1 Introduction
The spirooxindole system is the core structure of many pharmacological agents and natural products [1], for example, cytostatic alkaloids such as spirotryprostatins A, B, pteropodine, strychnofoline, (–)-horsfiline and isopteropodine have been shown to modulate the function of muscarinic serotonin receptors [2].
Among the heterocyclic spirooxindole ring system, these ones, together with substituted fused 4H-chromenes, have gained great importance due to their spasmolitic, anticoagulant, diuretic, anticancer, and antinaphylactic activities [3]. There have been several reports available in the literature on the synthesis of spirooxindoles with fused 4H-chromenes via multicomponent condensation reactions. The general procedure for the synthesis of these compounds involves the condensation of isatin, active methylene component, and 1,3-dicarbonyl compound in the presence of different catalysts, such as InCl3 or InCl3/SiO2 [4], triethylbenzyl ammonium chloride (TEBA) [5], NH4Cl [6], NEt3 [7], ethylenediamine diacetate [8], β-cyclodextrin [9], l-proline [10], HAuCl4·3H2O [11], MgO [12], [BMIm]BF4 [13], and sodium stearate [14]. Although all of these methods are effective, some of them have drawbacks such as long reaction times [5,9], high cost of the catalyst [6], harsh reaction conditions [4] and unreusability of the catalyst [5–8]. Therefore, there is still a demand for simple and facile methodologies for the preparation of spirochromene compounds with more efficiency and shorter reaction times.
Ionic liquids (ILs), considered as being a relatively recent magical chemical due their unique properties, have a large variety of applications in all areas of chemical synthesis. The unique properties of ILs, such as a negligible vapor pressure, good thermal stability, tunable viscosity, miscibility with water and with organic solvents, as well as good extractability for various organic compounds and metal ions, mainly depend on their special structures [15]. Protic ionic liquids (PILs) are a subclass of ILs family formed by an equimolar combination of a Brønsted acid and a Brønsted base and have an additional tunable feature as a result of proton transfer, proton activity. Environmentally considerable properties of PILs motivated chemists to investigate their application to other fields such as catalysis [16–21]. However, there are some limitations associated with the use of ILs as catalysts, such as a lack of potential activation sites on the substrates and their sensitivity to air and moisture, leading to the inactivation of the reaction. Therefore, the design of more active PILs that can also be employed as catalysts has been an important topic in this research field.
In continuation of our studies on the development of new catalysts and methods for organic synthesis [22], herein we report the highly efficient and simple methods for the synthesis of some spirochromene derivatives from isatin derivatives, alkyl-malonates and 1,3-dicarbonyl compounds in the presence of [TBD][TFA] ionic liquid as a catalyst at room temperature under solvent-free conditions (Scheme 1).
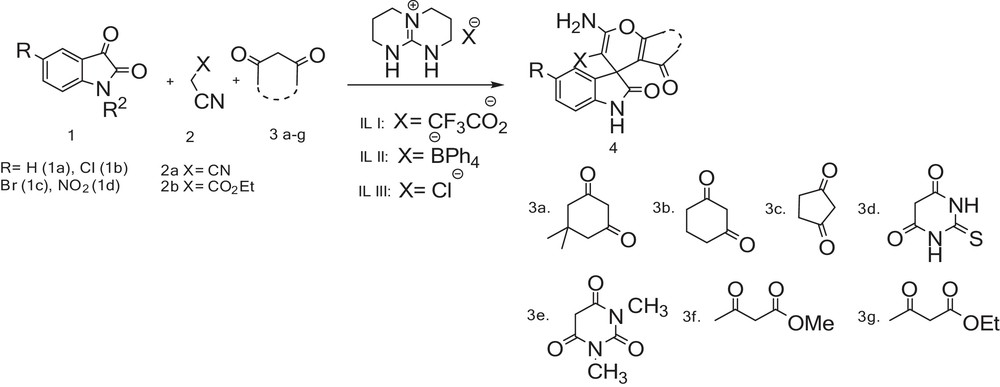
Synthesis of spirochromenes catalyzed by [TBD]-based ionic liquids.
2 Results and discussion
Initially, three triazabicyclodecene-based ionic liquids (Fig. 1, I–III) were synthesized and used as catalysts for the synthesis of spirochromene compounds.
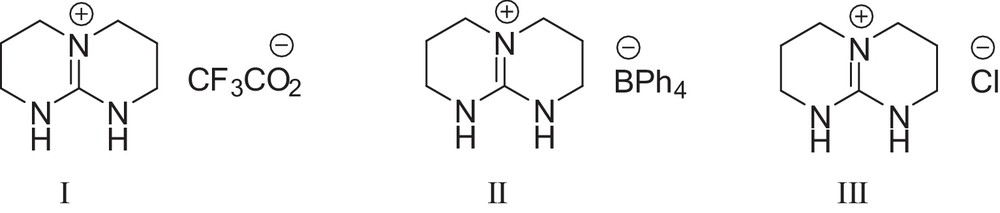
Structurally related TBD based protic ionic liquids.
In order to determine optimum reaction conditions, the reaction of isatin 1 (1 mmol) as a model compound with malononitrile 2 (1 mmol) and dimedone (1 mmol) was examined under different reaction conditions and shown in Table 1. Different catalyst loadings were examined and, as it is clear from Table 1, when an amount of 20 mol% of IL I was used as a catalyst under solvent-free conditions at room temperature after 20 min, the expected spirochromene was obtained at a yield of 85% (Table 1, entry 1). Lowering the catalyst loading to 5 mol%, an excellent yield of the product was obtained in shorter reaction times (Table 1, entries 2–4). The model reaction was also studied in different organic solvent such as ethanol, acetonitrile, tetrahydrofuran, toluene, and dichloromethane. In all solvents, the reaction procedure afforded the product with a moderate yield in longer reaction times (Table 1, entries 5–9). Although ionic liquids II and III are effective in this reaction, they give lower yields than IL I (Table 1, entries 10 and 11). When the model reaction was carried out in the presence of TBD and CF3CO2H, only a moderate yield of spirochromene was obtained in longer reaction times (Table 1, entries 12 and 14). This clearly evidences the significant role of IL I in this reaction. From the above observations, the best yields were obtained under solvent-free reaction conditions at room temperature (Table 1, entry 4).
Optimization of reaction conditions for the synthesis of spirochromene 4a.
| Entry | Catalyst | Solvent | Time (min) | Yield (%)a |
| 1 | IL I, 20 mol% | – | 20 | 85 |
| 2 | IL I, 15 mol% | – | 15 | 90 |
| 3 | IL I, 10 mol% | – | 10 | 95 |
| 4 | IL I, 5 mol% | – | 5 | 98 |
| 5 | IL I, 5 mol% | EtOH | 5 | 65 |
| 6 | IL I, 5 mol% | CH3CN | 30 | 70 |
| 7 | IL I, 5 mol% | THF | 25 | 85 |
| 8 | IL I, 5 mol% | Ph–CH3 | 40 | 72 |
| 9 | IL I, 5 mol% | CH2Cl2 | 35 | 65 |
| 10 | IL II, 5 mol% | – | 35 | 95 |
| 11 | IL III, 5 mol% | – | 5 | 88 |
| 12 | TBD, 5 mol% | – | 25 | 65 |
| 13 | TBD, 10 mol% | – | 30 | 75 |
| 14 | CF3CO2H, 5 mol% | – | 35 | 60 |
a Isolated yield.
In order to evaluate the generality and applicability of this methodology, the reaction of isatin with 1,3-dicarbonyl and active methylene compounds were concentrated using [TBD][TFA] (5 mol%) at room temperature under solvent-free conditions for the synthesis of a series of tetrahydrospiro [chromene-4,3′-indoline] 4a–4t derivatives, as shown in Table 2.
Synthesis of spirochromene derivatives catalyzed by [TBD][TFA] ionic liquids.
| Entry | R1 | R2 | X | 1,3-dicarbonyl compounds | Product | Time (min) | Yielda (%) | References |
| 1 | H | H | CN | 3a | 4a | 5 | 98 | [6] |
| 2 | H | H | CN | 3b | 4b | 6 | 96 | [6] |
| 3 | H | H | CN | 3c | 4c | 8 | 92 | [23] |
| 4 | H | H | CN | 3d | 4d | 8 | 95 | [17] |
| 5 | H | H | CN | 3e | 4e | 12 | 90 | [10b] |
| 6 | H | H | CN | 3f | 4f | 18 | 96 | [12] |
| 7 | H | H | CN | 3g | 4g | 20 | 94 | [10a] |
| 8 | Br | H | CN | 3a | 4h | 3 | 92 | [10a] |
| 9 | Br | H | CN | 3g | 4i | 15 | 90 | [13] |
| 10 | Cl | H | CN | 3a | 4j | 5 | 95 | [10a] |
| 11 | NO2 | H | CN | 3a | 4k | 2 | 98 | [9] |
| 12 | H | CH3 | CN | 3a | 4l | 5 | 95 | [11] |
| 13 | H | CH3 | CN | 3b | 4m | 8 | 92 | [11] |
| 14 | H | CH3 | CN | 3c | 4n | 10 | 90 | [11] |
| 15 | H | Et | CN | 3d | 4o | 10 | 90 | – |
| 16 | H | Benzyl | CN | 3a | 4p | 8 | 95 | [13] |
| 17 | H | CH2CO2Et | CN | 3b | 4q | 8 | 92 | – |
| 18 | H | H | CO2Et | 3b | 4r | 7 | 96 | [13] |
| 19 | H | H | CO2Et | 3g | 4s | 8 | 92 | [11] |
a Isolated yield.
Several types of isatins, including either electron-withdrawing or electron-donating groups, malononitrile or cyanoacetic ester and cyclic 1,3-diketones were used in this reaction. It was observed that all these cyclic 1,3-diketones as well as active methylene compounds were tolerated for this reaction, which affords satisfactory yield of desired products (Table 2). The possibility of recycling the catalyst was also examined. For this reason, the reaction of isatin with malononitrile and dimedone in the presence of [TBD][TFA] was studied. After completion of the reaction (monitored by TLC), EtOAc was added to the reaction mixture, and [TBD][TFA] was removed by filtration and washed with EtOAc (2 × 5 ml). The recovered catalyst was reused in six subsequent runs, affording yields from 90 to 98% of the product, without significant loss of activity.
A plausible mechanism is shown in Fig. 2. Two pathways are proposed for the synthesis of spirooxindole derivatives (A and B). In path A, the guanidinium core of salts could form doubly H-bonded motifs with the carbonyl group of isatin, thus enhancing their electrophilicity; then the nucleophilic attack of malononitrile on the carbonyl carbon produces the isatylidene malononitrile I. The electron-deficient Knoevenagel adduct I could be subsequently attacked via Michael addition of dimedone II to give intermediate III. In path B, isatin could form doubly H-bonded motifs with the catalyst and react with dimedone to afford the aldol adduct IV followed by dehydration and nucleophilic attack of malononitrile to afford intermediate III. In both paths, intermediate III affords, through an intermolecular cyclization by nucleophilic attack of the hydroxyl group on the cyano one, the final product 4a.
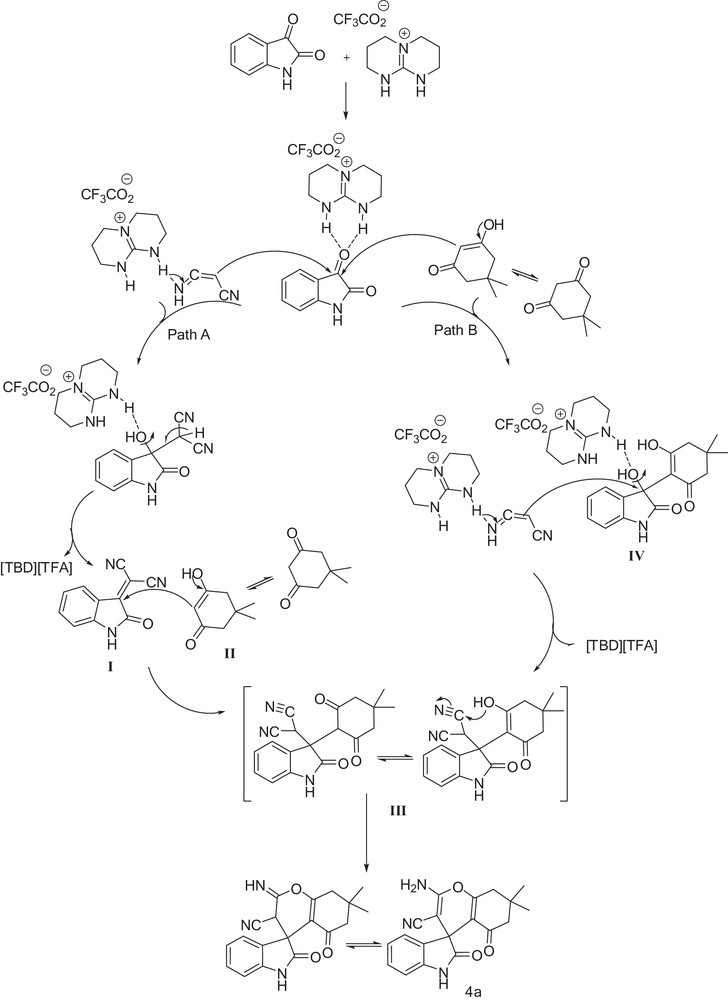
Proposed mechanism for the synthesis of spirooxindole 4a via paths A and B.
3 Experimental
All reagents were obtained from Fluka (Germany) and were used without further purification. Melting points were measured on an Electrothermal 9100 apparatus. Elemental analyses for C, H and N were performed using a Heraeus CHN-O-Rapid analyzer. Mass spectra were recorded on a FINNIGAN-MATT 8430 mass spectrometer operating at an ionization potential of 20 eV. 1H-, 13C NMR spectra were recorded on a Bruker DRX-400 AVANCE spectrometer. IR spectra were recorded as KBr pellets on a Shimadzu IR-460 spectrometer.
3.1 General procedure for the synthesis of 1,2,3,4,6,7,8,9-octahydropyrimido[1,2-a]pyrimidin-5-ium 2,2,2-trifluoroacetate IL (I)
Trifluoroacetic acid (92 mg, 0.81 mmol) was added dropwise to a suspension of triazabicyclo[4.4.0]dec-5-ene (70 mg, 0.50 mmol) in dry Et2O (5 mL) and the resulting mixture was vigorously stirred for 30 min. The solid formed was filtered off under reduced pressure and it was washed with several portions of dry Et2O to give IL I (119 mg, 94%) as a white solid. Spectral data: M.p. 161–163 °C; 1H NMR (DMSO-d6, 400 MHz) δH = 3.27 (4H, t, J = 6.0 Hz, 2 × CH2), 3.18 (4H, t, J = 5.6 Hz, 2 × CH2), 1.87 (4H, quintet., J = 6.0 Hz, 2 × CH2). 13C NMR (DMSO-d6, 100 MHz) δC = 159.2 (q, 2JC–F = 35.9 Hz, CF3CO2), 151.1 (CN3), 116.2 (q, 1JC–F = 291.7 Hz, CF3CO2), 46.6 (CH2), 37.9 (CH2), 20.7 (CH2).
3.2 General procedure for the synthesis of spirooxindole derivatives
To a magnetically stirred mixture of isatins (1 mmol) and IL I (12.6 mg, 0.05 mmol) were added alkyl-malonates (1 mmol) or 1,3-dicarbonyl compounds (1 mmol) and then the reaction mixture was stirred at room temperature. After completion of the reaction (monitored by TLC), EtOAc was added to the reaction mixture, and [TBD][TFA] was removed by filtration and washed with EtOAc (2 × 5 ml). The filtrate was evaporated under reduced pressure to give the solid product as a residue in almost pure form. If necessary, the product could further be purified by recrystallization from ethanol. The physical data (mp, IR, NMR) of known compounds were found to be identical with those reported in the literature. Spectroscopic data for selected examples are shown below.
3.2.1 7’-Amino-1-ethyl-2,4’-dioxo-2’-thioxo-1’,2’,3’,4’-tetrahydrospiro[indoline-3,5’ pyrano[2,3-d]pyrimidine]-6’-carbonitrile (4o)
White powder; mp 257–259 °C; yield: 0.329 g (90%); IR (KBr): 3452, 3327, 3169, 2212, 1689, 1626, 1588, 1468, 1389 cm−1; 1H NMR (400 MHz, DMSO-d6): δ = 1.13 (t, J = 6.9 Hz, 3 H, CH3), 3.72 (q, J = 7.8 Hz, 2 H,CH2), 6.98 (t, J = 7.3 Hz, 1 Harom), 7.05 (d, J = 7.5 Hz, 1 Harom), 7.25–7.29 (m, 2 Harom), 7.45 (s, 2 H, NH2), 12.35 (s, 1 H, NH), 13.86 (br s, 1 H, NH); 13C NMR (100 MHz, DMSO-d6): δ = 12.6, 34.9, 46.6, 57.6, 91.9, 108.7, 117.1, 122.8, 123.4, 128.3, 133.9, 143.1, 154.5, 159.1, 159.6, 174.6, 175.7; MS (EI): m/z: 367 (M+); anal. calcd. (%) for C17H13N5O3S: C, 55.58; H, 3.57; N, 19.06. Found: C, 55.57; H, 3.58; N, 19.10.
3.2.2 Ethyl-2-(2-amino-3-cyano-2’,5-dioxo-5,6,7,8-tetrahydrospiro[chromene-4,3’-indoline]-1’-yl)acetate (4q)
White powder; mp 236–238 °C; yield: 0.367 g (92%); IR (KBr): 3369, 3296, 3177, 2984, 2192, 1750, 1710, 1673, 1605, 1457, 1419, 1381 cm−1; 1H NMR (400 MHz, DMSO-d6): δ = 1.17 (t, J = 7.4 Hz, 3 H, CH3), 1.88–1.93 (m, 2 H, CH2), 2.18–2.22 (m, 2 H, CH2), 2.66–2.70 (m, 2 H, CH2), 4.12 (q, J = 7.2 Hz, 2 H, CH2), 4.40 and 4.54 (ABq, J = 16.4 Hz, 2 H, CH2), 6.96 (d, J = 7.2 Hz, 1 Harom), 7.2 (d, J = 7.2 Hz, 1 Harom), 7.10 (d, J = 7.2 Hz, 1 Harom), 7.25 (t, J = 7.4 Hz, 1 Harom), 7.24 (s, 2 H, NH2); 13C NMR (100 MHz, DMSO-d6) δ: 14.4, 20.2, 27.3, 36.8, 42.2, 46.7, 57.2, 61.3, 109.1, 112.2, 117.2, 123.1, 123.6, 128.7, 133.8, 142.9, 159.3, 166.7, 168.2, 178.0, 195.3; MS (EI): m/z: 393 (M+); anal. calcd. (%) for C21H19N3O5: C, 64.12; H, 4.87; N, 10.68. Found: C, 64.13; H, 4.82; N, 10.58.
4 Conclusion
In conclusion, a simple, efficient, and eco-friendly procedure is described for the synthesis of spirochromene derivatives at room temperature using [TBD][TFA] as a reusable catalyst. The present approach offers the following advantages: simple methodology, clean and mild reaction conditions, high atom economy, short reaction times, low loading of catalyst, high yield, and excellent purity of the product.
Acknowledgments
This research is supported by the Islamic Azad University, Ayatollah Amoli Branch, Amol, Iran.


