Throughout history, the discoverers of the elements have given them various names, which sometimes inform us about their properties, sometimes about the natural substances from which these elements were extracted, or sometimes about the circumstances of their discovery. Etymology thus brings us into the history of science. We shall mention in this text the names of chemists who have made a particularly significant contribution to the list of known elements to date. Among these chemists, Lavoisier holds a prominent place.
The text will present successively: names related to Lavoisier's fundamental discoveries; names derived from astronomy, then from mythology; names of botanical origin; others linked to geography; and finally, names taken directly from ancient Greek. In addition, this presentation will pay tribute to Mendeleev, the great architect of the periodic table in 1869, whose greatest genius was to predict the existence of elements still unknown and actually discovered later. The epilogue will also pay tribute to the French geologist Chancourtois, who presented his Vis tellurique to the Académie des sciences in 1862, the first demonstration of the periodicity of the elements.
1. The names accredited to Lavoisier
Lavoisier was a revolutionary theorist, if one dares to say, considering the fate that the French Revolution reserved for him, as well as a brilliant experimenter.

Lavoisier (1743–1794).
Copyrighted work available under Creative Commons Attribution only license CC BY 4.0.
His work, beginning in the 1770s, led to the first evidence of chemical elements as such, well-known today by the names of carbon, oxygen, hydrogen, and nitrogen. These names are attested and justified in two founding texts: the Méthode de nomenclature chimique (1787) by MM. de Morveau, Lavoisier, Berthollet and Fourcroy, as well as Lavoisier's Traité élémentaire de chimie (1789), in which he establishes the very notion of the chemical element, under the term "simple substance".
Lavoisier demonstrated the existence of the element carbon from his experiments on the combustion of diamond, carried out at a high temperature in the hearth of a solar furnace (equipped with an 80 cm diameter lens built by the German glassmaker Tschirnhaus) [1].
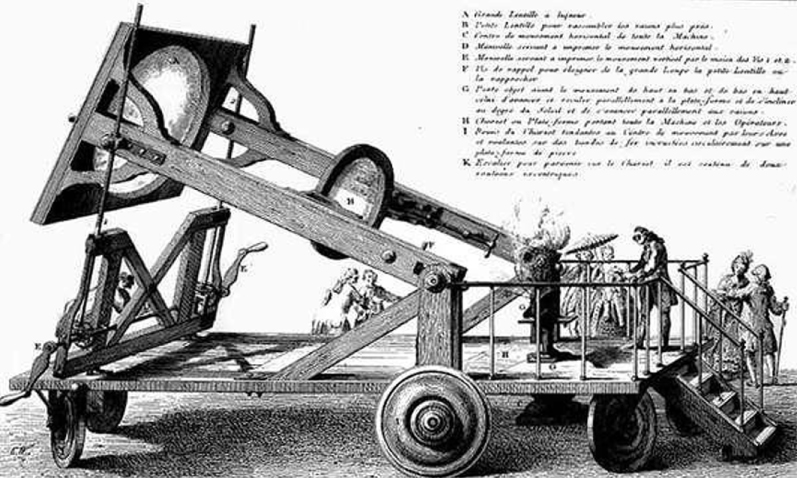
Solar furnace with Trudaine's lens, used for public experiments on the combustion of diamonds.
(This file was provided to Wikimedia Commons by the Science History Institute).
Noting that the gas from this combustion was the same as that obtained by burning coal, he established the existence of a common element, first called "carbonaceous substance," then with de Morveau:
— carbon, from Latin carbo, carbonis "charcoal."
Oxygen and hydrogen were isolated soon afterwards thanks to Lavoisier's experiments on the decomposition and recomposition of water.
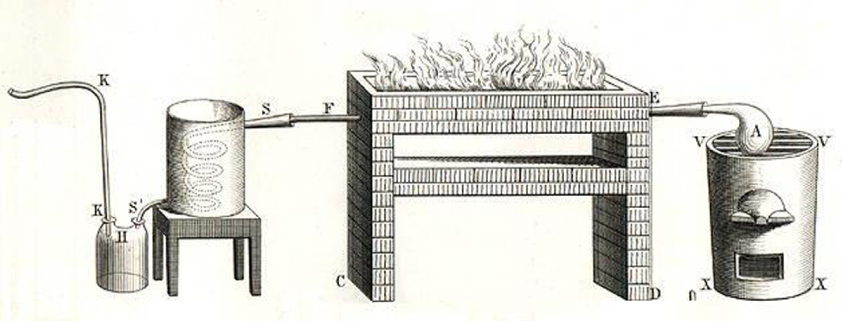
Lavoisier's device for studying water decomposition (Traité élémentaire de chimie, 1789)
Digital copy of the Dumas & Grimaux edition text, 1862, BNF, author: LaosLos (from a .pdf file).
Lavoisier gave the two gases produced by the decomposition of water the following names:
— oxygen meaning "which generates acid," from the Greek oxus "acute, pungent, sour," hence also oxos, "vinegar". He assumed that oxygen was present in acetic acid, nitric acid, sulfuric acid, etc.
- hydrogen meaning "which generates water," implying "with oxygen".
However, water contains much more oxygen in mass than hydrogen, and it is now known that the acid function is because of hydrogen, not oxygen. The relevance of the names oxygen and hydrogen has moreover been disputed since 1787, but of course no one dared to change, or even exchange, the names accredited to Lavoisier.
Having finally shown that air was composed of oxygen and of another gas (nitrogen), which "needed a unique name," Lavoisier opted in 1787 for:
— azote (French, in English: nitrogen), from the Greek azotikos "no life."
This time, however, Chaptal, as early as 1790, preferred the name nitrogen, meaning "which generates nitric acid," a name that the Anglo-Saxon world adopted very quickly, hence the symbol N adopted by the international community in the end.
In short, Lavoisier put an end to the reign of the four elements of antiquity by replacing three of these elements (earth, water, and air) with four chemical elements: carbon, oxygen, hydrogen, and nitrogen. These four elements appear in the first table (not yet periodic) of elements which he published in 1789, along with 19 others, including sulphur, phosphorus, arsenic, and 16 metals, of which only 7 were known in antiquity.
2. Astronomically inspired names
— The magic of the number 7
The Ancients, and later the alchemists, cultivated a sort of numerology based on the number 7 by associating the 7 metals known since time immemorial to the 7 non-fixed celestial bodies they could observe (the Sun, the Moon and the five planets identified at the time, Saturn being the farthest visible to the naked eye), bearing the names of 7 mythological Greco-Latin deities. After some hesitation for certain planets, the subsequent correspondences were imposed [2, p. 154]: The Sun and the brilliance of gold; the Moon and its silver light; the red of Mars and the iron used for war; the yellow of the planet Venus and a copper mirror (an abundant metal in Cyprus, the island of Venus); the white of Jupiter, a lightning bolt, and tin; the pallor of Saturn whose slowness (orbiting the Sun in 30 years) matches with with the heaviness of the dull metal that is lead; and on the contrary, the velocity of Mercury (orbiting the Sun in three months) evokes the fluidity of quicksilver (later named mercury) and the agility of traders.
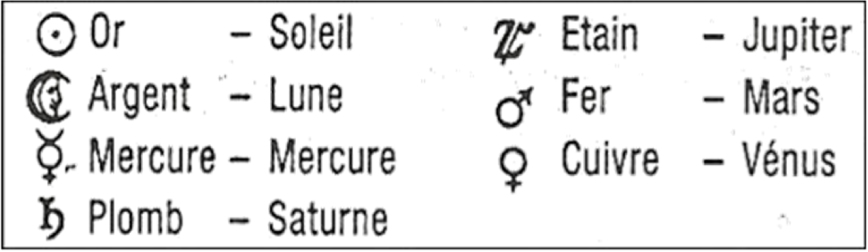
Alchemist table.
This metal-celestial body-divinity relationship prevailed for a very long time as the first metals identified beyond the 7 of Antiquity appeared only at the end of the Middle Ages, with zinc, antimony, then a few others. As for the first planet beyond Saturn, it was only discovered in 1781 by Herschel. It was named Uranus, the name of Saturn's father in mythology.
— The planet Uranus and uranium metal
In 1792, the German chemist Martin Klaproth identified a new metal in pitchblende [3, pp. 160, 172]. Having pointed out that a dozen or so metals had already been discovered beyond the 7 of antiquity without being able to pair them with new celestial bodies, he hastened to associate the new planet with his new metal, which he named uranium (originally uran).

Martin Klaproth (1743–1817).
Wikimedia Commons; author: Ambroise Tardieu (engraving) after original portrait by Eberhard-Siegfried Henne.
— Transuranic planets and transuranic metals
Beyond Uranus, two other planets were subsequently discovered, still named in the mythological tradition: Neptune in 1846 and Pluto in 1930 (which became a dwarf planet in 2006). These planets, called transuranic planets, are located beyond the solar system that had been observed since 1781, like an extension into the unknown, which the irregularities of the orbit of Uranus suggested. Similarly, the so-called transuranic elements have been discovered. Located beyond uranium, the heaviest of the natural elements, an extension into another unknown. These discoveries were made during nuclear physics research in the 1940s in the United States, mainly under the aegis of Glenn Seaborg (1912–1999) at Berkeley. Thus, after uranium (element 92), neptunium (element 93) and plutonium (element 94) were named after the planets Neptune and Pluto. This research demonstrated successive decay reactions of the following type:
— mythological, Uranus being the grandfather of Neptune and Pluto,
— astronomical; uranium, neptunium and plutonium appear in the order of the planets,
— radiochemical; from parent nucleus to daughter nucleus.
As we have just seen, the names of astronomical origin also refer to Greco-Latin mythology. However, other names come directly from mythology, Greco-Latin or otherwise.
3. Names of mythological inspiration
Shortly after dedicating uranium to Uranus, Klaproth discovered another metal and extended its mythological metaphor by naming it titanium, in reference to the Titans, sons of Uranus.
We can also mention tantalum, so named by its discoverer, the Swedish chemist Ekeberg, because of the impossibility for this metal to react with the acids in which it is immersed, recalling the impossibility for Tantalus, plunged into Tartarus, to feed and drink: an unexpected consequence of Tantalus' torture.

The temptation of Tantalus in Tartarus, in the Underworld.
Bernard Picart engraving, The Temple of Muses (1733).
Greek mythology is not the only mythology to appear in the periodic table. Thorium and vanadium, named by the great Swedish chemist Berzelius after the Scandinavian deities Thor and Vanadis, can also me mentioned.

Vanadis and Thor, who inspired the names of vanadium and thorium.
Wikimedia Commons: James D. Penrose: Freyja and the Necklace (1890); Mårten E. Winge: Thor's Fight with the Giants (1872).
We can also find Germanic mythology, which explains the names of cobalt and nickel. The story takes place in the 18th century in the German mines, where the miners led a frighteningly difficult life, for many reasons. First of all, these miners were searching in vain for copper, and could not recognise nor use cobalt or nickel. Secondly, they were seriously intoxicated by roasting ores that were sulphides and arsenides. Lastly, gallery collapses were deadly.
In German legends, those responsible for all these accidents suffered by the miners were the Kobolds, small evil goblins who haunted the underground mines. The miners called the ores they found by the German name Kobold (in English kobold), which inspired the Swedish chemist G. Brandt to name the cobalt, which he identified in 1735.
As for nickel?
To appease the wicked kobolds, the miners would give them a nickname, often Nickel, a diminutive of Nicolas, the name of the most popular saint in the Germanic regions. Thus, German dictionaries define a Nickel as a particular Kobold. The miners even used the term Kupfernickel, i.e., "the little Nicolas of copper," who promises you copper and instead poisons you. It is understandable that when the Swedish chemist Cronstedt identified nickel in 1851, he named it after the name given to cobalt some 15 years earlier.
4. Names of botanical origin
Soda is a chemical product that is well known for being caustic (coming, through latin, from the Greek kaustikos, from the verb kaiein "to burn"). But what is the original meaning of the word soda?
Soda is first of all the ancient name of a plant, today named saltwort.

Common saltwort (Salsola soda).
GFDL license authorised by L. Rignanese on 2-November-2006.
Like salicornia (e.g. glasswort), it is a plant described as halophilic ("salt-loving"), growing on sea shores where soils are impregnated with salt.
There are many species of saltwort, characterised by their high concentration of mineral salts, which are now known to be mixtures of carbonates and nitrates, of sodium and potassium. Since ancient times, these plants have been burned to obtain various substances from their ashes, such as those known today as soda and potash.
The English chemist Humphry Davy used electrolysis on these substances to obtain metals which he named sodium and potassium in 1808. He derived potassium from the English "potash," formed from "pot" and "ash," from which the word potasse in French is also derived.

Humphry Davy (1778–1829).
Copyright: Wikipedia Commons.
As for sodium, Davy derived it from the Late Latin word soda, which simultaneously referred to soda the plant and to the substance obtained from its ashes. This word soda is of Arabic origin. It is related to the Late Latin sodanum, "migraine remedy," from the Arabic sudā meaning "migraine," without doubt in reference to the beneficial effect of the bicarbonate present in these plants. These were also called qali in Arabic, and with the article, al-qali, hence alkali, the name given to the basic salts derived from plant ashes, and the adjective alkaline. This time, the term is related to the verb meaning "to burn, to grill" in Arabic.

Berzelius (1779–1848).
Copyright: Wikipedia Commons.
Finally, a student of Berzelius discovered a new metal in 1817 from an ore found in Sweden. The metal was alkaline, like sodium and potassium. Berzelius called it lithium, from the Greek lithos, "stone," to recall that it had been "discovered in the mineral kingdom, while the other two (sodium and potassium) were discovered in the plant kingdom."
We now come to element names inspired by geography. This provides an opportunity to talk about the work of Mendeleev, beginning by describing the current status of the periodic table.
5. Names of geographical origin
— The current periodic table
The periodic table currently contains 118 elements, 90 of which are natural, from hydrogen (element 1) to uranium (element 92), except elements 43 and 61 which are obtained artificially, as are the transuranic elements from 93 to 118. In total, 90 natural elements and 28 artificial, all radioactive.
This table is presented in the form of 18 columns and 7 rows (Figure 1).
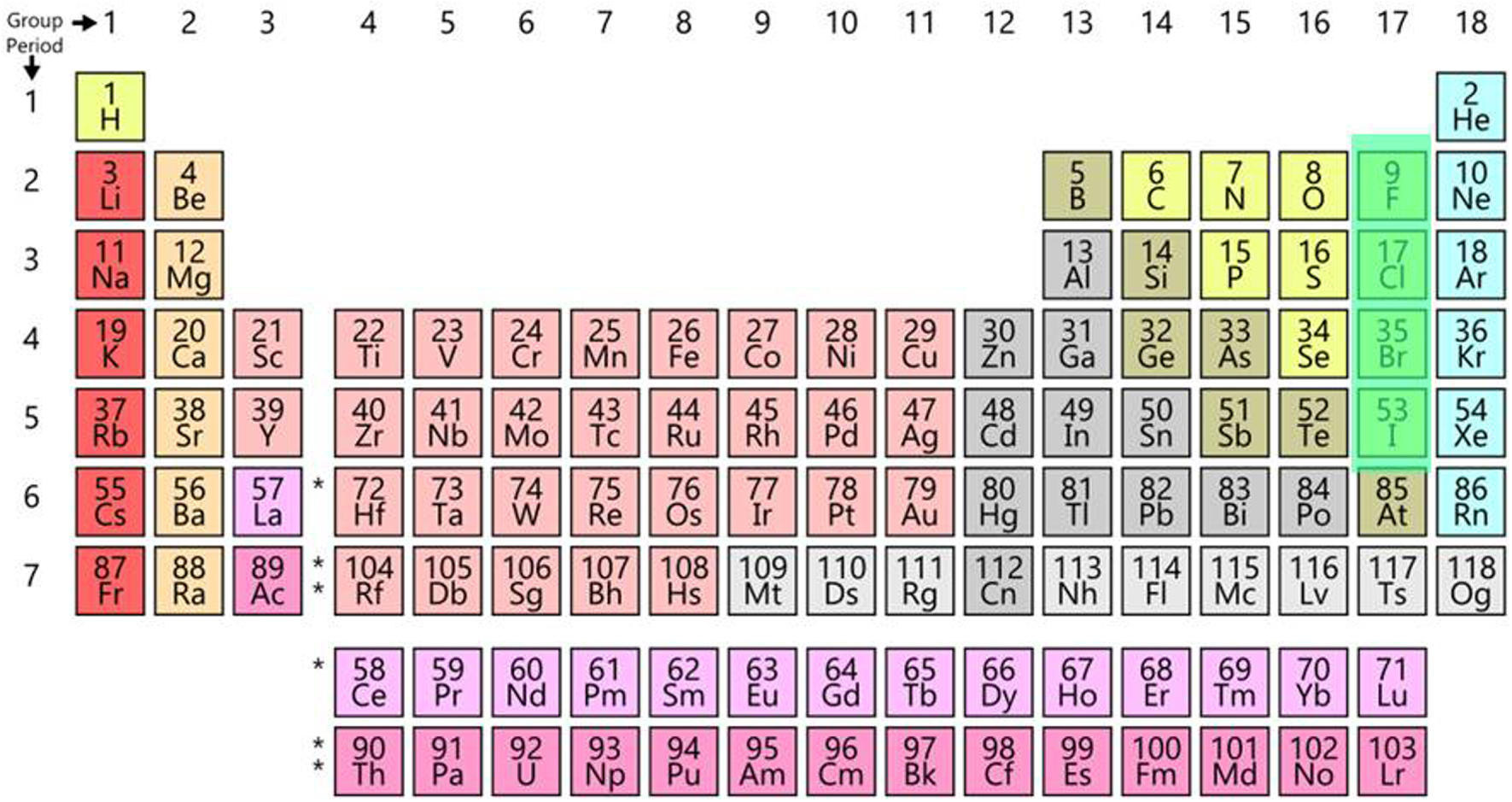
The current periodic table.
Let us recall the names of the main families:
column 1: alkali metals
column 2: alkaline earth metals
columns 3 to 12: transition metals, including rare-earth elements (in column 3 and row 6) and actinides, including transuranic elements (in row 7).
columns 12 to 16: post-transition metals, metalloids, and non-metals
column 17: halogens (except astatine, a metalloid)
column 18: noble gases (formerly rare gases)

The table drawn up by Mendeleev in 1872, following his 1869 publication. Mendeleev in 1861. Photo by Sergey Lvovich Levitsky.
— Mendeleev's publication
It is interesting to compare the current periodic table to Mendeleev's publications in 1869 and the following years, particularly his table from 1872 (Figure 2):
The table has 63 elements, 62 of which are valid since didymium (symbol Di) was proved in 1886 to be a mixture of two rare earth elements: praseodymium and neodymium.
This table was a considerable step forward in understanding the periodicity of chemical properties according to the atomic mass of the elements:
— on the one hand, the 62 elements known at that date were arranged in 17 rows, corresponding to the first 17 columns of the 18 in the current table. This classification is of remarkable relevance, the transition metals in particular being ranked in the right order for the first time.
— on the other hand, Mendeleev's genius was to foresee the existence of still unknown elements, actually discovered later and placed in the locations anticipated in his table. This forecast is shown schematically in the table of Figure 2 by five arrows (one could have placed a dozen). The first case appeared in 1875, with gallium, which took its place in line 13 between aluminium and indium, and which Mendeleev had anticipated under the name of eka-aluminium (eka = 1 in Sanskrit).

François Lecoq de Boisbaudran (1838–1912) and the cock of his family coat of arms.
Wikimedia Commons: unknown author.
— Gallium
It's quite the story that is linked to the name of gallium, discovered in 1875 using spectroscopy by François Lecoq de Boisbaudran. He proposed the name gallium in 1875 without giving the slightest justification, which is highly unusual. The international community has accepted gallium anyway, but at the same time, a somewhat controversial interpretation spread: the discoverer put himself forward by taking inspiration from his own name, symbolised proudly by a cock (in Latin, gallus) on the family coat of arms. Such an attitude had never been seen; even if elements were later named in honour of great scientists, it was avoided while they were still alive.
After giving free rein to interpretations, Lecoq de Boisbaudran revealed in 1877, in the Annales de Chimie et de Physique [4, p. 103], that he had named gallium "in honour of France (Gallia)." The problem is that this written explanation came late, and many continued to see gallium as a derivative of gallus "cock." However, the justification given by Lecoq de Boisbaudran was credible: almost thirty years earlier, the name of ruthenium was given based on the Late Latin name Ruthenia, of Russia. Moreover, by dedicating gallium to France, he was honouring his country during a period of great tension with Germany. However, in 1886 the German chemist Winkler discovered a new element, which he unequivocally named germanium, from Germania, the Latin name for Germany. This was seen as a response to Lecoq de Boisbaudran, and a posteriori, it strengthened the connection between gallium and Gallia.
In summary, it can be accepted that gallium refers explicitly to France, the nationality of its discoverer, and implicitly to his name. In this regard, the Latin gallus "cock" may simply mean "Gallic (bird)," the cock having been considered typically Gallic by the Romans, who joked about the pun Gallus gallus, "gallic cock" (which remains the sports emblem of France!). In both cases, gallium flatters the national pride of France!
6. Names taken directly from Ancient Greek
— An extraordinary event
During the solar eclipse of 1868, astrophysicists detected an intense yellow ray in the spectrum of the solar corona, which was initially thought to be sodium, then was later attributed to a new element, unknown on Earth at the time. Logically, this "extraterrestrial" element was named helium, from the Greek helios, "Sun." It was not until 1882 that helium was detected in a volcanic rock, then, in 1895, the English chemist William Ramsey successfully isolated it from uranium ore.

Total solar eclipse
— The discovery of noble gases (formerly rare gases)
In fact, when he discovered helium, Ramsay was seeking in uranium ore the gas he had isolated the previous year (1894). As this gas appeared inert, unable to react with other substances, he named it argon, from the Greek argos, "inactive," itself formed by the privative prefix a- and ergon, "action, work" (from which we obtain energy...). It appeared that helium and argon were gases of a new nature, paving the way for the discovery of other gases, which Ramsay isolated using an increasingly potent distillation of liquefied air.
Ramsay was also a linguist, and he derived (in English, borrowed from other languages) the names of these gases from the Greek adjectives neos, argos, kruptos, and xenos, in the neutral form (suffix -on) to reflect the extreme neutrality of these monoatomic gases:
neos "new" → neon
argos "inert" → argon
kruptos "hidden" → krypton
xenos "strange" → xenon
The name of krypton is understandable because this gas was found "hidden" in argon (therefore impure). The name xenon is the result of a more complicated history.

The bluish colour of xenon headlights
— Xenon
The Greek xenos means first of all "foreign", hence also by metaphor, "unknown, stranger." However, Ramsay used the adjective stranger, not foreigner [5 p. 106]. But then, why strange? Because of the unexpected blue colour in the emission spectrum of this new gas. They first looked for a name evoking blue, but while avoiding radicals/etymological roots that had already been used for a long time in organic chemistry. Thus for example * cyanon, from the Greek kuanous, "blue" was not suitable because this radical had already been used for a long time in the word cyanide. Finally, for lack of an available "blue" radical, the idea was to base the name on the Greek xenos, still unused in chemistry, xenon in this case meaning "strange (because of an unexpected blue colour)."

William Ramsay (1852–1916).
Wikimedia Commons: Popular Science Monthly Vol. 67, 1905, unknown author.
— The 18th column
All these new elements posed a problem: they did not fit in any free space in the existing periodic table at the time, as it only had 17 columns. The existence of an 18th column was not anticipated by Mendeleev, nor by anyone else. It was Ramsay and Rayleigh who proposed it to accommodate helium and argon, then neon, krypton, and xenon shortly thereafter. Initially, Mendeleev even rejected the idea, only to finally acknowledge in a 1902 letter that the 18th column was justified, and constituted "a glorious confirmation of the general character of the application of the periodic law" [6, p. 144].
The Vis tellurique of Chancourtois as an epilogue
Mendeleev was not the first to demonstrate a periodicity in the properties of the elements according to their mass. Based on the dates of publication, this credit goes to the geologist Chancourtois, who proposed in 1862 a classification of elements based on a helix traced on a cylinder, known as “Vis tellurique" (i.e. Telluric screw, as a reference to Tellurium, as well as to a theory about elements of the Earth). This first periodic system was not as efficient as the one published by Mendeleev in 1869, but it was nonetheless a major innovation.

Alexandre-Émile Béguyer de Chancourtois (1820–1886).
Wikimedia Commons: date 15 February 2007.
Chancourtois had the idea of ordering the elements by increasing mass by aligning them along 45° inclined segments to constitute a table that can be described as oblique.

The Vis tellurique of Chancourtois, preserved at the École des Mines de Paris
Thus, Chancourtois correctly placed the first two or three elements of columns 1, 2, 13, 14, 15, 16, and 17: sodium was, for example, placed below lithium, calcium below magnesium, silicon below carbon, chlorine below fluorine, etc. He then rolled up the table on a cylinder, so that the 45° segments formed a continuous helix, the chemical families then being on the generatrices of the cylinder.
However, this system lost all relevance from the third turn of the helix onwards, because Chancourtois had not classified transition metals correctly, which Mendeleev would later accomplish by prefiguring columns 3 to 12 of the current table.
Even though there were other contributors, including Lothar Meyer in Germany, we can consider that the three main builders of the periodic table were Chancourtois, for the initial idea; Mendeleev for the understanding of the essentials of the table; and Ramsay for the 18th column, which can be qualified as the keystone of the system.
This suggests the following metaphorical diagram:
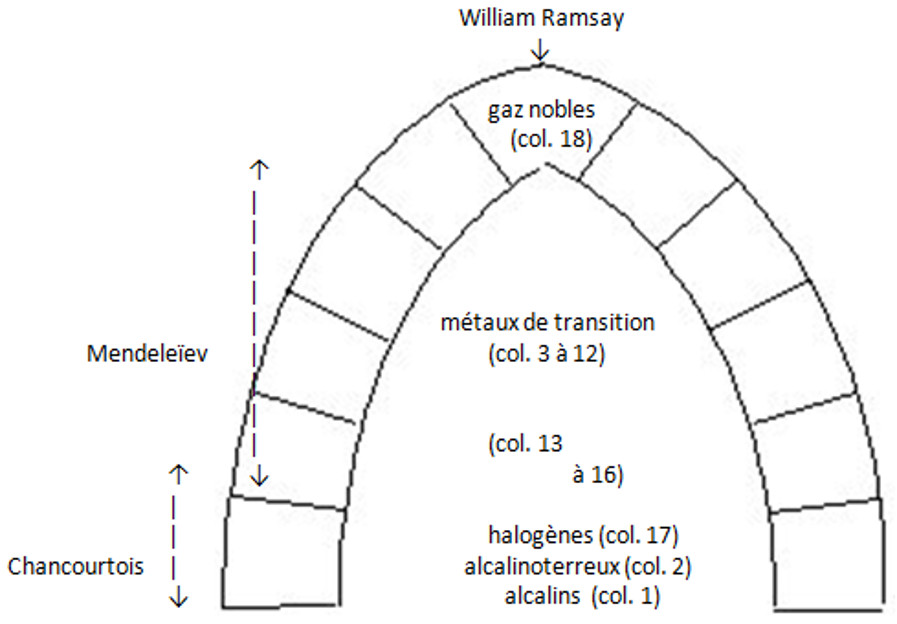
The periodic table is a construction whose leading architects were Chancourtois (1820–1886), Mendeleev (1834–1907) and Ramsay (1852–1916).
The table was gradually filled thanks to the discoverers of elements, of which those appearing in this article were among the biggest contributors:
Lavoisier first with the initial 23 elements in 1789, Martin Klaproth, Humphry Davy, Berzelius, Lecoq de Boisbaudran, William Ramsay and his column of noble gases, and Glenn Seaborg and his line of transuranic elements.
We owe the discovery of nearly two thirds of the hundred or so chemical elements that constitute our universe to these great figures in the story of chemistry.
For more information:
La Prodigieuse histoire du nom des éléments, 2018, in English, The Amazing History of Element Names, 2020, EDP Sciences/SCF, Paris, by Pierre Avenas, with the collaboration of the scientific journalist Minh-Thu Dinh-Audouin, foreword by Jacques Livage.



 CC-BY 4.0
CC-BY 4.0