1 Introduction
Multicomponent reactions (MCRs) can be defined as special types of organic reactions in which three or more starting materials are employed in an atom-economic, convergent, efficient and timesaving manner to obtain a final product in a one-step procedure.
The 3-amino-2-oxindole moiety has drawn great attention of chemists and pharmacologists in the past few decades [1], owing to its potency and wide spectrum of biological activities, like anti-fungal [2], anti-microbial [3], anti-tubercular [4], and anti-tumor [5] functions. Besides, the 1,4-naphthoquinone unit also displays a fascinating array of biological applications, such as anti-cancer [6], anti-platelet [7], and radical scavenging [8] activities.
Therefore, developing a single molecular framework possessing biologically versatile oxindole and naphthoquinone units can be particularly promising as well as strongly desired, since they may create new medicinal properties or enhance biological activities [9,10].
As far as we know, there is no report available in the literature describing the synthesis of 3-amino-2-oxindole derivatives with 1,4-naphthoquinone fragments [11–14]. All these facts have prompted us to design a novel and highly practical approach that allows convenient formation of a wide range of 2-(3-amino-2-oxoindolin-3-yl)-3-hydroxynaphthalene-1,4-dione derivatives incorporating the above-mentioned two moieties (Scheme 1).
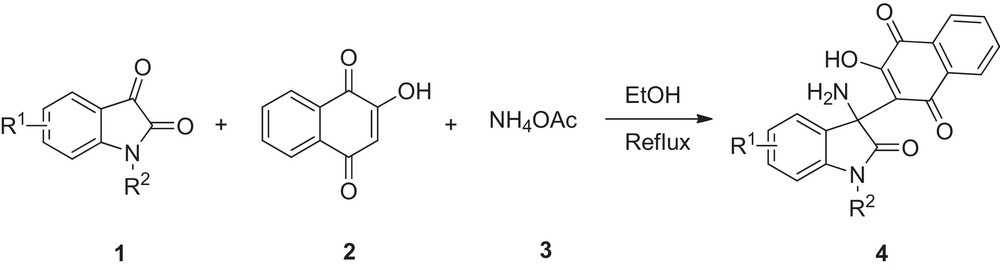
Synthesis of 2-(3-amino-2-oxoindolin-3-yl)-3-hydroxynaphthalene-1,4-dione derivatives.
2 Results and discussion
Initially, we employed isatin 1a, 2-hydroxy-1,4-naphthoquinone 2 and ammonium acetate 3 as a model reaction to establish the feasibility of the strategy and optimize the reaction conditions. To our delight, when the reaction was attempted without a catalyst, it was found that the highest yield of product was obtained after 2 h (Table 1, entry 6). Using ammonium hydroxide instead of ammonium acetate with 20 mol% HOAc resulted in a lower yield (Table 1, entry 5). Then we investigated the amount of ammonium acetate; the results indicated that 1.5 equiv of NH4OAc was the best choice for completing the reaction, since decreasing the amount of NH4OAc led to a lower yield (90%), while the use of excessive NH4OAc had no impact either on the product yield (99%) or on the rate of the reaction (Table 1, entries 6–8). Next, various solvents, such as MeOH, i-PrOH, H2O, DCM, CH3CN and THF were examined and EtOH emerged as the solvent of choice in terms of product yield (Table 1, entries 6,9–14).
Optimization of reaction conditions for the synthesis of 4aa.
| Entry | Catalystb | Solvent | Time (h) | Temp (°C) | Yieldf (%) |
| 1 | TsOH·H2O | EtOH | 2 | 78 | 80 |
| 2 | HOAc | EtOH | 2 | 78 | 93 |
| 3 | HCl | EtOH | 2 | 78 | 70 |
| 4 | Cs2CO3 | EtOH | 2 | 78 | 78 |
| 5 | HOAc | EtOH | 2.5 | 78 | 82c |
| 6 | – | EtOH | 2 | 78 | 99 |
| 7 | – | EtOH | 2 | 78 | 90d |
| 8 | – | EtOH | 2 | 78 | 99e |
| 9 | – | MeOH | 1.5 | 64 | 95 |
| 10 | – | i-PrOH | 2 | 82 | 88 |
| 11 | – | H2O | 1.5 | 90 | 70 |
| 12 | – | DCM | 2 | 39 | 60 |
| 13 | – | CH3CN | 3 | 80 | 94 |
| 14 | – | THF | 4 | 65 | 83 |
a Reactions were performed with isatin 1a (1 mmol), 2-hydroxy-1,4-naphthoquinone 2 (1 mmol) and ammonium acetate 3 (1.5 mmol) in the specified solvent (5 mL) under reflux conditions.
b Using 20 mol% catalyst.
c Ammonium acetate was replaced by ammonium hydroxide (1.5 equiv).
d Ammonium acetate (1.3 equiv) used.
e Ammonium acetate (1.7 equiv) used.
f Isolated yields.
Encouraged by the efficiency of the reaction protocol described above, the scope and specificity of this protocol were further investigated under the optimal reaction conditions (Table 1, entry 5). Thus, a broad range of structurally diverse isatins afforded the corresponding products in good to excellent yields; the results are depicted in Table 2. From these results, we could see that isatins 1 bearing substituents at the 4-position gained a lower yield due to the steric hindrance (Table 2, entries 2 and 3). Substrates having electron-withdrawing or electron-donating groups at the 5-position proceeded smoothly to give the desired compounds in excellent yields (Table 2, entries 4–9). A slight decrease in the yield was also observed when the 1-position (Table 2, entry 13) or 7-position was substituted (Table 2, entries 3, 10 and 12).
Preparation of 2-(3-amino-2-oxoindolin-3-yl)-3-hydroxynaphthalene-1,4-dione derivatives 4a.
| Entry | R1 | R2 | Product | Time (h) | Yieldb (%) |
| 1 | H | H | 4a | 2 | 99 |
| 2 | 4-Br | H | 4b | 3 | 86 |
| 3 | 4,7-diCl | H | 4c | 4 | 83 |
| 4 | 5-F | H | 4d | 2 | 99 |
| 5 | 5-Cl | H | 4e | 2 | 97 |
| 6 | 5-Br | H | 4f | 2 | 93 |
| 7 | 5-I | H | 4g | 2 | 90 |
| 8 | 5-CH3 | H | 4h | 2 | 96 |
| 9 | 5-OCH3 | H | 4i | 2 | 96 |
| 10 | 5,7-diCH3 | H | 4j | 4 | 83 |
| 11 | 6-Cl | H | 4k | 2 | 97 |
| 12 | 7-CF3 | H | 4l | 2 | 81 |
| 13 | H | Bn | 4m | 3 | 80 |
a Reactions were conducted with 1 (1 mmol), 2 (1 mmol) and 3 (1.5 mmol) in EtOH (5 mL) under reflux for the indicated time.
b Isolated yields.
Finally, the structural elucidation was unequivocally determined by single-crystal X-ray analysis of 4e (Fig. 1).

(Color online.) ORTEP drawing of 4e1.
Taking all this data and previous studies into consideration, two reaction mechanisms may be tentatively proposed (Scheme 2). The former route shows that the isatin CO group is activated by NH4+ with elimination of NH3 at first. Next, Michael addition of 2-hydroxy-1,4-naphthoquinone 2 to intermediate A occurs to provide intermediate B, which is attacked by AcO− to form intermediate C with the elimination of HOAc. Finally, NH3 reacts with intermediate C to give the desired product 4 with the elimination of H2O [15,16]. The latter path indicates that isatin 1 converts to intermediate E by reacting with ammonium acetate 3 with elimination of H2O and HOAc. Intermediate F results from the subsequent addition of 2-hydroxy-1,4-naphthoquinone 2 to intermediate E and undergoes tautomerization to generate the target product 4 [17].
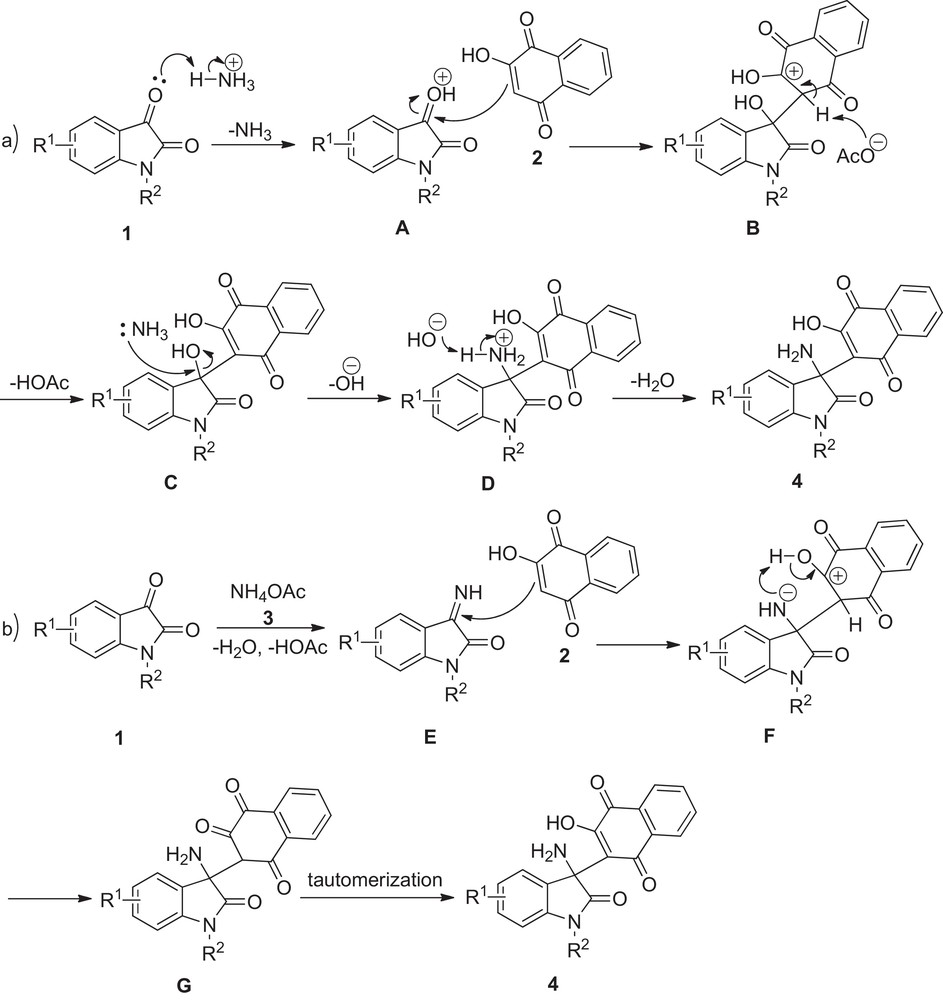
The plausible reaction mechanism.
3 Conclusion
In summary, we have successfully demonstrated a concise, facile and straightforward synthetic method for the preparation of 2-(3-amino-2-oxoindolin-3-yl)-3-hydroxynaphthalene-1,4-dione derivatives under catalyst-free conditions. Prominent advantages within this new method are operational simplicity, overall good to excellent yields and easy work-up procedures. Further studies on the application of this procedure are ongoing in our laboratory.
4 Experimental
4.1 General procedure
A mixture of isatin (1 mmol), 2-hydroxy-1,4-naphthoquinone (1 mmol) and ammonium acetate (1.5 mmol) in EtOH (5 mL) was stirred magnetically under reflux for the appropriate time (Table 2). After completion of the reaction, as indicated by TLC, the formed precipitate was collected by filtration and washed with hot EtOH to furnish pure 2-(3-amino-2-oxoindolin-3-yl)-3-hydroxynaphthalene-1,4-dione derivatives in good to excellent yields without column chromatographic purification.
Acknowledgments
We gratefully acknowledge financial support from the Foundation of Natural Science of Zhejiang Province (No. LY12B02016) and the State Key Laboratory Breeding Base of Green Chemistry-Synthesis Technology, Zhejiang University of Technology (People's Republic of China).
1 CCDC-1003513 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.


