m, CH2), 2.62–2.80 (2 H, m, CH2), 3.38–4.02 (4 H, m, CH2NH and CH2N), 5.36 (1 H, s, CH), 7.11–7.68 (5 H, m, 5 CH of Ph), 9.61 (1 H, s, NH). 13C NMR (125.7 MHz, CDCl3): δC (ppm) 20.30 (CH2), 26.88 (CH2), 31.29 (CH), 36.84 (CH2), 42.34 (CH2NH), 43.45 (CH2N), 102.59 (CCO), 115.99 (C–NO2), 125.92 (CH of Ph), 128.21 (2 CH of Ph), 128.47 (2 CH of Ph), 136.50 (Cipso of Ph), 144.99 (NCN), 151.93 (C–N), 196.69 (CO). MS: m/z (%) = 294 (37), 217 (100), 152 (20), 115 (22), 77 (37), 55 (25). Anal. calcd for C17H17N3O3 (311.33): C, 65.58; H, 5.50; N, 13.50%. Found: C, 65.56; H, 5.51; N, 13.49%.
4.3.7 2-Methyl-4-nitro-5-phenyl-1,2,3,5,6,7,8,9-octahydro-imidazo[1,2-a]quinoline-6-one (4g)
Yellow powder (yield 71%); mp: 247 °C (decomp); IR (KBr) (νmax, cm−1): 3159 (NH), 1649 (CO), 1582 and 1392 (C–NO2). 1H NMR (500.1 MHz, CDCl3): δH (ppm) 1.47 (3 H, br, CH3), 1.76–1.93 (2 H, m, CH2), 2.24–2.42 (2 H, m, CH2), 2.57–2.65 (2 H, m, CH2), 3.53–3.66 (2 H, m, CH2N), 4.17–4.23 (1 H, m, CHNH), 5.38 (1 H, s, CH), 7.10–7.30 (5 H, m, 5 CH of Ph), 8.70 (1 H, s, NH). 13C NMR (125.7 MHz, CDCl3): δC (ppm) 20.25 (CH3), 21.14 (CH2), 27.32 (CH2), 31.59 (CH), 37.91 (CH2), 48.34 (CHNH), 50.13 (CH2N), 104.76 (CCO), 116.88 (C–NO2), 126.35 (CH of Ph), 128.04 (2 CH of Ph), 128.32 (2 CH of Ph), 135.83 (Cipso of Ph), 144.33 (NCN), 151.65 (C–N), 196.40 (CO). MS: m/z (%) = 325 (M+, 2), 294 (82), 217 (90), 156 (70), 91 (100). Anal. calcd for C18H19N3O3 (325.36): C, 65.58; H, 5.50; N, 13.50%. Found: C, 65.56; H, 5.51; N, 13.49%.
4.3.8 4-Nitro-5-(4-nitrophenyl)-1,2,3,5,6,7,8,9-octahydro-imidazo[1,2-a]quinoline-6-one (4h)
Yellow powder (yield 87%); mp: 245 °C (decomp); IR (KBr) (νmax, cm−1): 3378 (NH), 1654 (CO), 1532 and 1337 (C–NO2). 1H NMR (500.1 MHz, CDCl3): δH (ppm) 1.73 (1 H, br, CH2), 1.93 (1 H, br, CH2), 2.17–2.65 (4 H, m, CH2), 3.81–3.84 (2 H, m, CH2NH), 3.99–4.05 (1 H, m, CH2N), 4.15–4.18 (1 H, m, CH2N), 5.19 (1 H, s, CH), 7.48 (2 H, d, 3JH,H = 7.9 Hz, 2 CH of Ar), 8.04 (2 H, d, 3JH,H = 8.0 Hz, 2 CH of Ar), 9.48 (1 H, s, NH). 13C NMR (125.7 MHz, CDCl3): δC (ppm) 20.61 (CH2), 25.67 (CH2), 36.21 (CH), 38.17 (CH2), 43.96 (CH2NH), 45.21 (CH2N), 106.80 (CCO), 113.95 (C–NO2), 123.33 (2 CH of Ar), 129.63 (2 CH of Ar), 146.31 (Cipso of Ar), 151.68 (NCN), 152.14 (C–N), 152.63 (Cipso of Ar), 193.88 (CO). MS: m/z (%) = 356 (M+, 8), 338 (6), 310 (13), 264 (10), 234 (100), 188 (43), 132 (31), 83 (21), 55 (47). Anal. calcd for C17H16N4O5 (356.33): C, 57.30; H, 4.53; N, 15.72%. Found: C, 57.28; H, 4.55; N, 15.71%.
4.3.9 5-(4-Chlorophenyl)-4-nitro-1,2,3,5,6,7,8,9-octahydro-imidazo[1,2-a]quinoline-6-one (4i)
Yellow powder (yield 85%); mp: 243 °C (decomp); IR (KBr) (νmax, cm−1): 3380 (NH), 1660 (CO), 1533 and 1348 (C–NO2). 1H NMR (500.1 MHz, CDCl3): δH (ppm) 1–73–1.94 (2 H, m, CH2), 2.17–2.25 (2 H, m, CH2), 2.48–2.63 (2 H, m, CH2), 3.79–3.85 (2 H, m, CH2NH), 4.02–4.14 (2 H, m, CH2N), 5.07 (1 H, s, CH), 7.13–7.27 (4 H, m, 5 CH of Ar), 9.41 (1 H, s, NH). 13C NMR (125.7 MHz, CDCl3): δC (ppm) 20.26 (CH2), 26.88 (CH2), 36.79 (CH), 37.20 (CH2), 43.87 (CH2NH), 45.21 (CH2N), 107.31 (CCO), 113.62 (C–NO2), 128.00 (2 CH of Ar), 130.14 (2 CH of Ar), 130.98 (Cipso of Ar), 143.94 (Cipso of Ar), 151.55 (NCN), 151.79 (C–N), 193.88 (CO). MS: m/z (%) = 328 (90), 293 (66), 217 (100), 152 (25), 111 (27), 77 (16), 20 (46). Anal. calcd for C17H16ClN3O3 (345.78): C, 59.05; H, 4.66; N, 10.25%. Found: C, 59.10; H, 4.64; N, 110.28%.
Acknowledgements
We gratefully acknowledge financial support from the Research Council of Tarbiat Modares University.


