1. Introduction
In the current Anthropocene epoch, insect populations face drastic decline (“insectageddon”; [1]) and bees (Hymenoptera: Anthophila) are no exception [2]. Bees are among the most important groups of pollinators for wild plants and agricultural crops. They support the seed set of more than 85% of wildflower species [3] and benefit the production of roughly 75% of the 115 most important crops worldwide [4, 5]. In Europe, although biotic pollination is crucial for only 12% of the cropland area, it enables more than 31% of the EU income from crop production [6], reaching more than $17 billion annually [7]. Moreover, bees provide nutrient-rich food for human populations [8]. They therefore play a compelling role in natural ecosystems as well as in human societies [9, 10, 11], making their persistence a matter of both scientific and societal importance.
The consensus is that bee population decline is the product of multiple factors that can act singly or in combination, the most known being diseases and parasites, invasive species, intensive agriculture, climate change, and pesticides (i.e., insecticides, herbicides and fungicides) [12, 13]. Among these factors, land use (i.e., agricultural intensification and its associated landscape simplification) is considered an important driver of pollinator decline [14, 15, 16]. Indeed, in the ongoing human-driven landscape homogenisation (i.e., diminishing resource quality) and natural habitat destruction (i.e., diminishing resource quantity [17]), bee populations face serious challenges to find suitable feeding and nesting resources [18, 19, 20]. Besides, human activities also influence natural bee-pathogen dynamics, either increasing or decreasing pathogen prevalence, typically through the global trade of commercial pollinators [21] and landscape modification [22]. Such human-disturbed host-pathogen dynamics also contribute to bee population decline [23], since bees suffer from taxonomically diverse pathogens and parasites [24] that play crucial roles in shaping their communities [25]. A dearth of suitable resources may then be even more worrying as bees depend on specific flower species not only to meet their nutritional demand, but also to deal with parasite infection, most likely relying on some beneficial specialised metabolites in pollen and nectar [26, 27]. For example, flavonoids, a broad class of specialised metabolites widely found in pollen [28], have been shown to display antioxidant and antimicrobial properties [29]. The flavonoid kaempferol and the isoflavonoid biochanin A have been found to reduce Vairimorpha spp. (formerly Nosema spp.; Microsporidia: Nosematidae) load in honey bees [30] and bumble bees [31], respectively. Flavonoids have even been shown to help bees face other stressors, such as pesticide exposure (e.g., [32, 33]).
Issues could arise when plant species with medicinal properties decline, as Koch et al. [34] raised concerns regarding worldwide heathland decline since the norisoprenoid callunene found in Calluna vulgaris (Ericales: Ericaceae) nectar helps bumble bee wipe out the obligate intestinal parasite Crithidia sp. (Euglenozoa: Trypanosomatidae). This parasite is known to cause premature death in food-stressed bumble bees [35] as well as impair foraging efficiency [36], decrease likelihood of reproduction [37], decrease queen bee’s survival to diapause [38] and reduce successful colony initiation [39]. In addition, bees’ immunocompetence is a cornerstone in the nutrition-infection interplay and this parameter has often been assessed through fat body content, a crucial tissue involved in energy metabolism and antimicrobial peptide production in insects [40]. Both nutrition and infection, including specialised metabolites (e.g., [41, 42]) and Crithidia sp. [43], have been shown to impact this organ. As a consequence of the nutritional and parasite stress undergone by bees, there has been an unanimous concern over the loss of bee populations and an urge for conservation strategies [44, 45], which have led to an increasing number of research around bees (Supplementary Figure S1) to promote suitable floral resources.
Most studies focusing on bee resources have principally considered herbaceous plants (e.g., [46]) but recently, Donkersley [47] underlined the importance of neglected woody species for pollinators, notably because (i) they provide wooden nesting materials and sites, (ii) they offer refuge during wind and rain, (iii) they produce a great amount of floral resources (i.e., in three dimensions) and (iv) they show an extended flowering time. Wood et al. [48] even showed that woody species are as suitable (e.g., Fabaceae species) or even more suitable than some herbaceous species (e.g., Asteraceae species) for bumble bee colony development. Considering the phenology is also crucial since bumble bee queens establish their colony in early spring and hence rely on early flowering plants for brood development. One remarkable tree species is the willow (Salix spp.; Salicales: Salicaceae) that represents an important resource for spring bee species, particularly in wetlands and wet heathlands, blooming in early spring and offering a huge amount of nutritive and easily accessible pollen (e.g., [49, 50, 51]). However, riparian zones and their associated willows are declining, notably as a consequence of stream diversions, groundwater pumping, extended drought, and replacement with less desirable shrubs and trees [52, 53, 54], thereby threatening bumble bee colony establishment. Hence, bumble bee queens must rely on other tree resources in early spring to initiate their brood with abundant and nutritive pollen, but also to deal with parasites such as Crithidia sp.
Among spring-blooming tree species, bumble bees can forage on fruit trees in orchards. For instance, mass-flowering orchard species such as sweet cherry (Prunus avium; Rosales: Rosaceae), apple (Malus domestica; Rosales: Rosaceae) and pear (Pyrus communis; Rosales: Rosaceae) trees represent important resources for early emerging bee pollinators [55, 56, 57]. Indeed, human populations rely increasingly on mass-flowering crops, including fruit crops [17], but their consequences for pollinator-parasite dynamics remain unclear [58, 59]. Besides, bumble bees can also benefit from non-crop trees used in hedgerows such as the hawthorn (Crataegus monogyna; Rosales: Rosaceae) known as a dominant woody species in hedgerows [60], benefiting insect pollinators [61, 62] and especially bumble bees [63]. Indeed, to address pollinator decline in Europe, agri-environment schemes have been introduced, including the implementation of hedgerows along field boundaries to provide additional food resources and nesting sites for bees [64, 65]. However, despite the well-founded importance of such trees for pollinators, the suitability of their pollen—including their specialised metabolites—for social bee species development as well as their potential benefits to deal with parasites remain poorly explored [48].
To address this gap, we evaluated how tree pollen found either in mass-flowering orchards (i.e., sweet cherry, apple and pear) or in hedgerows (i.e., hawthorn) and their respective flavonoids impact healthy and parasite-challenged buff-tailed bumble bees Bombus terrestris (Hymenoptera: Apidae), the second-most economically important bee pollinator worldwide [66]. For the parasite challenge, we used the trypanosomatid gut parasite Crithidia sp. (Euglenozoa: Trypanosomatidae), the most prevalent parasite species in bumble bee populations [67, 68] on which non-flavonoid specialised metabolites were found to have an effect in previous studies (e.g., [27, 69]). Specifically, we provided B. terrestris microcolonies with tree pollen diets or flavonoid extract-laced control diets, and observed the effects at the microcolony and individual levels. We also assessed whether impacts varied between healthy and infected bumble bees, and the differences in parasite load.
2. Materials and methods
2.1. Experimental design
The “hedgerow” experiment (i.e., hawthorn pollen) was conducted from April to June 2021 while the “orchard” experiment (i.e., mix of sweet cherry, apple, and pear pollen; hereafter “orchard mix”) was conducted from April to June 2022. In both experiments, B. terrestris microcolonies were distributed among a 2 × 3 factorial design (i.e., six treatments), with infection (uninfected or infected) crossed with diet (control, natural or flavonoid). The control diet consisted of willow Salix sp. pollen, the natural diet consisted of hawthorn pollen or orchard mix, and the flavonoid diet consisted of willow Salix sp. pollen laced with flavonoid extract from hawthorn pollen or orchard mix. Honey bee-collected willow Salix sp. pollen was purchased from the company “Ruchers de Lorraine” (Nancy, France) while hawthorn pollen and the orchard mix were purchased from the company “Pollenergie” (Saint-Hilaire-de-Lusignan, France). Pollen was ground and mixed with sugar syrup (water:sucrose 35:65 w/w) to be provided to microcolonies as candies (see Supplementary Section 1 for flavonoid extraction and details about diet preparation).
For both experiments, a set of five colonies was ordered from Biobest bvba (Westerlo, Belgium; the five colonies in the two experiments were different). Fifteen and ten queenless B. terrestris microcolonies were established for each treatment in the hedgerow and orchard experiments, respectively (i.e., three and two microcolonies per colony, respectively). Each microcolony was made of five workers (inoculated or not) placed in distinct plastic boxes (10 cm × 16 cm × 16 cm). For the hedgerow experiment, microcolonies (n = 90) were initiated with 1 g of willow pollen for three days before being fed with their respective diet while in the orchard experiment, microcolonies (n = 60) were directly provided with their respective diet upon the onset of the bioassay. Every microcolony was fed ad libitum with its respective diet for 35 days using pollen candies (1–3 g depending on the size of the microcolony) and sugar syrup (water:sucrose 35:65 w/w). Pollen candies were freshly prepared and renewed every other day while weighing pollen left and syrup container to determine resource collection. Collection data were corrected for evaporation using controls placed in bee-free boxes. Dead workers and ejected larvae were checked every other day. Workers that died during the experiment were removed, weighed and replaced by new tagged workers originating from the same colony. Microcolonies were reared in a dark room (27 ± 1 °C; 60 ± 10% humidity) and manipulated under red light to minimise disturbance. Upon the end of the experiment, the masses of adult individuals were noted, the brood was carefully dissected, and the masses of individuals in each developmental stage were recorded. No mortality among emerged males was observed during the experiments.
2.2. Parasite inoculation and monitoring
Three B. terrestris queens were collected in March 2021 and determined to be infected by Crithidia sp. through faecal inspection under a microscope (it is impossible to differentiate the cryptic species C. bombi and C. expoeki under a microscope [70]). Contaminated faeces were mixed with sugar syrup (water:sucrose 50:50 w/w) and the resulting solution was provided in caps to two commercial B. terrestris colonies continuously fed with willow pollen. These two colonies were used as parasite reservoirs for the hedgerow experiment. For a year, infection was transmitted seven times through commercial colonies and thus, in March 2022, two other infected colonies were used as parasite reservoirs for the orchard experiment. For microcolony inoculation, faeces were collected from 30 workers from the two colonies used as parasite reservoirs. In the hedgerow experiment, parasite load was measured every three days after inoculation (i.e., 11 measurements) while in the orchard experiment, parasite load was measured at days 3, 5, 7, 9, 11, 15, 19 and 34 after inoculation (i.e., eight measurements). The inoculum preparation, the inoculation protocol and the parasite load monitoring are fully described in Gekière et al. [42]. Briefly, faeces from workers from the parasite reservoirs were collected and purified following a modified “triangulation” method [71]. Then, workers used in the microcolonies were starved for five hours and inoculated with 25,000 Crithidia sp. cells placed in 10 μL of sugar syrup (water:sucrose 60:40 w/w) in the tip of a 10 mL syringe. Finally, workers that consumed the inoculum were placed in their respective microcolonies. To avoid any confounding effect due to handling and starvation stress, uninoculated workers were also isolated and starved for five hours before being placed in their microcolonies. Parasite load was monitored by counting Crithidia sp. cells found in faeces under a phase contrast microscope. In the hedgerow experiment, faeces were collected from a randomly chosen worker in every infected microcolony whereas in the orchard experiment, faeces were collected from the exact same worker in every microcolony over the experiment. Uninfected microcolonies were checked to be free of parasite at the end of the experiment through faecal examination under a microscope.
2.3. Microcolony parameters
To estimate the performance and development of the microcolonies [72], we analysed (i) pollen and syrup collection as well as (ii) colony growth (i.e., the total mass of non-isolated larvae, isolated and pre-defecating larvae, isolated and post-defecating larvae, pupae, non-emerged and emerged males). We also analysed stress parameters based on (iii) larval ejection (i.e., the number of larvae removed from the brood by workers over the experiment divided by the number of hatched offspring), (iv) pollen efficacy (i.e., the mass of alive hatched offspring divided by the total mass of collected pollen), (v) pollen dilution (i.e., the total mass of collected syrup divided by the total mass of collected pollen) and (vi) worker mortality (i.e., the number of dead workers over the experiment).
2.4. Individual parameters
To estimate the immunocompetence of bumble bee individuals [73], we measured abdominal fat body content of two workers and two males per microcolony (hedgerow: n = 360; orchard: n = 280) at the end of the experiment following Ellers [74]. The abdomens were first removed from the thorax and dehydrated in an incubator at 70 °C for three days and weighed. They were then placed for one day in 2 mL of diethyl ether in order to solubilise the lipids before being washed twice and incubated at 70 °C for seven days and then weighed again. The mass difference before and after lipid solubilisation corresponds to the mass of the fat body. Fat body content is calculated by dividing the fat body mass by the dry abdomen mass prior to solubilisation.
2.5. Statistical analyses
We built distinct models for the hedgerow and orchard experiments since these two experiments were conducted with a one-year interval and included their respective controls.
To test for differences in total mass of hatched alive offspring and in pollen dilution, we built linear mixed-effect models (LMMs) and checked for linearity, normality and homoscedasticity of residuals. Pollen dilution was log-transformed in the orchard experiment to meet the previous assumptions. To test for differences in pollen efficacy, larval ejection and fat body content, we built generalised linear mixed-effect models (GLMMs) and checked the deviance residual diagnostics to ensure goodness of fit. Pollen efficacy and fat body content were assessed using a beta error structure with a logit link, since they are continuous variables restricted to the interval [0, 1]. For pollen efficacy, we discarded two outliers in the orchard experiments. Larval ejection was assessed using a binomial error structure with a logit link with the number of ejected larvae considered as the number of “successes” and the total number of hatched alive offspring considered as the number of “failures” (cbind() argument). LMMs and GLMMs included diet, parasite and their interaction as fixed effects and colony of origin as random effect. For larval ejection, microcolony nested within colony was used as a random effect to deal with overdispersion (i.e., observation-level random effect). Besides, for fat body content, we included caste and microcolony nested within colony as random effects to avoid pseudo-replication. Cumulative pollen and syrup collection, as well as parasite load were assessed using generalised additive mixed-effect models (GAMMs), to relax the GLMM restriction that relationships must be a weighted sum, by allowing covariates to vary according to non-linear spline functions [75]. Cumulative syrup collection and log-transformed cumulative pollen collection were fitted using a Gamma error structure with a log link. Square root-transformed parasite load was fitted using a Gaussian location-scale error structure with an identity link for the mean and a logb link for the standard deviation. Pollen collection GAMM included diet, parasite and their interaction as fixed effects and microcolony nested within colony as random effect to account for repeated measures, while parasite load GAMM only considered diet as fixed effect (since infection was only assessed in infected treatments). The goodness-of-fit was checked using diagnostic information and plots. Survival analyses were conducted using mixed-effect proportional hazards regression models (Cox models) after checking for proportional hazard assumptions. Cox models included diet, parasite and their interaction as fixed effects and colony as random effect. When models highlighted a significant effect of diet (p-value < 0.05), we ran between-diet contrasts conditioned on the infection status because we were not interested by the impacts of the parasite itself in this study (i.e., uninfected treatments were never contrasted to infected treatments). Error due to multiple comparisons was controlled using the one stage false discovery rate (FDR) method and the corrected p-values are presented. All analyses and graphs were run in R version 4.1.3 [76] using the packages mgcv [77], glmmTMB [78], emmeans [79], DHARMa [80], survival [81], survminer [82], coxme [83], ggpubr [84] and ggplot2 [85]. Detailed statistical outputs from the models can be found in Supplementary Table S3.
3. Results
3.1. Hedgerow experiment
3.1.1. Resource collection
Irrespective of the infection, we found a lower cumulative pollen collection in microcolonies fed the natural diet than in microcolonies fed either the control (Uninfected: t = −3.278, p = 0.003; Infected: t = −4.334, p < 0.001) or supplemented diet (Uninfected: t = −2.165, p = 0.046; Infected: t = −3.217, p = 0.002), while the cumulative pollen collection in microcolonies fed one of the two latter diets did not differ from each other (Supplementary Figure S2A,B). Regarding syrup, there was no significant impact of diet on cumulative collection (Supplementary Figure S3A,B).
3.1.2. Reproduction
The only difference in mass of alive hatched offspring was detected in uninfected microcolonies, whereby we found a lower mass of alive hatched offspring in microcolonies fed the supplemented diet when compared to microcolonies fed either the control (t = −4.324, p < 0.001) or natural diet (t = −3.547, p = 0.001). In these uninfected microcolonies, the mass of alive hatched offspring did not differ between microcolonies fed the control diet and microcolonies fed the natural diet (Supplementary Figure S4A,B).
3.1.3. Stress response
In the uninfected treatments, we observed a higher larval ejection in microcolonies fed the supplemented diet than in microcolonies fed the control (t = 2.300,p = 0.036) or natural diet (t = 2.464, p = 0.036), while the larval ejection in microcolonies fed the one of the two latter diets did not differ between each other (Supplementary Figure S5A). By contrast, in the infected treatments, we found a higher larval ejection in microcolonies fed the supplemented diet (supplemented vs. control: t = 5.594, p < 0.001; supplemented vs. natural: t = 2.567, p = 0.012), an intermediate larval ejection in microcolonies fed the natural diet (natural vs. control: t = 3.219, p = 0.003), and a lower larval ejection in microcolonies fed the control diet (Supplementary Figure S5B). Regarding the pollen efficacy, irrespective of the infection, it was higher in microcolonies fed the natural diet than in microcolonies fed the control diet (Uninfected: t = 7.313, p < 0.001; Infected: t = 10.771, p < 0.001) or supplemented diet (Uninfected: t = 7.771, p < 0.001; Infected: t = 8.523, p < 0.001), while the pollen efficacy in microcolonies fed one of the two latter diets did not differ between each other (Figure 1A,B). Regarding the pollen dilution, irrespective of the infection, it was lower in microcolonies fed the control diet when compared to microcolonies fed either the natural (Uninfected: t = −11.544, p < 0.001; Infected: t = −4.684, p < 0.001) or supplemented diet (Uninfected: t = −12.735, p < 0.001; Infected: t = −3.750, p = 0.001), while the pollen dilution in microcolonies fed one of the two latter diets did not differ between each other (Supplementary Figure S6A,B).
Parameters recorded in the hedgerow experiment from uninfected (A,C,E) and infected (B,D,F) microcolonies. Control diet = Salix sp. pollen. Natural diet = Crataegus monogyna pollen. Flavo diet = Control pollen laced with flavonoids from natural pollen. (A,B) Pollen efficacy, defined as the mass of alive hatched offspring divided by total mass of collected pollen per microcolony. (C,D) Fat body content in bumble bee individuals. ▴: Female individuals. ∙: Male individuals. (E,F) Kaplan–Meier survival curves for microcolonies across diets. Note that y-axis have been truncated for easier interpretation. Two treatments sharing a letter are not significantly different (GLMM). n.s. Not significant.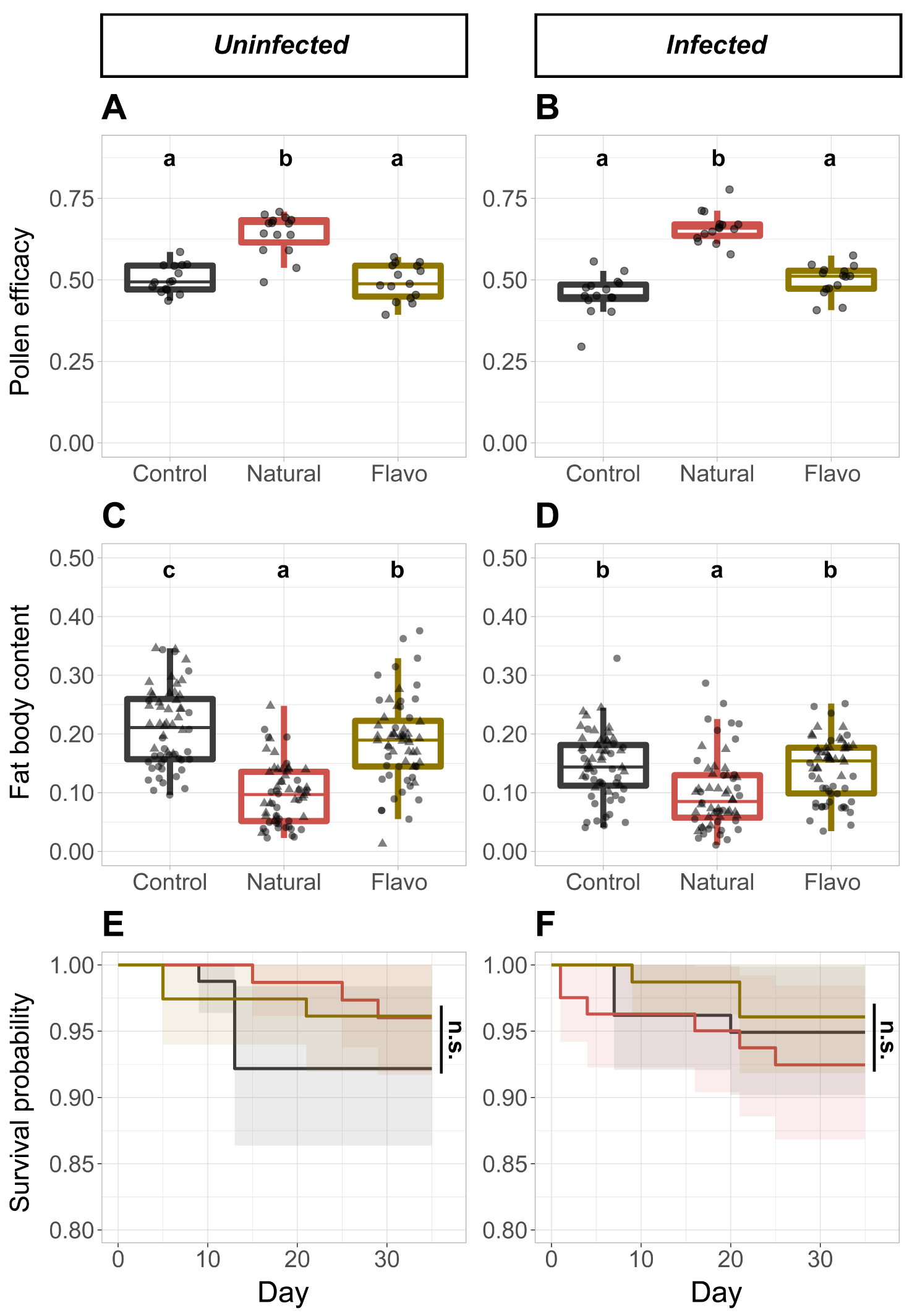
3.1.4. Immunity and health
In the uninfected treatments, we found a higher fat body content in individuals housed in microcolonies fed the control diet (control vs. natural: t = 9.112, p < 0.001; control vs. supplemented: t = 2.117, p = 0.035), an intermediate fat body content in individuals housed in microcolonies fed the supplemented diet (supplemented vs. natural: t = 7.065, p < 0.001), and a lower fat body content in individuals housed in microcolonies fed the natural diet (Figure 1C). By contrast, in the infected treatments, we observed a lower fat body content in individuals housed in microcolonies fed the natural diet than in individuals housed in microcolonies fed the control (t = −5.027, p < 0.001) or supplemented diet (t = −4.634, p < 0.001), while fat body content in individuals housed in microcolonies fed one of the two latter diets did not differ between each other (Figure 1D). Regarding the other parameters, we did not find any significant impact of diets on worker mortality (Figure 1E,F) or on parasite load (Figure 2A).
Parasite load in Crithidia-infected Bombus terrestris individuals. Control diet = Salix sp. pollen. Flavonoid diet = Control pollen laced with flavonoids from natural pollen. (A) Hedgerow experiment (i.e., natural diet = Crataegus monogyna pollen). (B) Orchard experiment (i.e., natural diet = mix of Prunus avium, Malus domestica and Pyrus communis pollen). Two treatments sharing a letter are not significantly different (GAMM). n.s. Not significant.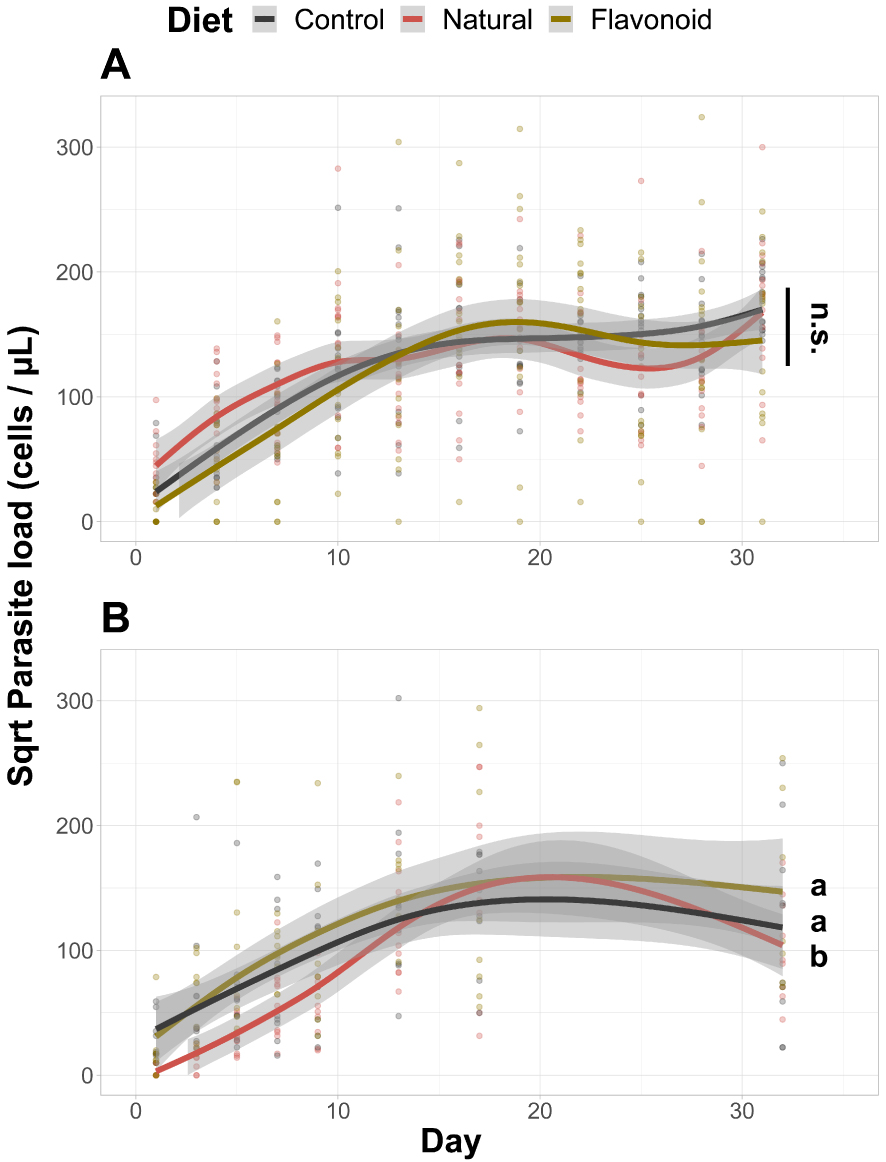
3.2. Orchard experiment
3.2.1. Resource collection
We found that uninfected microcolonies fed the supplemented diet had a lower cumulative pollen collection when compared to uninfected microcolonies fed either the control (t = −9.272, p < 0.001) or natural diet (t = −9.352, p < 0.001), while the cumulative pollen collection in microcolonies fed one of the two latter diets did not differ between each other (Supplementary Figure S2C). On the contrary, there was no difference in the cumulative pollen collection across diets in infected microcolonies (Supplementary Figure S2D). Regarding syrup, there was no significant impact of diet on cumulative collection (Supplementary Figure S3C,D).
3.2.2. Reproduction
In the uninfected treatments, we found a higher mass of alive hatched offspring in microcolonies fed the natural diet (natural vs. control: t = 5.086, p < 0.001; natural vs. supplemented: t = 9.853, p < 0.001), an intermediate total mass of alive hatched offspring in microcolonies fed the control diet (control vs. supplemented: t = 4.767, p < 0.001), and a lower total mass of alive hatched offspring in microcolonies fed the supplemented diet (Supplementary Figure S4C). By contrast, in the infected treatments, we found a higher mass of alive hatched offspring in microcolonies fed the natural when compared to microcolonies fed the control (t = 3.036, p = 0.006) or supplemented diet (t = 4.824, p < 0.001), while the mass of alive hatched offspring in microcolonies fed one of the two latter diets did not differ between each other (Supplementary Figure S4D).
3.2.3. Stress response
Regarding the larval ejection, there was no significant difference among diets, irrespective of the infection (Supplementary Figure S5C,D). Regarding the pollen efficacy, irrespective of the infection, it was lower in microcolonies fed the supplemented diet compared to microcolonies fed either the control (Uninfected: t = −3.472, p = 0.002; Infected: t = −3.725, p = 0.002) or natural diet (Uninfected: t = −4.436, p < 0.001; Infected: t = −2.839, p = 0.010), while the pollen efficacy in microcolonies fed the one of the two latter diets did not differ between each other (Figure 3A,B). Regarding pollen dilution, in uninfected microcolonies, we observed a higher pollen dilution in microcolonies fed the supplemented diet (supplemented vs. control: t = 2.893, p = 0.006; supplemented vs. natural: t = 6.114, p < 0.001), an intermediate pollen dilution in microcolonies fed the control diet (control vs. natural: t = 3.221, p = 0.003), and a lower pollen dilution in microcolonies fed the natural diet (Supplementary Figure S6C). By contrast, in infected microcolonies, we observed a higher pollen dilution in microcolonies fed the control diet (control vs. natural: t = 4.973, p < 0.001; control vs. supplemented: t = 2.770, p = 0.012), an intermediate pollen dilution in microcolonies fed the supplemented diet (supplemented vs. natural: t = 2.203, p = 0.032), and a lower pollen dilution in microcolonies fed the natural diet (Supplementary Figure S6D).
Parameters recorded in the orchard experiment from uninfected (A,C,E) and infected (B,D,F) microcolonies. Control diet = Salix sp. pollen. Natural diet = mix of Prunus avium, Malusdomestica and Pyrus communis pollen. Flavo diet = Control pollen laced with flavonoids from natural pollen. (A,B) Pollen efficacy, defined as the mass of alive hatched offspring divided by total mass of collected pollen per microcolony. (C,D) Fat body content in bumble bee individuals. ▴: Female individuals. ∙: Male individuals. (E,F) Kaplan–Meier survival curves for microcolonies across diets. Note that y-axis have been truncated for easier interpretation. Two treatments sharing a letter are not significantly different (GLMM). n.s. Not significant.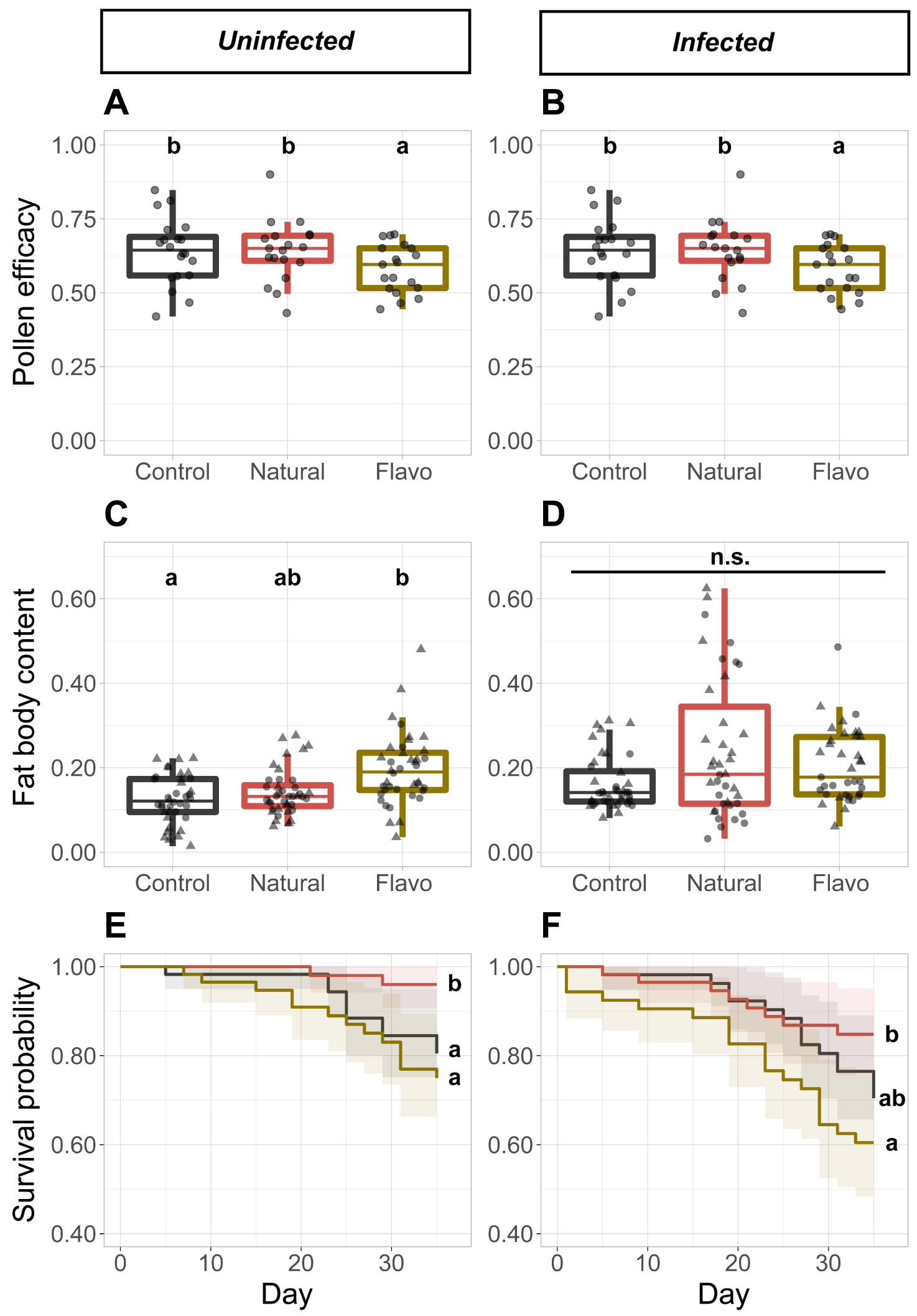
3.2.4. Immunity and health
The only difference in fat body content was detected in uninfected microcolonies, whereby individuals housed in microcolonies fed the supplemented diet showed higher fat body content than individuals housed in microcolonies fed the control diet (t = 2.979, p = 0.010; Figure 3C,D). Regarding mortality, it was higher in microcolonies fed the supplemented diet than in microcolonies fed the natural diet, irrespective of the infection (Uninfected: HR = 7.012, z = −2.564, p = 0.031; Infected: HR = 3.067, z = −2.676, p = 0.022; Figure 3E,F). We also observed a higher mortality in uninfected microcolonies fed the control diet than in uninfected microcolonies fed the natural diet (HR = 5.230, z = 2.136, p = 0.049; Figure 3E). Regarding the parasite load, it was lower in individuals housed in microcolonies fed the natural diets over individuals housed in microcolonies fed either the control (t = −2.188, p = 0.045) or supplement diet (t = −3.145, p = 0.006), while parasite load in individuals housed in microcolonies fed one of the two latter diets did not differ between each other (Figure 2B).
4. Discussion
4.1. Hedgerow and orchard pollen as suitable diets
Based on the mass of alive hatched offspring, hedgerow (i.e., hawthorn) and orchard (i.e., sweet cherry, apple and pear) pollen were as adequate or even better than willow pollen, respectively, for the microcolony development of the buff-tailed bumble bee. For the hedgerow experiment, these results are in line with Wood et al. [48] who found no beneficial effect of hawthorn pollen over willow pollen for bumble bee microcolony development, despite a higher protein-to-lipid ratio and higher amino acid content in the former. For the orchard experiment, these results are in line with Aupinel et al. [86] and Genissel et al. [87] who highlighted a higher offspring production and a reduced oophagy in microcolonies fed sweet cherry pollen when compared to microcolonies fed willow pollen, likely due to higher protein, amino acid and sterol content in sweet cherry pollen [88, 89]. Especially, the latter contains a 20-times higher concentration of 24-methylenecholesterol than willow pollen [88, 89], a crucial sterol for bee moulting and ovary development [90]. To our knowledge, no study has ever assessed the impact of pure apple or pear tree pollen on bumble bee microcolony development. Barraud et al. [91] showed that a mix of apple, pear and willow pollen did not lead to significant differences in microcolony development when compared to pure willow pollen, suggesting that apple or pear tree pollen should not have drastic negative consequences for bumble bee development (e.g., toxic phytochemicals). Quinet et al. [57] suggested that apple and pear trees are complementary for bumble bees as they produce a huge quantity of pollen and nectar, respectively. Hedgerow and orchard pollen suitability for bumble bees was further confirmed by a low mortality rate in microcolonies.
By contrast, immunocompetence was slightly impacted by the natural pollen treatments, as they were as adequate or less adequate than willow pollen for fat body content of the buff-tailed bumble bee. It could be due to quantitative or qualitative reasons: (i) bumble bees collected slightly less natural than control pollen, or (ii) natural pollen differ in their metabolite content when compared to control pollen. Differences in nutrient intakes are likely to influence fat body development [92, 93, 94, 95], as in Gekière et al. [42] wherein sunflower pollen reduced fat body content when compared to willow pollen. By contrast, another study conducted by our team showed that bumble bees fed natural heather pollen (Calluna vulgaris; Ericales: Ericaceae) had higher fat body content than bumble bees fed willow pollen (Tourbez et al. [96]). However, many studies did not find any influence of the pollen diet on fat body content [78, 97, 98, 99, 100]. Overall, nutritional compounds underlying fat body development remain obscure and definitely warrant further attention. Likewise, we must stress that there is an increasing uncertainty regarding the adequacy of fat body content as a proxy for immunocompetence, as results are inconsistent and hard to interpret. Although fat bodies have been shown as the main sites of antimicrobial peptide production [101] and the main organs for lipid metabolization [102]—two crucial steps in immune responses—no study has ever properly demonstrated a correlation between fat body content and immunocompetence in insects (Gekière, Dewaele, et al. [103]).
4.2. Natural pollen diets shape resistance towards parasite infection
Regarding pollen medicinal effects, we found that orchard pollen slightly increased bumble bee resistance towards infection (i.e., decreased load), which could be due to the poor adaptation of our parasite strains for orchard pollen. Indeed, Crithidia sp. require pollen to thrive in the gut lumen and specific strains are likely selected when bumble bees are chronically fed the same pollen diet [104]. Because our strains were sampled from stock bumble bees fed willow pollen for more than a year (2021–2022), it is likely that these strains thrived better on willow pollen than orchard pollen. This interpretation is in line with the hedgerow experiment wherein bumble bees fed willow pollen did not differ in their parasite load from bumble bees fed hawthorn pollen, likely because parasite strains were freshly acquired from the wild (spring 2021), and did not develop for more than two weeks in stock colonies fed willow pollen.
Although we focussed on the chemical roles of natural pollen on Crithidia-infected bumble bees in this study, we would like to remind that the impacts of natural pollen on gut parasites could be purely mechanical. In 2018, Adler and her team showed that sunflower pollen (Asterids: Asteraceae) reduced Crithidia sp. load in Bombus impatiens [105] but further research failed to identify any specialised metabolites accountable for such effects [106]. Recently, they found that sunflower medicinal effects were due to its spiny exine, rather than to its chemical profile [107]. Here, the pollen species we used belong to the Rosaceae family which harbours smooth exines [108]. Hedgerow and orchard pollen were consequently unlikely to display any medicinal effects through their morphological features.
4.3. Slight detrimental impacts of flavonoid extracts
An intriguing observation to underline is the different results obtained in microcolonies fed flavonoid diets when compared to microcolonies fed natural diets, whereas both diets harbour the same flavonoid profiles. We propose that although pollen grains containing these phytochemicals are constantly ingested by bumble bees, it could be that these phytochemicals are not readily bioavailable in the bumble bee gut (e.g., because of the pollen wall). For instance, Omar et al. [109] demonstrated that honey bees digested 60% of the proteins found in corn pollen when fed raw pollen, while honey bees fed with crushed pollen digested >70% of the proteins. Further, some phytochemicals are confined in the pollen wall and are therefore likely to never be digested (e.g., the biopolymer sporopollenin) [110]. By treating pollen with chemical solvents, we presumably rendered bioavailable phytochemicals that would not be naturally digested in the bee gut, or at lower concentrations. Besides, as we used willow pollen in the mix for flavonoid diets, we exposed bumble bees not only to flavonoid extracts but also to flavonoids naturally occurring in willow pollen. Using willow pollen in the mix for flavonoid diets also provided bumble bees with central metabolites (e.g., lipids) different from the ones in the natural diets. This limitation prevents us from drawing clear conclusions on hedgerow and orchard flavonoid consequences on bumble bees. As already stressed in previous studies (e.g., [95, 99]), this research further calls for the development of controlled pollen substitutes for laboratory experiments on bees.
In our experiment, flavonoid extracts mostly had detrimental impacts for the microcolony development and the survival of the buff-tailed bumble bee. We are not aware of any studies tackling the influence of these specialised metabolites on bumble bee microcolony development, but flavonoids did not increase mortality rate in honey bees [111]. Given that flavonoids are ubiquitous in pollen species [112], we expect that they should have only minor effects on bumble bee colonies, which somewhat contrasts with our results. This is however in accordance with a previous research conducted in our lab, wherein we found that phenolamide extracts—a major class of phenylpropanoid metabolites evolutionarily conserved across angiosperms—had detrimental impacts on bumble bee microcolony development [42]. In bees, larval development is highly shaped by pollen properties, whether it is due to pollen metabolite content or harmful morphological features, because bee species differ in their abilities to deal with specific pollen species [113, 114]. Our experiment does not enable us to disentangle if the impeded offspring production in microcolonies fed flavonoid diets is caused by either altered worker behaviours or phytochemical unsuitability for the larvae. Yet, one may suggest that it is due to phytochemical unsuitability because our results showed that pollen collection did not seem to explain offspring production. Further experiments using larval rearing in vitro [115] and isolating pollen wall [116] are required to directly assess diet phytochemical and morphological suitability for bumble bee larval development.
Strikingly, flavonoid extracts mostly had beneficial consequences on fat body content, although this parameter must be interpreted with caution as previously discussed. Based on Ellers [74], we hypothesise that it could be explained by a trade-off between immunocompetence and reproduction, whereby egg-laying workers allocated their fat resources in their ovaries instead of their body fat. Indeed, higher body fat content was found in microcolonies fed flavonoid diets which had a lower reproductive success. We could argue that we should not observe this trade-off in our results since it is assumed that only one worker lays egg in microcolonies [117], and that we did not target these pseudo-queens for fat body analyses. However, personal observations and previous studies showed that several workers could have well-developed ovaries in queen-less microcolonies [118], which is consistent with our results.
Surprisingly, flavonoids did not reduce parasite load, whereas flavonoids were shown to have antimicrobial properties (e.g., Vairimorpha spp. load reduction in honey bees [30] and bumble bees [31]). As far as we know, there is no report of flavonoid impacts on Crithidia sp. in bumble bees, but Palmer-Young et al. [119] studied flavonoid impacts in vitro on C. mellificae (host: honey bees) and C. fasciculata (host: mosquitoes). They found no effect on Crithidia spp. growth except for the flavonoid chrysin, which hampered C. fasciculata but not C. mellificae. They suggested a flavonoid resistance for C. mellificae since this parasite is recurrently exposed to flavonoids in pollen and propolis [120], while C. fasciculata is only exposed to flavonoids found in nectar [112]. Despite the flavonoid antimicrobial properties [29], it is hence perfectly reasonable to assume that Crithidia sp. is able to deal with flavonoid exposure.
4.4. Bee conservation strategies and perspectives
Overall, our results are supporting the development of orchards and hedgerows as mitigation strategies to face decline of wetlands, wet heathlands and their associated willow trees due to human activities and extreme climate events [52, 53, 54], since bumble bees greatly rely on willows for colony initiation [49, 50, 51]. Given the phenology of the investigated tree species (Supplementary Figure S7), our results suggest that bumble bees could lean on sweet cherry, pear and apple tree pollen for colony initiation in early spring, while hawthorn pollen could rather be used by older bumble bee colonies later in the season. However, although sweet cherry, apple and pear orchards have been increasingly harvested worldwide since 1960, a worrying decline has been observed for the last 20 years (Supplementary Figure S8 [121]). Besides, there is no data around hedgerow management, and it is therefore impossible to tell whether hedgerows will significantly contribute to bumble bee diets in future springs. Moreover, even though flavonoids did not help bumble bees reduce infection, they did not show any harmful effects and remain important dietary phytochemicals to deal with environmental stress, such as agrochemical exposure [33, 122]. In conclusion, trees represent nutritional resources of tremendous importance for bumble bees, especially early flowering species that are crucial to sustain colony initiation. Sweet cherry, apple and pear trees bloom in early spring, and are good candidates to offset the ongoing decline of early flowering willows, while hawthorn is a suitable resource for the late season. In addition to preserving early blooming tree species, conservation strategies should further consider specialised metabolite profiles, especially flavonoids, when designing agri-environmental schemes to support wild bee health.
Conflicts of interest
Authors have no conflict of interest to declare.
Author contributions
Conceptualisation, AG and MV; formal analysis, AG, AM and MV; funding acquisition, DM, PG and MV; investigation, AG, AM, CT and MB; methodology, AG, IS and MV; resources, DM and PG; supervision, DM and MV; visualisation, AG; writing—original draft, AG with the help of MV; writing—review and editing, AG, AM, IS, CT, MB, DM, PG and MV.
Funding
This work was part of the ARC “Actions de Recherche Concertées” project “METAFLORE”, 2019–2023. AG is supported by a F.R.S.-FNRS PhD grant “Aspirant”. The PhD grant of IS is supported by the ARC project METAFLORE. The PhD grant of CT is supported by the University of Mons (UMons).
Acknowledgements
We would like to thank D. Evrard and L. Verdy for their help in logistics as well as the numerous people that helped during microcolony dissection. Many thanks to L. Marin for her help in daily monitoring. We are very grateful to the Laboratory of Cellular Biology (UMons) for their help in microscopic analyses.




 CC-BY 4.0
CC-BY 4.0