1 Introduction
Water is a versatile solvent in many ways, and performing organic reactions in this medium is now of great interest [1]. It is undoubtedly the most inexpensive among various solvents used in organic synthesis. The lack of inflammable, explosive, mutagenic, and carcinogenic properties is a favorable aspect of water in academic laboratories as well as in industry. Furthermore, water is now regarded as one of the most suitable solvents from an environmental point of view [2]. 2-Amino-4H-chromenes and their derivatives are of significant importance as they possess a wide range of biological activities, such as antimicrobial [3], antiproliferative [4], sex pheromone [5], antitumor [6] ones; they can be used in cancer therapy, and are beneficial to the central nervous system [7]. Several methods have been reported for the synthesis of 2-amino-4H-chromene derivatives using malononitrile, resorcinol and aldehydes. Various catalysts, such as piperidine [8], cetyltrimethylammonium bromide (CTABr) [9], TFE [10], Ca(OH)2 [11], MgAl/HT [12] and tungstic acid functionalized mesoporous SBA-15 [13] have been used for these reactions. Also, the Yb(OTf)3-catalyzed reactions of 5-alkylidene Meldrum's acids with phenols for the one-pot synthesis of 2-substituted chromones was reported [14]. Most of these methods require a long reaction time, high temperatures, and afford unsatisfactory yields. In order to make the reaction simple and green, herein, K2CO3 has been successfully applied to perform the three-component reaction of resorcinol, 1-(2,4-dihydroxyphenyl)ethanone or 2,4-dihydroxybenzophenone with benzaldehydes and malononitrile in water at 70 °C to provide a series of novel 2-amino-3-cyano-4H-chromene derivatives in excellent yields.
2 Results and discussion
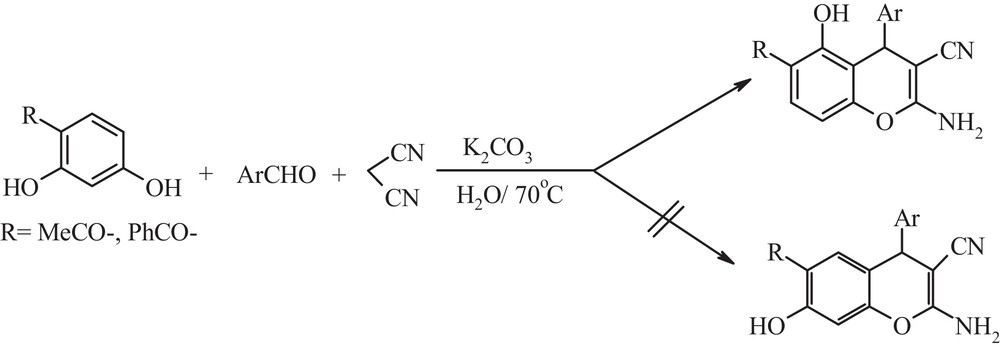
A variety of 2-amino-3-cyano-4H-chromenes was prepared from resorcinol 1-(2,4-dihydroxyphenyl)ethanone or 2,4-dihydroxybenzophenone with benzaldehydes and malononitrile in the presence of K2CO3 (25 mol%) in water as a green solvent at 70 °C in excellent yields (Table 1, entries 1–11). It is worth mentioning that the corresponding 2-amino-3-cyano-4H-chromenes were isolated by crystallization from the crude filtrate. The reactions worked well with almost all benzaldehydes with electron-donating or electron-withdrawing substituents. During the synthesis of the 2-amino-4H-chromene derivatives from resorcinol, the product 2-amino-5-hydroxy-4H-chromene was not observed; this may be due to steric hindrance by the two adjacent ortho-hydroxyls and the enol to keto tatumeric forms between hydroxyl groups in resorcinol (Table 1, 4a–e) [15]. In the reaction of 1-(2,4-dihydroxyphenyl)ethanone or 2,4-dihydroxybenzophenone with benzaldehydes and malononitrile, the substituted group enters the 2-position between the hydroxyl groups in the 1-(2,4-dihydroxyphenyl)ethanone or 2,4-dihydroxybenzophenone molecule and the product 6-acetyl-2-amino-5-hydroxy-4H-chromene or 2-amino-6-benzoyl-5-hydroxy-4H-chromene was observed, whereas the expected 6-acetyl-2-amino-7-hydroxy-4H-chromene or 2-amino-6-benzoyl-7-hydroxy-4H-chromene as a regioisomer was not produced (Scheme 1). The regioselectivity of 5a–g may be defined by the chelation of the o-hydroxy group in the 1-(2,4-dihydroxyphenyl)ethanone or 2,4-dihydroxybenzophenone molecule via intramolecular hydrogen bond formation between the acetyl or the benzoyl carbonyl group with the adjacent hydroxyl group and this chelation makes more reactive the 2-position of the 1-(2,4-dihydroxyphenyl)ethanone or 2,4-dihydroxybenzophenone molecule that reacted with substituted 2-benzylidenemalononitrile intermediates to give 2-amino-5-hydroxy-4H-chromene derivatives (Table 1, 5a–g) [11].
Synthesis of 2-amino-3-cyano-4H-chromene derivatives catalyzed by K2CO3a.
| Entry | R | Ar | Product | Time (min) | Yields (%)b |
| 1 | H | C6H5 | 4a | 25 | 87 |
| 2 | H | 4-Cl–C6H4 | 4b | 20 | 92 |
| 3 | H | 4-Br–C6H4 | 4c | 20 | 96 |
| 4 | H | 4-F–C6H4 | 4d | 15 | 98 |
| 5 | H | 4-NO2–C6H4 | 4e | 15 | 94 |
| 6 | CH3CO | 4-Cl–C6H4 | 5a | 25 | 82 |
| 7 | CH3CO | 4-Br–C6H4 | 5b | 25 | 96 |
| 8 | CH3CO | 4-F–C6H4 | 5c | 15 | 98 |
| 9 | CH3CO | 4-NO2–C6H4 | 5d | 15 | 92 |
| 10 | PhCO | C6H5 | 5e | 25 | 90 |
| 11 | PhCO | 4-Br–C6H4 | 5f | 20 | 94 |
| 12 | PhCO | 4-MeO–C6H4 | 5g | 30 | 86 |
a Reaction and conditions: resorcinols (1 mmol), aldehyde (1 mmol), malononitrile (1 mmol) and K2CO3 (0.25 mmol) in H2O (5 mL) at 70 °C.
b All yields refer to isolated products.
A pathway for synthesis of 2-amino-5-hydroxy-4H-chromene derivatives.
In a plausible mechanism, it is assumed that the reaction may proceed initially through a Knoevenagel condensation between arylaldehyde and malononitrile to form intermediate I. Next, a Michael addition of activated resorcinol derivative II (K2CO3 abstracts an acidic proton from the resorcinol derivative) to intermediate I affords III. Intermediate III converts to IV after tautomerisation. Then, intermediate IV converts into V via deprotonation of an acidic proton of the resorcinol derivative by K2CO3. Finally, the desired product VII is obtained after intramolecular cyclization and rearomatization from VI (Scheme 2).

A plausible mechanism for the 2-amino-3-cyano-5-hydroxy-4H-chromens synthesis.
3 Conclusion
We have developed an efficient and one-pot procedure for the synthesis of some novel 2-amino-3-cyano-4H-chromene derivatives by three-component condensation of resorcinol, 1-(2,4-dihydroxyphenyl)ethanone or 2,4-dihydroxybenzophenone, benzaldehydes and malononitrile using K2CO3 as a cheap and efficient catalyst in water as a green solvent.
4 Experimental
All chemicals were purchased from Merck chemical company. The melting points were recorded on an electrothermal melting point apparatus. The NMR spectra were recorded in CDCl3 with TMS as an internal standard on a Bruker Avance DRX 400 MHz spectrometer. FT–IR spectra were determined on an SP-1100, P-UV-Com instrument. The products were separated by simple filtration and identified by FT–IR, 1H NMR and 13C NMR spectra.
4.1 General procedure for the preparation of 2-amino-3-cyano-4H-chromenes
A mixture of arylaldehyde (1 mmol), resorcinol, 1-(2,4-dihydroxyphenyl)ethanone or 2,4-dihydroxybenzophenone (1 mmol), malononitrile (1 mmol) and K2CO3 (25 mol%) were dissolved in H2O (5 mL) and was heated at 70 °C for the stipulated time. The progress of the reaction is monitored by TLC. After completion of the reaction, the corresponding product was obtained through simple filtering, and the crude solid residue recrystallized from ethanol to afford the highly pure 2-amino-4H-chromene derivatives.
4.2 Spectra data of the representative compounds
4.2.1 6-Acetyl-2-amino-4-(4-chlorophenyl)-5-hydroxy-4H-chromene-3-carbonitrile (5a)
Mp: 290–292 °C. FT–IR (KBr): 1606, 1550 (aromatic CC stretch), 1107 (C–O stretch), 1662 (CO stretch), 3323, 3446 (NH2 and OH stretch), 2198 (CN stretch) cm−1. 1H NMR (400 MHz, CDCl3): δ: 2.50 (s, 3H), 4.70 (s, 1H), 6.80 (d, 1H, J = 8.8 Hz), 7.10 (s, 2H, NH2), 7.30–7.60 (m, 5H), 7.90 (d, 1H, J = 8.8 Hz), 13 (s, 1H) ppm. 13C NMR (100 MHz, DMSO-d6): δ: 205.0, 160.5, 159.8, 154.2, 144.2, 132.6, 131.7, 129.5, 128.9, 120.3, 116.4, 111.6, 107.9, 57.1, 36.0, 27.1 ppm.
4.2.2 6-Acetyl-2-amino-4-(4-bromophenyl)-5-hydroxy-4H-chromene-3-carbonitrile (5b)
Mp: 285-287 °C. FT–IR (KBr): 1606, 1550 (aromatic CC stretch), 1107 (C–O stretch), 1662 (CO stretch), 3325, 3440 (NH2 and OH stretch), 2198 (CN stretch) cm−1. 1H NMR (400 MHz, CDCl3): δ: 2.80 (s, 3H), 4.70 (s, 1H), 6.70 (d, 1H, J = 8.8 Hz), 7 (s, 2H, NH2), 7.10–7.50 (m, 5H), 7.90 (d, 1H, J = 8.8 Hz), 13.0 (s, 1H) ppm. 13C NMR (100 MHz, DMSO-d6): δ: 205.0, 160.5, 159.8, 154.2, 144.6, 132.6, 131.8, 129.9, 120.3, 120.2, 111.5, 107.9, 57.1, 38.1, 27.1 ppm.
4.2.3 6-Acetyl-2-amino-4-(4-fluorophenyl)-5-hydroxy-4H-chromene-3-carbonitrile (5c)
Mp: 278–280 °C. FT–IR (KBr): 1606, 1550 (aromatic CC stretch), 1106 (ether C–O stretch), 1658 (CO stretch), 3336, 3442 (NH2 and OH stretch), 2195 (CN stretch) cm−1. 1H NMR (400 MHz, CDCl3): δ: 2.60 (s, 3H), 4.70 (s, 1H), 6.75 (d, 1H, J = 8.8 Hz), 7.10 (s, 2H, NH2), 7.10–7.20 (m, 5H), 7.90 (d, 1H, J = 8.8 Hz), 13.0 (s, 1H) ppm. 13C NMR (100 MHz, DMSO-d6): δ: 205.0, 162.6, 160.5, 160.2, 160.0, 154.2, 141.4, 141.4, 132.5, 129.5, 129.4, 120.4, 116.4, 115.7, 115.5, 111.9, 107.8, 57.5, 35.8, 27.0 ppm.
4.2.4 6-Acetyl-2-amino-5-hydroxy-4-(4-nitrophenyl)-4H-chromene-3-carbonitrile (5d)
Mp: 250-252 °C. FT–IR (KBr): 1606, 1550 (aromatic CC stretch), 1107 (C–O stretch), 1367, 1535 (NO2 stretch), 1662 (CO stretch), 3336, 3442 (NH2 and OH stretch), 2198 (CN stretch) cm−1. 1H NMR (400 MHz, CDCl3): δ: 2.60 (s, 3H), 4.70 (s, 1H), 6.75 (d, 1H, J = 8.8 Hz), 7.10 (s, 2H, NH2), 7.20, 7.10 (m, 5H), 7.90 (d, 1H, J = 8.8 Hz), 13.0 (s, 1H) ppm. 13C NMR (100 MHz, DMSO-d6): δ: 205.0, 160.6, 159.9, 154.1, 152.6, 145.7, 133.0, 129.0, 124.0, 120.0, 116.5, 110.8, 108.0, 56.4, 38.4, 27.0 ppm.
4.2.5 2-Amino-6-benzoyl-5-hydroxy-4-phenyl-4H-chromene-3-carbonitrile (5e)
Mp: 284–286 °C. FT–IR (KBr): 1602, 1585 (aromatic CC stretch), 1081 (C–O stretch), 1652 (CO stretch), 3323, 3436 (NH2 and OH stretch), 2185 (CN stretch) cm−1. 1H NMR (400 MHz, CDCl3): δ: 4.80 (s, 1H), 6.70 (d, 1H, J = 8.8 Hz), 7.14 (s, 2H, NH2), 7.17–7.33 (m, 5H), 7.48 (d, 1H, J = 8.8 Hz), 7.53–7.65 (m, 5H), 12.50 (s, 1H) ppm. 13C NMR (100 MHz, DMSO-d6): δ: 200.0, 160.7, 160.0, 154.3, 145.2,137.6, 133.9, 132.6, 129.3, 128.9, 127.6, 120.5, 116.4, 112.8, 108.1, 57.6, 36.6 ppm.
4.2.6 2-Amino-6-benzoyl-4-(4-bromophenyl)-5-hydroxy-4H-chromene-3-carbonitrile (5f)
Mp: 248–250 °C. FT–IR (KBr): 1606 (aromatic CC stretch), 1080 (C–O stretch), 1656 (CO stretch), 3336, 3443 (NH2 and OH stretch), 2196 (CN stretch) cm−1. 1H NMR (400 MHz, CDCl3): δ: 4.80 (s, 1H), 6.72–6.75 (d, 1H, J = 8.8 Hz), 7.19 (s, 2H, NH2), 7.14–7.53 (m, 5H), 7.55–7.65 (m, 5H), 12.40 (s, 1H) ppm. 13C NMR (100 MHz, DMSO-d6): δ: 200.0, 160.7, 159.9, 154.1, 144.6, 137.5, 134.0, 132.6, 131.8, 130.1, 129.7, 129.0, 120.3, 116.5, 112.2, 108.7, 103.2, 57.1, 36.0 ppm.
4.2.7 2-Amino-6-benzoyl-5-hydroxy-4-(4-methoxyphenyl)-4H-chromene-3-carbonitrile (5g)
Mp: 232–233 °C. FT–IR (KBr): 1606, 1510 (aromatic CC stretch), 1071 (C–O stretch), 1658 (CO stretch), 3330, 3436 (NH2 and OH stretch), 2195 (CN stretch) cm−1. 1H NMR (400 MHz, CDCl3): δ: 4.80 (s, 1H), 6.80 (d, 1H, J = 8.8 Hz), 7.16 (s, 2H, NH2), 7.17–7.35 (m, 5H), 7.50 (d, 1H, J = 8.8 Hz), 7.53–7.68 (m, 5H), 12.50 (s, 1H) ppm. 13C NMR (100 MHz, DMSO-d6): δ: 200.1, 160.8, 159.9, 158.4, 154.2, 137.6, 133.7, 133.7, 132.6, 129.3, 128.9, 128.7, 120.5, 116.3, 114.3, 113.2, 108.0, 56.0, 35.8 ppm.
Acknowledgments
We are grateful to Islamic Azad University, Rasht Branch, for financial assistance of this work.



Vous devez vous connecter pour continuer.
S'authentifier